Odekon M. Encyclopedia of paleoclimatology and ancient environments
Подождите немного. Документ загружается.

Interstadials during the Weichselian
Many different interstadials are defined in Europe based on
palynology. The interstadials that occurred during, for
instance, the Weichselian Glac ial have been named after the
place where the interstadial was described for the first time.
Many of these Weichselian interstadial stratotypes have
been defined in NW Europe and are described by Behre
(1989), Zagwijn (1989), and de Beaulieu and Reille ( 1992)
(Table I4). Dansgaard et al. (1993) described 24 interstadial
phases in the GRIP oxygen isotope record from the Greenland
ice, characterized by a sudden i ncrease and gradually decrease
of oxygen isotope values. Recent evidence points to a total of
25 interstadial phases that are resolved in the Greenland ice
core (NG RIP Members, 2004). These so-called Dansgaard-
Oeschger interstadial events can be correlated with low per-
centages of the planktonic cold water foraminifera Neoglobo-
quadrina pachyderma in high-resolution records from the
North Atlantic (Bond et al., 1993).
Wim Z. Hoek
Bibliography
Behre, K.E., 1989. Biostratigraphy of the last glacial period in Europe.
Quaternary Sci. Rev., 8,25–44.
Bond, G., Broecker, W., Johnsen, S., McManus, J., Labeyrie, L., Jouzel, J.,
and Bonani, G., 1993. Correlations between climate records from North
Atlantic sediments and Greenland ice. Nature, 365, 143–147.
Dansgaard, W., Johnsen, S.J., Clausen, H.B., Dahl-Jensen, D., Gun-
destrup, N.S., Hammer, C.U., Hvi dberg, C.S., Steffensen, J.P.,
Sveinbjörnsdottir, A.E., Jouzel, J., and Bond, G., 1993. Evidence
for general instability of past climate from a 250-kyr ice-core record.
Nature, 364, 218–220.
de Beaulieu, J.L., and Reille, M., 1992. The last climatic cycle at La
Grande Pile (Vosges, France): A new pollen profile. Quaternary
Science Reviews, 11, 431–438.
Jessen, K., and Milthers, V., 1928. Stratigraphical and palaeontological
studies of interglacial freshwater deposits in Jutland and north-west
Germany. Danmarks Geologiske Undersøgelse II, 48, 380pp.
Gibbard, P.L., and West, R.G., 2000. Quaternary chronostratigraphy: The
nomenclature of terrestrial sequences. Boreas, 29, 329–336.
Lowe, J.J., and Walker, M.J.C., 1997. Reconstructing Quaternary Environ-
ments. London, UK: Longman, 446pp.
North Greenland Ice Core Project (NGRIP) Members, 2004. High-
resolution record of Northern Hemisphere climate extending into the
last interglacial period. Nature, 431, 147–151.
Zagwijn, W.H., 1989. Vegetation and climate during warmer intervals in
the Late Pleistocene of western and central Europe. Quaternary Int.,
3–4,57–67.
Cross-references
Bølling-Allerød Interstadial
Millennial Climate Variability
Dansgaard-Oeschger Cycles
Ice Cores, Antarctica and Greenland
Oxygen Isotopes
Palynology
Pollen Analysis
IRON AND CLIMATE CHANGE
Iron availability limits the ability of phytoplankton in some
parts of the surface ocean to photosynthesize biomass from dis-
solved CO
2
in seawater. An important source of iron to phyto-
plankton is the deposition of dust from continents. The
sensitivity of dust deposition to climatic factors such as aridity
and windiness makes iron a possible link between climate and
the CO
2
concentration in the atmosphere. Iron climate linkages
and their biogeochemical ramifications are explored further in a
recent article by Jickels et al. (2005).
The element iron is abundant in the core and crust of the
Earth. In the early Earth, the oceans contained high concentra-
tions of dissolved iron in the form of Fe
2+
. However, as the
oceans became oxidized by increasing O
2
concentrations in
the atmosphere, iron oxidized to the relatively insoluble state
Fe
3+
, and deposited into sedimentary structures known as
Banded Iron Formations. Except for anoxic regions such as in
sediments or isolated basins such as the Black Sea, dissolved
iron concentrations have remained low in the oceans ever since.
