Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

666 Chapter 13 Reacting Mixtures and Combustion
SOLUTION
Known: Methane gas reacts completely with 140% of the theoretical amount of air. Combustion products exit the reactor at
1 atm and a specified temperature. The environment is also specified.
Find: Determine the flow exergy of the combustion products, in Btu per lbmol of fuel, for each of two given temperatures.
Assumptions:
1. The combustion products are modeled as an ideal gas mixture at all states considered.
2. Neglect the effects of motion and gravity.
Analysis: For 140% theoretical air, the reaction equation for complete combustion of methane is
The flow exergy is given by Eq. 13.47, which involves chemical and thermomechanical contributions. Since the com-
bustion products form an ideal gas mixture when at T
0
, p
0
(assumption 1) and each component is present within the en-
vironment, the chemical exergy contribution, per mole of fuel, is obtained from the following expression patterned after
Eq. 13.41a
From the reaction equation, the mole fractions of the components of the products are
Substituting these values together with the respective environmental mole fractions, we obtain
Applying ideal gas mixture principles, the thermomechanical contribution to the flow exergy, per mole of fuel, is
Since p p
0
, each of the logarithm terms drop out, and with and data at T
0
from Table A-23, the thermomechanical
contribution reads
Then, with and from Table A-23 at T 480 and 1560K, respectively, the following results are obtained
Adding the two contributions, the flow exergy of the combustion products at each of the specified states is
This is a hypothetical state for the combustion products because condensation of some of the water vapor present would
occur were the products brought to T
0
, p
0
. An exergy evaluation explicitly taking such condensation into account is con-
sidered in Bejan, Tsatsaronis and Moran, Thermal Design and Optimization, p. 129, p. 138.
The chemical contribution to the flow exergy is relatively unimportant in the higher-temperature case, amounting only
to about 4% of the flow exergy. Chemical exergy accounts for about half of the exergy in the lower-temperature case,
however.
T 1560 K: e
f
408,603 kJ per kmol of fuel
T 480 K: e
f
35,462 kJ per kmol of fuel
T 1560 K: h
h
0
T
0
1s s
0
2 390, 853 kJ per kmol of fuel
T 480 K: h
h
0
T
0
1s s
0
2 17,712 kJ per kmol of fuel
s
°h
0.83h1T 2 8682 2981s°1T 2 205.03324
O
2
10.533h1T 2 8669 2981s°1T 2 191.50224
N
2
23h1T 2 9904 2981s°1T 2 188.7224
H
2
O
h h
0
T
0
1s s
0
2 3h1T 2 9364 2981s°1T 2 213.68524
CO
2
s°h
0.83h1T 2 h1T
0
2 T
0
1s°1T 2 s°1T
0
2 R ln1y
O
2
p
y
O
2
p
0
224
O
2
10.533h1T 2 h1T
0
2 T
0
1s°1T 2 s°1T
0
2 R ln1y
N
2
p
y
N
2
p
0
224
N
2
23h1T 2 h1T
0
2 T
0
1s°1T 2 s°1T
0
2 R ln1y
H
2
O
p
y
H
2
O
p
0
224
H
2
O
h h
0
T
0
1s s
0
2 3h1T 2 h1T
0
2 T
0
1s°1T 2 s°1T
0
2 R ln1y
CO
2
p
y
CO
2
p
0
224
CO
2
e
ch
17,750 kJ per kmol of fuel.
0.7348, y
O
2
0.0558.
y
CO
2
0.0698, y
H
2
O
0.1396, y
N
2
e
ch
RT
0
c1 ln a
y
CO
2
y
e
CO
2
b 2 ln a
y
H
2
O
y
e
H
2
O
b 10.53 ln a
y
N
2
y
e
N
2
b 0.8 ln a
y
O
2
y
e
O
2
bd
CH
4
2.81O
2
3.76N
2
2S CO
2
2H
2
O 10.53N
2
0.8O
2
❷
❶
❶
❷

13.9 Exergetic (Second Law) Efficiencies of Reacting Systems 667
Devices designed to do work by utilization of a combustion process, such as vapor and gas
power plants and internal combustion engines, invariably have irreversibilities and losses as-
sociated with their operation. Accordingly, actual devices produce work equal to only a frac-
tion of the maximum theoretical value that might be obtained in idealized circumstances. The
vapor power plant exergy analysis of Sec. 8.6 and the combined cycle exergy analysis of
Example 9.13 provide illustrations.
The performance of devices intended to do work can be evaluated as the ratio of the actual
work developed to the maximum theoretical work. This ratio is a type of exergetic (second law)
efficiency. The relatively low exergetic efficiency exhibited by many common power-producing
devices suggests that thermodynamically more thrifty ways of utilizing the fuel to develop power
might be possible. However, efforts in this direction must be tempered by the economic imper-
atives that govern the practical application of all devices. The trade-off between fuel savings and
the additional costs required to achieve those savings must be carefully weighed.
The fuel cell provides an illustration of a relatively fuel-efficient device. We noted previ-
ously (Sec. 13.4) that the chemical reactions in fuel cells are more controlled than the rapidly
occurring, highly irreversible combustion reactions taking place in conventional power-
producing devices. In principle, fuel cells can achieve greater exergetic efficiencies than many
such devices.
The example to follow illustrates the evaluation of an exergetic efficiency for an internal
combustion engine.
13.9 Exergetic (Second Law) Efficiencies
of Reacting Systems
EXAMPLE 13.15 Exergetic Efficiency of an Internal Combustion Engine
Devise and evaluate an exergetic efficiency for the internal combustion engine of Example 13.4. For the fuel, use the chemi-
cal exergy value determined in Example 13.12(a).
SOLUTION
Known: Liquid octane and the theoretical amount of air enter an internal combustion engine operating at steady state in sep-
arate streams at 25C, 1 atm, and burn completely. The combustion products exit at 890 K. The power developed by the en-
gine is 37 kW, and the fuel mass flow rate is 1.8 10
3
kg/s.
Find: Devise and evaluate an exergetic efficiency for the engine using the fuel chemical exergy value determined in Exam-
ple 13.12(a).
Schematic and Given Data: See Fig. E13.4.
Assumptions:
1. See the assumptions listed in the solution to Example 13.4.
2. The environment is the same as used in Example 13.12(a).
3. The combustion air enters at the condition of the environment.
4. Kinetic and potential energy effects are negligible.
Analysis: An exergy balance can be used in formulating an exergetic efficiency for the engine: At steady state, the rate at
which exergy enters the engine equals the rate at which exergy exits plus the rate at which exergy is destroyed within the en-
gine. As the combustion air enters at the condition of the environment, and thus with zero exergy, exergy enters the engine
only with the fuel. Exergy exits the engine accompanying heat and work, and with the products of combustion.

668 Chapter 13 Reacting Mixtures and Combustion
If the power developed is taken to be the product of the engine, and the heat transfer and exiting product gas are regarded
as losses, an exergetic efficiency expression that gauges the extent to which the exergy entering the engine with the fuel is
converted to the product is
where denotes the rate at which exergy enters with the fuel.
Since the fuel enters the engine at 25C and 1 atm, which correspond to the values of T
0
and p
0
of the environment,
and kinetic and potential energy effects are negligible, the exergy of the fuel is just the chemical exergy evaluated in
Example 13.12(a). There is no thermomechanical contribution. Thus
The exergetic efficiency is then
The “waste heat” from large engines may be utilizable by some other device—for example, an absorption heat pump. In
such cases, some of the exergy accompanying heat transfer and the exiting product gas might be included in the numera-
tor of the exergetic efficiency expression. Since a greater portion of the entering fuel exergy would be utilized in such
arrangements, the value of would be greater than that evaluated in the solution.
e a
37 kW
85.2 kW
b 0.434 143.4%2
E
#
F
m
#
F
e
ch
a1.8 10
3
kg
s
b a47,346
kJ
kg
b`
1 kW
1 kJ/s
` 85.2 kW
E
#
F
e
W
#
cv
E
#
F
❶
❶
In the next example, we evaluate an exergetic efficiency for a reactor.
EXAMPLE 13.16 Exergetic Efficiency of a Reactor
For the reactor of Example 13.9, determine the exergy destruction, in kJ per kmol of fuel, and devise and evaluate an exer-
getic efficiency. Consider (a) complete combustion with the theoretical amount of air (b) complete combustion with 400%
theoretical air. For the fuel, use the chemical exergy value determined in Example 13.12(a).
SOLUTION
Known: Liquid octane and air, each at 25C and 1 atm, burn completely in a well-insulated reactor operating at steady state.
The products of combustion exit at 1 atm pressure.
Find: Determine the exergy destruction, in kJ per kmol of fuel, and evaluate an exergetic efficiency for complete combus-
tion with (a) the theoretical amount of air, (b) 400% theoretical air.
Schematic and Given Data: See Fig. E13.9.
Assumptions:
1. See assumptions listed in Example 13.9.
2. The environment is the same as that in Example 13.12(a).
3. The combustion air enters at the condition of the environment.
4. Kinetic and potential energy effects are negligible.
Analysis: An exergy balance can be used in formulating an exergetic efficiency: At steady state, the rate at which exergy
enters the reactor equals the rate at which exergy exits plus the rate at which exergy is destroyed within the reactor. Since the
combustion air enters at the condition of the environment, and thus with zero exergy, exergy enters the reactor only with the
Chapter Summary and Study Guide 669
fuel. The reactor is well insulated, so there is no exergy transfer accompanying heat transfer. There is also no work . Ac-
cordingly, exergy exits only with the combustion products. An exergetic efficiency can be written as
where is the rate at which exergy enters with the fuel and is the rate at which exergy exits with the combustion
products. Using the exergy balance for the reactor, given above in words, the exergetic efficiency expression can be written
alternatively as
The exergy destruction term appearing in the last expression can be found from the relation
where T
0
is the temperature of the environment and is the rate of entropy production. The rate of entropy production is
evaluated in the solution to Example 13.9 for each of the two cases. For the case of complete combustion with the theoretical
amount of air
Similarly, for the case of complete combustion with 400% of the theoretical amount of air
Since the fuel enters the reactor at 25C, 1 atm, which correspond to the values of T
0
and p
0
of the environment, and ki-
netic and potential effects are negligible, the exergy of the fuel is just the chemical exergy evaluated in Example 13.12(a).
There is no thermomechanical contribution. Thus, for the case of complete combustion with the theoretical amount of air
Similarly, for the case of complete combustion with 400% of the theoretical amount of air
The calculated efficiency values show that a substantial portion of the fuel exergy is destroyed in the combustion process.
In the case of combustion with the theoretical amount of air, about 30% of the fuel exergy is destroyed. In the excess air
case, over 50% of the fuel exergy is destroyed. Further exergy destructions would take place as the hot gases are utilized.
It might be evident, therefore, that the overall conversion from fuel input to end use would have a relatively low exergetic
efficiency. The vapor power plant exergy analysis of Sec. 8.6 illustrates this point.
e 1
2,906,692
5,407,843
0.463 146.3%2
e 1
1,610,392
5,407,843
0.702 170.2%2
E
#
d
n
#
F
12982 197542 2,906,692
kJ
kmol
E
#
d
n
#
F
1298 K2 a5404
kJ
kmol
#
K
b 1,610,392
kJ
kmol
s
#
cv
E
#
d
n
#
F
T
0
s
#
cv
n
#
F
e
E
#
F
E
#
d
E
#
F
1
E
#
d
E
#
F
E
#
products
E
#
F
e
E
#
products
E
#
F
W
#
cv
❶
❶
Chapter Summary and Study Guide
In this chapter we have applied the principles of thermody-
namics to systems involving chemical reactions, with em-
phasis on systems involving the combustion of hydrocarbon
fuels. We also have extended the notion of exergy to include
chemical exergy.