However, iron is not absent in the oxic ocean. Fe
3+
is stabi-
lized in the dissolved form by complexing organic compounds
called chelators or siderophores (Rue and Bruland, 1995).
These compounds appear to be biogenic, but little is known
of their structure, lifetime, or origins. Similar compounds are
secreted in soil and freshwater systems to enable plants, fungi,
and bacteria to acquire iron. A vast majority of the iron in sea-
water is found to be complexed to siderophores. In addition,
the precipitation of Fe
3+
into solid form does not result in its
immediate removal by sinking when the particles generated
are very small. More than half of the Fe
3+
in seawater is found
in colloidal form, too small to sink but potentially too large to
be biologically useful. In particular, the diffusion coefficient of
colloidal complexed iron will be slower than that for truly dis-
solved iron, and its availability for biological uptake will be
correspondingly less (Wu et al., 2001).
In spite of its scarcity, iron is an essential co-factor in enzy-
matic systems such as the light-harvesting photosynthesis
systems, and in nitrogenase, the enzyme which fixes NH
4
+
from
molecular N
2
(Falkowskietal.,1998). Because of its low solubi-
lity in oxic seawater, iron has been shown to limit the production
of biomass in several regions of the surface ocean. These regions,
historically called High Nutrient Low Chlorophyll (HNLC)
regions, include the equatorial Pacific, the North Pacific, and
the Southern Ocean. When the iron concentration in the surface
ocean is artificially increased in these areas, the iron stimu-
lates increases in chlorophyll concentration, photosynthetic
efficiency, and phytoplankton growth rates (Coale et al.,
1996). These iron fertilization experiments constitute one of
the most dramatic breakthroughs in biological oceanography
in several decades.
Table I4 Interstadials during the Weichselian in Europe: Denmark (DK),
The Netherlands (NL), Germany (D) France (F), Great Britain (UK),
compared to GRIP Summit glacial interstadials IS as defined by
Dansgaard et al. (1993)
Terrestrial sequences Europe IS
Bølling-Allerød (DK), Windermere (UK) 1
Denekamp (NL) 8
Hengelo (NL) 12
Moershoofd (NL) 13
Glinde (D) 14
Oerel (D) 16
Odderade (D) St. Germain II (F) 21
Brørup (DK), St. Germain I (F), Chelford (UK) 23
Amersfoort (NL)
478 IRON AND CLIMATE CHANGE

The link between phytoplankton and the CO
2
concentration
of the atmosphere occurs via the biological pump in the ocean.
Photosynthesis produces particles that can leave their source
waters by sinking. The downward net flux of biological mate-
rial leaves the surface ocean depleted in nutrients such as
NO
3
2
and PO
4
3
, as well as dissolved CO
2
.CO
2
gas dissolves
into surface seawater, or evaporates into the atmosphere,
according to its concentration in seawater or air, and its solubi-
lity (mostly controlled by temperature). Depletion of CO
2
from
surface waters by biological activity therefore draws down the
CO
2
concentration of the atmosphere. In the present-day ocean,
Southern Ocean surface waters contain the majority of the poten-
tially biologically usable nutrients NO
3
2
and PO
4
3
. In addition,
because the Southern Ocean is a conduit to the largest oceanic
carbon reservoir – the deep ocean, atmospheric pCO
2
in models
is more sensitive to surface chemistry changes in the Southern
Ocean than other oceanographic regions. Therefore, the search
for an explanation for lower glacial pCO
2
values has centered
on deposition of iron onto the Southern Ocean.
There are arguments for and against the Southern Ocean fer-
tilization hypothesis. On the positive side, the deposition of
dust and Fe in Antarctic ice cores was indeed higher during
glacial times, by more than an order of magnitude. Most of this
dust appears to originate from a specific region in Patagonia,
but dust deposition increased globally by a factor of two or
more. The decline in Antarctic dust deposition is also one of
the earliest indicators of the coming deglaciation (suggesting
that dust decrease could be an ultimate cause for the other
aspects of the deglaciation) (Broecker and Henderson, 1998).