The first part of the chapter begins with a discussion of
concepts and terminology related to fuels, combustion air, and
products of combustion. The application of energy balances
to reacting systems is then considered, including control vol-
umes at steady state and closed systems. To evaluate the spe-
cific enthalpies required in such applications, the enthalpy of
formation concept is introduced and illustrated. The determi-
nation of the adiabatic flame temperature is considered as an
application.

670 Chapter 13 Reacting Mixtures and Combustion
Key Engineering Concepts
complete
combustion p. 620
air–fuel ratio p. 622
theoretical air p. 622
percent of theoretical
air p. 623
dry product
analysis p. 625
enthalpy of
formation p. 630
heating values p. 639
adiabatic flame
temperature p. 642
fuel cell p. 645
absolute entropy p. 648
chemical exergy p. 655
standard chemical
exergy p. 659
Exercises: Things Engineers Think About
1. In a balanced chemical reaction equation, is mass conserved?
Are moles conserved?
2. If a hydrocarbon is burned with the theoretical amount of air,
can the combustion be incomplete?
3. If a hydrocarbon is burned with less than the theoretical
amount of air, can the combustion be complete?
4. For the case of Example 13.1(b), is the air–fuel mixture rich
or lean?
5. What is the equivalence ratio for the reaction of Example 13.2?
6. Why is the combustion of hydrocarbons considered a con-
tributor to global warming?
7. When applying the energy balance to a reacting system, why
is it essential that the enthalpies of each reactant and product be
evaluated relative to a common datum?
8. Why are some enthalpy of formation values in Tables A-25
positive and others negative?
9. If you knew the higher heating value of a hydrocarbon, C
a
H
b
,
how would you determine its lower heating value?
10. Why are some high-efficiency, residential natural gas fur-
naces equipped with drain tubes?
11. For a given fuel, how would the adiabatic flame temperature
vary if the percent of theoretical air were increased? Why?
12. In which case would the adiabatic flame temperature be
higher, complete combustion of methane (CH
4
) with the theoret-
ical amount of oxygen (O
2
), or complete combustion with the
theoretical amount of air, all initially at 298 K, 1 atm?
13. Why is combustion inherently an irreversible process?
14. What irreversibilities are present in the internal combustion
engine of Example 13.4? The combustion chamber of Example
13.5?
15. What is an advantage of using standard chemical exergies?
A disadvantage?
16. How might you define an exergetic efficiency for the
hydrogen–oxygen fuel cell of Fig. 13.3b
17. How might you define an exergetic efficiency for the reac-
tor of Example 13.5?
The use of the second law of thermodynamics is also dis-
cussed. The absolute entropy concept is developed to provide
the specific entropies required by entropy balances for sys-
tems involving chemical reactions. The related Gibbs func-
tion of formation concept is introduced. The first part of the
chapter also includes a discussion of fuel cells.
In the second part of the chapter, we extend the exergy con-
cept of Chap. 7 by introducing chemical exergy. The standard
chemical exergy concept is also discussed. Means are developed
and illustrated for evaluating the chemical exergies of hydro-
carbon fuels and other substances. The presentation concludes
with a discussion of exergetic efficiencies of reacting systems.
The following list provides a study guide for this chapter.
When your study of the text and end-of-chapter exercises has
been completed, you should be able to
write out the meaning of the terms listed in the margin
throughout the chapter and understand each of the re-
lated concepts. The subset of key concepts listed below
is particularly important.
determine balanced reaction equations for the combus-
tion of hydrocarbon fuels, including complete and
incomplete combustion with various percentages of
theoretical air.
apply energy balances to systems involving chemical
reactions, including the evaluation of enthalpy using
Eq. 13.9 and the evaluation of the adiabatic flame
temperature.
apply entropy balances to systems involving chemical
reactions, including the evaluation of the entropy
produced.
evaluate the chemical exergy of hydrocarbon fuels and
other substances using Eqs. 13.35 and 13.36, as well as
the standard chemical exergy using Eqs. 13.44 and
13.45.
apply exergy analysis, including chemical exergy and the
evaluation of exergetic efficiencies.

Problems: Developing Engineering Skills 671
Problems: Developing Engineering Skills
Working with Reaction Equations
13.1 A vessel contains a mixture of 60% O
2
and 40% CO on a
mass basis. Determine the percent excess or percent deficiency
of oxygen, as appropriate.
13.2 One hundred kmol of butane (C
4
H
10
) together with 4000
kmol of air enter a furnace per unit of time. Carbon dioxide,
carbon monoxide, and unburned fuel appear in the products of
combustion exiting the furnace. Determine the percent excess
or percent deficiency of air, whichever is appropriate.
13.3 Propane (C
3
H
8
) is burned with air. For each case, obtain
the balanced reaction equation for complete combustion
(a) with the theoretical amount of air.
(b) with 20% excess air.
(c) with 20% excess air, but only 90% of the propane being
consumed in the reaction.
13.4 Methane (CH
4
) burns completely with the stiochiometric
amount of hydrogen peroxide (H
2
O
2
). Determine the balanced
reaction equation.
13.5 A fuel mixture with the molar analysis 40% CH
3
OH, 50%
C
2
H
5
OH, and 10% N
2
burns completely with 33% excess air.
Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio, both on a molar and mass basis.
13.6 A natural gas with the molar analysis 78% CH
4
, 13% C
2
H
6
,
6% C
3
H
8
, 1.7% C
4
H
10
, 1.3% N
2
burns completely with 40%
excess air in a reactor operating at steady state. If the molar
flow rate of the fuel is 0.5 kmol/h, determine the molar flow
rate of the air, in kmol/h.
13.7 A fuel mixture with the molar analysis of 20% CH
4
, 40%
H
2
, 40% NH
3
burns completely with 150% of theoretical
oxygen. Determine the balanced reaction equation.
13.8 Coal with the mass analysis 77.54% C, 4.28% H, 1.46% S,
7.72% O, 1.34% N, 7.66% noncombustible ash burns com-
pletely with 120% of theoretical air. Determine
(a) the balanced reaction equation.
(b) the amount of SO
2
produced, in kg per kg of coal.
13.9 A coal sample has a mass analysis of 80.4% carbon, 3.9%
hydrogen (H
2
), 5.0% oxygen (O
2
), 1.1% nitrogen (N
2
), 1.1%
sulfur, and the rest is noncombustible ash. For complete com-
bustion with 120% of the theoretical amount of air, determine
the air–fuel ratio on a mass basis.
13.10 A sample of dried feedlot manure is being tested for use
as a fuel. The mass analysis of the sample is 42.7% carbon,
5.5% hydrogen (H
2
), 31.3% oxygen (O
2
), 2.4% nitrogen (N
2
),
0.3% sulfur, and 17.8% noncombustible ash. The sample is
burned completely with 120% of theoretical air. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio on a mass basis.
13.11 A sample of dried Appanoose County coal has a mass
analysis of 71.1% carbon, 5.1% hydrogen (H
2
), 9.0% oxygen
(O
2
), 1.4% nitrogen (N
2
), 5.8% sulfur, and the rest noncom-
bustile ash. For complete combustion with the theoretical
amount of air, determine
(a) the amount of SO
2
produced, in kg per kg of coal.
(b) the air–fuel ratio on a mass basis.
13.12 Octane (C
8
H
18
) burns completely with 120% of theoret-
ical air. Determine
(a) the air–fuel ratio on a molar and mass basis.
(b) the dew point temperature of the combustion products, in
C, when cooled at 1 atm.
13.13 Butane (C
4
H
10
) burns completely with 150% of theoret-
ical air. If the combustion products are cooled at 1 atm to tem-
perature T, plot the amount of water vapor condensed, in kmol
per kmol of fuel, versus T ranging from 20 to 60C.
13.14 A gaseous fuel mixture with a molar analysis of 72%
CH
4
,9% H
2
, 14% N
2
, 2% O
2
, and 3% CO
2
burns completely
with moist air to form gaseous products at 1 atm consisting of
CO
2
,H
2
O, and N
2
only. If the dew point temperature of the
products is 60C, determine the amount of water vapor pres-
ent in the combustion air, in kmol per kmol of fuel mixture.
13.15 Acetylene (C
2
H
2
) enters a torch and burns completely
with 110% of theoretical air entering at 25C, 1 atm, 50%
relative humidity. Obtain the balanced reaction equation, and
determine the dew point temperature of the products, in C, at
1 atm.
13.16 Ethane (C
2
H
6
) enters a furnace and burns completely with
130% of theoretical air entering at 25C, 85 kPa, 50% relative
humidity. Determine
(a) the balanced reaction equation.
(b) the dew point temperature of the combustion products, in
C, at 85 kPa.
13.17 A liquid fuel mixture that is 40% octane (C
8
H
18
) and 60%
decane (C
10
H
22
) by mass is burned completely with 10% ex-
cess air at 25C, 1 atm, 80% relative humidity.
(a) Determine the equivalent hydrocarbon composition, C
a
H
b
,
of a fuel that would have the same carbon–hydrogen ratio
on a mass basis as the fuel mixture.
(b) If the combustion products are cooled to 25C at a pres-
sure of 1 atm, determine the amount of water vapor that
condenses, in kg per kg of fuel mixture.
13.18 Carbon burns with 80% theoretical air yielding CO
2
, CO,
and N
2
only. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio on a mass basis.
(c) the analysis of the products on a molar basis.
13.19 Propane (C
3
H
8
) reacts with 80% of theoretical air to form
products including CO
2
, CO, H
2
O, and N
2
only. Determine
(a) the balanced reaction equation.
(b) the air–fuel ratio on a mass basis.
(c) the analysis of the products on a dry molar basis.
672 Chapter 13 Reacting Mixtures and Combustion
13.20 Hexane (C
6
H
14
) burns with air to give products with the
dry molar analysis of CO
2
, 11.5%; CO, 2.4%; O
2
, 2.0%; H
2
,
1.6%; N
2
, 82.5%. Determine the air–fuel ratio on a molar
basis.
13.21 The components of the exhaust gas of a spark-ignition
engine using a fuel mixture represented as C
8
H
17
have a dry
molar analysis of 8.7% CO
2
, 8.9% CO, 0.3% O
2
, 3.7% H
2
,
0.3% CH
4
, and 78.1% N
2
. Determine the equivalence ratio.
13.22 Decane (C
10
H
22
) burns with 95% of theoretical air, pro-
ducing a gaseous mixture of CO
2
, CO, H
2
O, and N
2
. Determine
(a) the air–fuel ratio on a molar basis.
(b) the analysis of the products on a dry molar basis.
13.23 Butane (C
4
H
10
) burns with air, giving products having the
dry molar analysis 11.0% CO
2
, 1.0% CO, 3.5% O
2
, 84.5% N
2
.
Determine
(a) the percent theoretical air.
(b) the dew point temperature of the combustion products, in
C, at 1 bar.
13.24 A fuel oil having an analysis on a mass basis of 85.7%
C, 14.2% H, 0.1% inert matter burns with air to give products
with a dry molar analysis of 12.29% CO
2
; 3.76% O
2
; 83.95%
N
2
. Determine the air–fuel ratio on a mass basis.
13.25 Ethyl alcohol (C
2
H
5
OH) burns with air. The product gas
is analyzed and the laboratory report gives only the following
percentages on a dry molar basis: 6.9% CO
2
, 1.4% CO, 0.5%
C
2
H
5
OH. Assuming the remaining components consist of O
2
and N
2
, determine
(a) the percentages of O
2
and N
2
in the dry molar analysis.
(b) the percent excess air.