On the other hand are arguments against the Southern Ocean
fertilization hypothesis. Several models of iron geochemistry
in the ocean conclude that the dominant iron source to the
Southern Ocean surface is from below, in the upwelling water,
and that increasing the negligible deposition flux will have little
effect (Archer and Johnson, 2000). Fertilization experiments in
the Southern Ocean have not resulted in increased sinking
of organic matter into deep waters, but rather an increased stand-
ing biomass stock in surface waters as the added iron is effi-
ciently recycled (Boyd et al., 2000). Trace element tracers for
sea surface nutrient concentration in the Southern Ocean have
not shown a substantial drawdown during glacial time (Boyle,
1992). Furthermore, the pCO
2
sensitivity to the Southern Ocean
decreases with increasing model complexity and realism, such
that even a complete drawdown of Southern Ocean nutrients
would not be enough to generate glacial pCO
2
values (Archer
et al., 2000). Finally, an increase in the biological pump would
result in a decrease in the O
2
concentration of the deep ocean,
which is not apparent in the sedimentary record.
With these counter-arguments in mind, the community is
also investigating the possibility that an increased glacial iron
supply might stimulate an increase in nitrogen fixation, result-
ing in an increase in the nitrate inventory of the ocean
(Broecker and Henderson, 1998). A release from nitrogen lim-
itation might result in a stronger biological pump, although
phosphorus availability could limit this effect. In addition, iron
fertilization is being explored as a potential fix for rising CO
2
concentrations resulting from fossil fuel consumption.
David Archer
Bibliography
Archer, D.E., and Johnson, K., 2000. A model of the iron cycle in the
ocean. Global Biogeochem. Cycles, 14, 269–279.
Archer, D., Eshel, G., Winguth, A., Broecker, W.S., Pierrehumbert, R.T.,
Tobis, M., and Jacob, R., 2000. Atmospheric pCO
2
sensitivity to the
biological pump in the ocean. Global Biogeochem. Cycles, 14,
1219–1230.
Boyd, P.W., Watson, A.J., Law, C.S., Abraham, E.R., Trull, T., Murdoch, R.,
Bakker, D.C.E., Bowie, A.R., Buesseler, K.O., Chang, H., Charette, M.,
Croot, P., Downing, K., Frew, R., Gall, M., Hadfield, M., Hall, J.,
Harvey, M., Jameson, G., LaRoche, J., Liddicoat, M., Ling, R.,
Maldonado, M.T., McKay, R.M., Nodder, S., Pickmere, S., Pridmore, R.,
Rintous, S., Safi, K., Sutton, P., Strzepek, R., Tannaberger, K., Turner, S.,
Waite, A., and Zeldis, J., 2000. A mesoscale phytoplankton bloom
in the polar Southern Ocean stimulated by iron fertilization. Nature, 407,
695–702.
Boyle, E.A., 1992. Cadmium and d
13
C paleochemical ocean distributions
during the Stage 2 glacial maximum. Annu. Rev. Earth Planet. Sci.,
20, 245–287.
Broecker, W.S., and Henderson, G., 1998. The sequence of event surround-
ing termination II and their implications for the cause of glacial-interglacial
CO
2
changes. Paleoceanography, 13,352–364.
Coale, K.H., Johnson, K.S., Fitzwater, S.E., Gordon, R.M., Tanner, S.,
Chavez, F.P., Ferioli, L., Sakamoto, C., Rogers, P., Mil lero, F.,
Steinberg, P., Nightingale, P., Cooper, D., Cochlan, W.P., Landry, M.
R., Consta ntinou, J., Rollwagen, G., Trasvina, A., and Kudela, R.,
1996. A massive phytoplan kton bloom induced by an ecosystem-scale
iro n fertilization experiment in the equatorial Pacific Ocean. Nature,
383,495–501.