13.26 A fuel oil with the mass analysis 87% C, 11% H, 1.4%
S, 0.6% inert matter burns with 120% of theoretical air. The
hydrogen and sulfur are completely oxidized, but 95% of the
carbon is oxidized to CO
2
and the remainder to CO.
(a) Determine the balanced reaction equation.
(b) For the CO and SO
2
, determine the amount, in kmol per
10
6
kmol of combustion products (that is, the amount in
parts per million).
13.27 Pentane (C
5
H
12
) burns with air so that a fraction x of the
carbon is converted to CO
2
. The remaining carbon appears as
CO. There is no free O
2
in the products. Develop plots of the
air–fuel ratio and the percent of theoretical air versus x, for x
ranging from zero to unity.
13.28 For the following mixture, determine the equivalence
ratio and indicate if the mixture is lean or rich:
1 kmol of butane (C
4
H
10
) and 32 kmol of air.
13.29 Octane (C
8
H
18
) enters an engine and burns with air to
give products with the dry molar analysis of CO
2
, 10.5%; CO,
5.8%; CH
4
, 0.9%; H
2
, 2.6%; O
2
, 0.3%; N
2
, 79.9%. Determine
the equivalence ratio.
13.30 Methane (CH
4
) burns with air to form products consist-
ing of CO
2
, CO, H
2
O, and N
2
only. If the equivalence ratio is
1.25, determine the balanced reaction equation.
Applying the First Law to Reacting Systems
13.31 Propane (C
3
H
8
) at 25C, 1 atm enters a combustion cham-
ber operating at steady state and burns completely with the the-
oretical amount of air entering at the same conditions. If the
products exit at 25C, 1 atm, determine the rate of heat trans-
fer from the combustion chamber, in kJ per kmol of fuel.
Kinetic and potential energy effects are negligible.
13.32 Methane gas (CH
4
) at 25C, 1 atm enters a steam gener-
ator operating at steady state. The methane burns completely
with 140% of theoretical air entering at 127C, 1 atm. Prod-
ucts of combustion exit at 427C, 1 atm. In a separate stream,
saturated liquid water enters at 8 MPa and exits as superheated
vapor at 480C with a negligible pressure drop. If the vapor
mass flow rate is 3.7 10
5
kg/h, determine the volumetric
flow rate of the methane, in m
3
/h.
13.33 Liquid ethanol (C
2
H
5
OH) at 25C, 1 atm enters a com-
bustion chamber operating at steady state and burns with air
entering at 227C, 1 atm. The fuel flow rate is 25 kg/s and
the equivalence ratio is 1.2. Heat transfer from the combus-
tion chamber to the surroundings is at a rate of 3.75 10
5
kJ/s. Products of combustion, consisting of CO
2
, CO, H
2
O(g),
and N
2
, exit. Ignoring kinetic and potential energy effects,
determine
(a) the exit temperature, in K.
(b) the air–fuel ratio on a mass basis.
13.34 Benzene gas (C
6
H
6
) at 25C, 1 atm enters a combustion
chamber operating at steady state and burns with 95%
theoretical air entering at 25C, 1 atm. The combustion prod-
ucts exit at 1000 K and include only CO
2
, CO, H
2
O, and N
2
.
Determine the mass flow rate of the fuel, in kg/s, to provide
heat transfer at a rate of 1000 kW.
13.35 The energy required to vaporize the working fluid pass-
ing through the boiler of a simple vapor power plant is pro-
vided by the complete combustion of methane with 110% of
theoretical air. The fuel and air enter in separate streams at
25C, 1 atm. Products of combustion exit the stack at 150C,
1 atm. Plot the mass flow rate of fuel required, in kg/h per MW
of power developed by the plant versus the plant thermal effi-
ciency, . Consider in the range 30–40%. Kinetic and
potential energy effects are negligible.
13.36 Methane (CH
4
) at 25C, enters the combustor of a sim-
ple open gas turbine power plant and burns completely with
400% of theoretical air entering the compressor at 25C, 1 atm.
Products of combustion exit the turbine at 577C, 1 atm. The
rate of heat transfer from the gas turbine is estimated as 10%
of the net power developed. Determine the net power output,
in MW, if the fuel mass flow rate is 1200 kg/h. Kinetic and
potential energy effects are negligible.
13.37 Octane gas (C
8
H
18
) at 25C enters a jet engine and burns
completely with 300% of theoretical air entering at 25C,
1 atm with a volumetric flow rate of 42 m
3
/s. Products of com-
bustion exit at 990 K, 1 atm. If the fuel and air enter with neg-
ligible velocities, determine the thrust produced by the engine
in kN.
Problems: Developing Engineering Skills 673
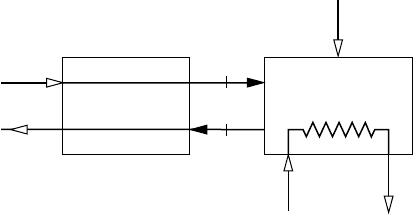
13.38 Figure P13.38 provides data for a boiler and air preheater
operating at steady state. Methane (CH
4
) entering the boiler at
25C, 1 atm is burned completely with 170% of theoretical air.
Ignoring stray heat transfer and kinetic and potential energy
effects, determine the temperature, in C, of the combustion
air entering the boiler from the preheater.
13.45 For each of the following fuels, plot the adiabatic flame
temperature, in K, versus percent excess air for complete com-
bustion in a combustor operating at steady state. The reactants
enter at 25C, 1 atm.
(a) carbon.
(b) hydrogen (H
2
).
(c) liquid octane (C
8
H
18
).
13.46 Propane gas (C
3
H
8
) at 25C, 1 atm enters an insulated re-
actor operating at steady state and burns completely with air
entering at 25C, 1 atm. Plot the adiabatic flame temperature
versus percent of theoretical air ranging from 100 to 400%.
Why does the adiabatic flame temperature vary with increas-
ing combustion air?
13.47 Methane gas (CH
4
) at 25C, 1 atm enters an insulated re-
actor operating at steady state and burns completely with x%
of theoretical air entering at 25C, 1 atm. Plot the adiabatic
flame temperature for x ranging from 100 to 400%.
13.48 Methane (CH
4
) at 25C, 1 atm, enters an insulated reac-
tor operating at steady state and burns with the theoretical
amount of air entering at 25C, 1 atm. The products contain
CO
2
, CO, H
2
O, O
2
, and N
2
, and exit at 2260 K. Determine the
fractions of the entering carbon in the fuel that burn to CO
2
and CO, respectively.
13.49 Liquid methanol (CH
3
OH) at 25C, 1 atm enters an in-
sulated reactor operating at steady state and burns completely
with air entering at 100C, 1 atm. If the combustion products
exit at 1256C, determine the percent excess air used. Neglect
kinetic and potential energy effects.
13.50 A mixture of gaseous octane (C
8
H
18
) and 200% of theo-
retical air, initially at 25C, 1 atm, reacts completely in a rigid
vessel.
(a) If the vessel were well-insulated, determine the tempera-
ture, in C, and the pressure, in atm, of the combustion
products.
(b) If the combustion products were cooled at constant vol-
ume to 25C, determine the final pressure, in atm, and the
heat transfer, in kJ per kmol of fuel.
13.51 A 5 10
3
kg sample of liquid benzene (C
6
H
6
) together
with 20% excess air, initially at 25C and 1 atm, reacts com-
pletely in a rigid, insulated vessel. Determine the temperature,
in C, and the pressure, in atm, of the combustion products.
Applying the Second Law to Reacting Systems
13.52 Carbon monoxide (CO) at 25C, 1 atm enters an insu-
lated reactor operating at steady state and reacts completely
with the theoretical amount of air entering in a separate stream
at 25C, 1 atm. The products of combustion exit as a mixture
at 1 atm. For the reactor, determine the rate of entropy pro-
duction, in kJ/K per kmol of CO entering. Neglect kinetic and
potential energy effects.
13.53 Carbon monoxide (CO) reacts with water vapor in an
insulated reactor operating at steady state to form hydrogen
(H
2
) and carbon dioxide (CO
2
). The products exit as a mixture
Air
25°C, 1 atm
Combustion
gas at 662°C
Preheater
T = ?
797°C
Boiler
Feedwater Steam
CH
4
25°C, 1 atm
Figure P13.38
13.39 A closed rigid vessel initially contains a gaseous mixture
at 25C, 1 atm with the molar analysis of 25% ethylene (C
2
H
4
),
75% oxygen (O
2
). The mixture burns completely and the prod-
ucts are cooled to 500 K. Determine the heat transfer between
the vessel and its surroundings, in kJ per kmol of fuel present
initially, and the final pressure, in atm.
13.40 A closed, rigid vessel initially contains a gaseous mix-
ture of 1 kmol of pentane (C
5
H
12
) and 150% of theoretical air
at 25C, 1 atm. If the mixture burns completely, determine the
heat transfer from the vessel, in kJ, and the final pressure, in
atm, for a final temperature of 800 K.
13.41 Determine the enthalpy of combustion for gaseous bu-
tane (C
4
H
10
), in kJ per kmol of fuel and kJ per kg of fuel, at
25C, 1 atm, assuming
(a) water vapor in the products.
(b) liquid water in the products.
13.42 Determine the higher heating value, in kJ per kmol of
fuel and in kJ per kg of fuel, at 25C, 1 atm for
(a) liquid octane (C
8
H
18
).
(b) gaseous hydrogen (H
2
).
(c) liquid methanol (CH
3
OH).
(d) gaseous butane (C
4
H
10
).
Compare with the values listed in Table A-25.
13.43 For a natural gas with a molar analysis of 86.5% CH
4
,8%
C
2
H
6
,2% C
3
H
8
, 3.5% N
2
, determine the lower heating value,
in kJ per kmol of fuel and in kJ per kg of fuel, at 25C, 1 atm.
13.44 Liquid octane (C
8
H
18
) at 25C, 1 atm enters an insulated
reactor operating at steady state and burns with 90% of theo-
retical air at 25C, 1 atm to form products consisting of CO
2
,
CO, H
2
O, and N
2
only. Determine the temperature of the ex-
iting products, in K. Compare with the results of Example 13.8
and comment.

674 Chapter 13 Reacting Mixtures and Combustion
at 1 atm. For the reactor, determine the rate of entropy
production, in kJ/K per kmol of carbon monoxide entering.
Neglect kinetic and potential energy effects. Consider two
cases:
(a) the carbon monoxide and water vapor enter the reactor is
separate streams, each at 400 K, 1 atm.
(b) the carbon monoxide and water vapor enter the reactor as
a mixture at 400 K, 1 atm.
Explain why the answers in parts (a) and (b) differ.
13.54 A gaseous mixture of butane (C
4
H
10
) and 80% excess air
at 25C, 3 atm enters a reactor. Complete combustion occurs,
and the products exit as a mixture at 1200 K, 3 atm. Coolant
enters an outer jacket as a saturated liquid and saturated vapor
exits at essentially the same pressure. No significant heat trans-
fer occurs from the outer surface of the jacket, and kinetic and
potential energy effects are negligible. Determine for the jack-
eted reactor
(a) the mass flow rate of the coolant, in kg per kmol of fuel.
(b) the rate of entropy production, in kJ/K per kmol of fuel.
(c) the rate of exergy destruction, in kJ per kmol of fuel, for
T
0
25C.
Consider each of two coolants: water at 1 bar and ammonia at
10 bar.
13.55 Liquid ethanol (C
2
H
5
OH) at 25C, 1 atm enters a re-
actor operating at steady state and burns completely with
130% of theoretical air entering in a separate stream at 25C,
1 atm. Combustion products exit at 227C, 1 atm. Heat trans-
fer from the reactor takes place at an average surface tem-
perature T
b
. For T
b
ranging from 25 to 200C, determine the
rate of exergy destruction within the reactor, in kJ per kmol
of fuel. Kinetic and potential energy effects are negligible.