Falkowski, P.G., Barber, R.T., and Smetacek, V., 1998. Biogeochemical
controls and feedbacks on ocean primary production. Science, 281,
200–206.
Jickels, T.D., 2005. Global iron connections between desert dust, ocean
biogeochemistry, and climate. Science, 308,67–71.
Rue, E.L., and Bruland, K.W., 1995. Complexation of iron(III) by natural
organic ligands in the Central North Pacific as determined by a new
competitive ligand equilibration/ absorptive cathodic stripping voltam-
metric method. Mar. Chem., 50,117–138.
Wu, J., Boyle, E., Sunda, W., and Wen, L.-S., 2001. Soluble and colloidal
iron in the oligotrophic North Atlantic and North Pacific. Science, 293,
847–849.
Cross-references
Banded Iron Formations and the Early Atmosphere
Marine Carbon Geochemistry
ISOTOPE FRACTIONATION
Isotopes are atoms whose nuclei contain the same number of pro-
tons but a different number of neutrons. The term ‘isotope’ is
derived from a Greek word meaning equal place: the various iso-
topes of an element occupy the same position in the periodic table.
Isotopes of an element can be either stable or unstable (radio-
genic). Differences in chemical and physical properties arising
from variations in atomic mass of an element are called ‘isotope
effects’. The partitioning of isotopes between two substances or
two phases of the same substance with different proportions of iso-
topes is called ‘isotope fractionation’ (Hoefs, 1997; Criss, 1999).
The isotopic composition of materials contains informa-
tion that can be used, for example, to reconstruct the palaeo-
environment and to understand the hydrological cycle and
biochemical pathways.
History
In 1913, isotopes were discovered by J. J. Thomson who found
that the element neon has two different kinds of atoms with
atomic weights 20 and 22. A few years later, 212 of the 287
ISOTOPE FRACTIONATION 479

naturally occurring isotopes were known. The reason for the
existence of isotopes was, however, unclear until the discovery
of the neutron by Chadwick in 1932. The seminal papers by
Urey (1947) and Bigeleisen and Mayer (1947) on the thermo-
dynamic properties of isotopic substances provided the basis
for the utilization of stable isotopes in geology, geochemistry,
biogeochemistry, paleoceanography and elsewhere.
Notation, defini tions
Isotopic ratios, R, are measures of the relative abundances of
isotopes of an element, E; they are usually arranged so that
the lightest stable isotope (index a), which is often also the
most abundant isotope, appears in the denominator:
b
R =
b
E/
a
E
(stable boron isotopes are an exception:
11
B is about four times
more abundant than
10
B). The three stable oxygen isotopes
16
O,
17
O, and
18
O will be used to explain the notation and basic
definitions.
The oxygen isotope with mass number 16 contains 8 pro-
tons and 8 neutrons and is denoted by
16
O. It is by far the most
abundant (99.76%) of the three stable oxygen isotopes. For
example, the ratio of
17
Oto
16
O in water, or the ratio of
18
O
to
16
O in carbon dioxide can be written as:
17
R
H
2
O
¼
H
2
17
O½
H
2
16
O½
;
18
R
CO
2
¼
2C
18
O
18
O½þC
18
O
16
O½
2C
16
O
16
O½þC
18
O
16
O½
The isotopic composition, d, of a sample, determined by
mass spectrometric methods, is measured with respect to a
standard (std):
d
b
E
sample
¼
b
R
sample
b
R
std
1
1;000
where b is the atomic mass of the isotope and the factor 1,000 con-
verts the d value to per mil. The standards (cf. Hoefs, 1997) used
for stable oxygen isotopes are V-SMOW (Vienna-Standard Mean
Ocean Water;
18
R
V-SMOW
·10
6
= 2,005.20 0.43) and V-PDB
(Vienna-Pee-Dee Belemnite,
18
R
V-PDB
·10
6
= 2,067.1 2.1).
The fractionation factor, a, is defined as the ratio between
the isotopic ratio in compound X and that in compound