Let T
0
25C.
13.56 A gaseous mixture of ethane (C
2
H
6
) and the theoretical
amount of air at 25C, 1 atm enters a reactor operating at steady
state and burns completely. Combustion products exit at 627C,
1 atm. Heat transfer from the reactor takes place at an average
surface temperature T
b
. For T
b
ranging from 25 to 600C, de-
termine the rate of exergy destruction within the reactor, in kJ
per kmol of fuel. Kinetic and potential energy effects are neg-
ligible. Let T
0
25C.
13.57 Determine the change in the Gibbs function, in kJ per
kmol of methane, at 25C, 1 atm for
2H
2
O, using
(a) Gibbs function of formation data.
(b) enthalpy of formation data, together with absolute entropy
data.
13.58 Separate streams of hydrogen (H
2
) and oxygen (O
2
) at
25C, 1 atm enter a fuel cell operating at steady state, and liquid
water exits at 25C, 1 atm. The hydrogen flow rate is 2 10
4
kmol/s. If the fuel cell operates isothermally at 25C, deter-
mine the maximum theoretical power it can develop and the
accompanying rate of heat transfer, each in kW. Kinetic and
potentially energy effects are negligible.
CH
4
2O
2
S CO
2
13.59 Streams of methane (CH
4
) and oxygen (O
2
), each at 25C,
1 atm, enter a fuel cell operating at steady state. Streams of
carbon dioxide and water exit separately at 25C, 1 atm. If the
fuel cell operates isothermally at 25C, 1 atm, determine the
maximum theoretical work that it can develop, in kJ per kmol
of methane. Ignore kinetic and potential energy effects.
13.60 Streams of hydrogen (H
2
) and oxygen (O
2
), each at 1 atm,
enter a fuel cell operating at steady state and water vapor ex-
its at 1 atm. If the cell operates isothermally at (a) 300 K,
(b) 400 K, and (c) 500 K, determine the maximum theoretical
work that can be developed by the cell in each case, in kJ per
kmol of hydrogen flowing, and comment. Heat transfer with
the surroundings takes place at the cell temperature, and ki-
netic and potential energy effects can be ignored.
13.61 An inventor has developed a device that at steady state
takes in liquid water at 25C, 1 atm with a mass flow rate of
4 kg/h and produces separate streams of hydrogen (H
2
) and
oxygen (O
2
), each at 25C, 1 atm. The inventor claims that the
device requires an electrical power input of 14.6 kW when
operating isothermally at 25C. Heat transfer with the sur-
roundings occurs, but kinetic and potential energy effects can
be ignored. Evaluate the inventor’s claim.
13.62 Coal with a mass analysis of 88% C, 6% H, 4% O, 1% N,
1% S burns completely with the theoretical amount of air.
Determine
(a) the amount of SO
2
produced, in kg per kg of coal.
(b) the air–fuel ratio on a mass basis.
(c) For environmental reasons, it is desired to separate the SO
2
from the combustion products by supplying the products
at 340 K, 1 atm to a device operating isothermally at 340 K.
At steady state, a stream of SO
2
and a stream of the re-
maining gases exit, each at 340 K, 1 atm. If the coal is
burned at a rate of 10 kg/s, determine the minimum theo-
retical power input required by the device, in kW. Heat
transfer with the surroundings occurs, but kinetic and
potential energy effects can be ignored.
Using Chemical Exergy
13.63 For (a) carbon, (b) hydrogen (H
2
), (c) methane (CH
4
),
(d) carbon monoxide, (e) liquid methanol (CH
3
OH), (f) nitro-
gen (N
2
), (g) oxygen (O
2
), (h) carbon dioxide, and (i) water,
determine the chemical exergy, in kJ/kg, relative to the fol-
lowing environment in which the gas phase obeys the ideal gas
model:
Environment
T
0
298.15 K (25C), p
0
1 atm
Gas Phase: Component y
e
(%)
N
2
75.67
O
2
20.35
H
2
O(g) 3.12
CO
2
0.03
Other 0.83
Problems: Developing Engineering Skills 675
13.64 The accompanying table shows an environment consist-
ing of a gas phase and a condensed water phase. The gas phase
forms an ideal gas mixture.
Environment
T
0
298.15 K (25C), p
0
1 atm
Condensed Phase: H
2
O(l) at T
0
, p
0
Gas Phase: Component y
e
(%)
N
2
75.67
O
2
20.35
H
2
O(g) 3.12
CO
2
0.03
Other 0.83
(a) Show that the chemical exergy of the hydrocarbon C
a
H
b
can be determined as
(b) Using the result of part (a), repeat parts (a) through (c) of
Problem 13.63.
13.65 Showing all important steps, derive (a) Eq. 13.38,
(b) Eq. 13.39 (c) Eq. 13.40, (d) Eqs. 13.41a, b (e) Eqs.
13.44 a, b.
13.66 Using data from Tables A-25 and A-26, together with
Eq. 13.45, determine the standard molar chemical exergy, in
kJ/kmol, of
(a) ammonia (NH
3
).
(b) propane (C
3
H
8
).
13.67 The chemical exergies of common hydrocarbons C
a
H
b
can be represented in terms of their respective lower heating
value, by an expression of the form
where c
1
, c
2
, and c
3
are constants. Evaluate the constants rel-
ative to the environment of Problem 13.63 to obtain an ex-
pression valid for several selected (a) gaseous hydrocarbons,
(b) liquid hydrocarbons.
13.68 Evaluate the specific flow exergy of water vapor, in kJ/kg,
at 200C, 1 bar. Neglect the effects of motion and gravity. Per-
form calculations
(a) relative to the environment of Problem 13.63.
(b) using data from Table A-26.
13.69 Evaluate the specific flow exergy of an equimolar
mixture of oxygen (O
2
) and nitrogen (N
2
), in kJ/kg, at 20C,
1 atm. Neglect the effects of motion and gravity. Perform
calculations
e
ch
1LHV2
c
1
c
2
1b
a2 c
3
a
LHV
,
b
2
g
H
2
O1l2
d RT
0
ln c
1y
e
O
2
2
ab
4
1y
e
CO
2
2
a
d
e
ch
cg
F
aa
b
4
b g
O
2
ag
CO
2
(a) relative to the environment of Problem 13.63.
(b) using data from Table A-26.
13.70 A mixture having an analysis on a molar basis of 85%
dry air, 15% CO enters a device at 125C, 2.1 atm, and a ve-
locity of 250 m/s. If the mass flow rate is 1.0 kg/s, determine
the rate exergy enters, in kW. Neglect the effect of gravity. Per-
form calculations
(a) relative to the environment of Problem 13.63.
(b) using data from Table A-26.
Exergy Analysis of Reacting and
Psychrometric Systems
13.71 Carbon at 25C, 1 atm enters an insulated reactor oper-
ating at steady state and reacts completely with the theoretical
amount of air entering separately at 25C, 1 atm. For the re-
actor, (a) determine the rate of exergy destruction, in kJ per
kmol of carbon, and (b) evaluate an exergetic efficiency. Per-
form calculations relative to the environment of Problem 13.63.
Neglect the effects of motion and gravity.
13.72 Propane gas (C
3
H
8
) at 25C, 1 atm and a volumetric flow
rate of 0.03 m
3
/min enters a furnace operating at steady state
and burns completely with 200% of theoretical air entering at
25C, 1 atm. Combustion products exit at 227C, 1 atm. The
furnace provides energy by heat transfer at 227C for an in-
dustrial process. For the furnace, compare the rate of exergy
transfer accompanying heat transfer with the rate of exergy de-
struction, each in kJ/min. Let T
0
25C and ignore kinetic
and potential energy effects.
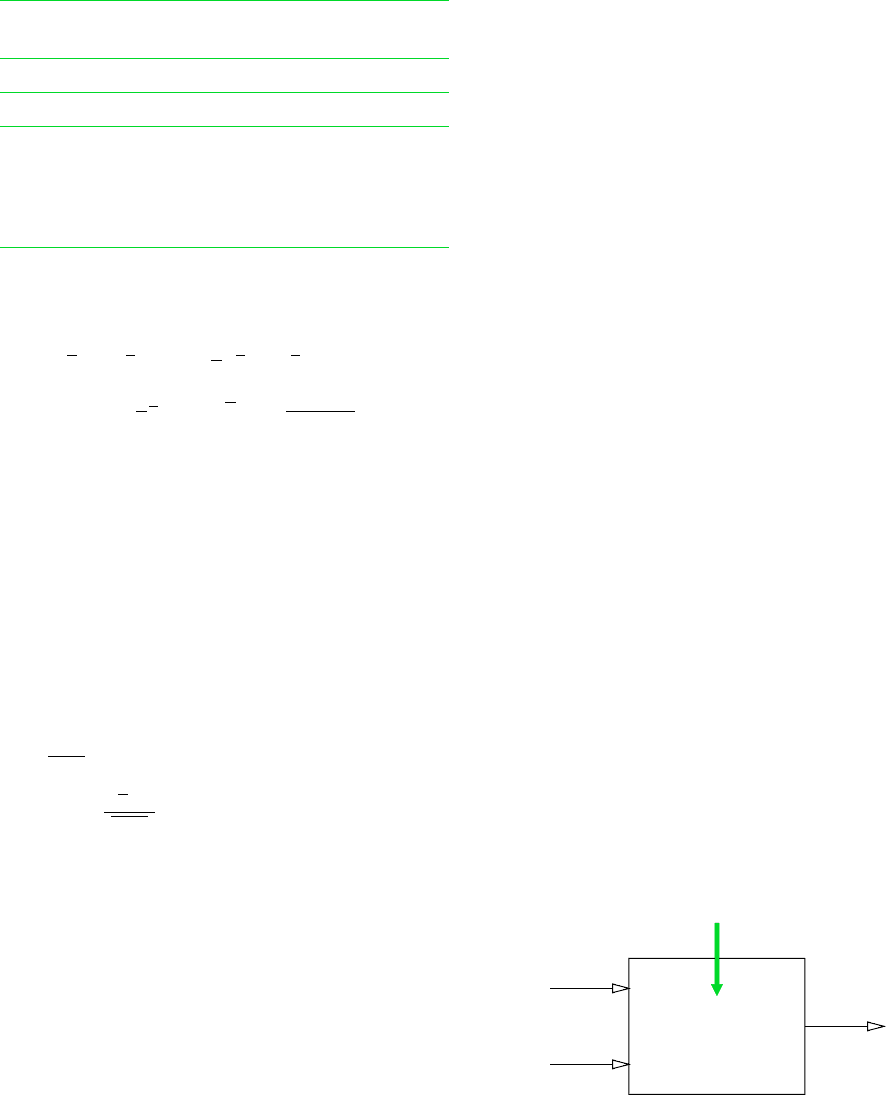
13.73 Figure P13.73 shows a coal gasification reactor making
use of the carbon–steam process. The energy required for the
endothermic reaction is supplied by an electrical resistor. The
reactor operates at steady state, with no stray heat transfers and
negligible kinetic and potential energy effects. Evaluate in Btu
per lbmol of carbon entering
(a) the required electrical input.
(b) the exergy entering with the carbon.
(c) the exergy entering with the steam.
(d) the exergy exiting with the product gas.
(e) the exergy destruction within the reactor.
Perform calculations relative to the environment of Problem
13.63. Ignore kinetic and potential energy effects.
Carbon (T
0
, p
0
)
Water vapor at
600°F, 1 atm
Electrical
input
Product gas at
1700°F, 1 atm
C + 1.25H
2
O(g) → CO + H
2
+ 0.25H
2
O(g)
Figure P13.73
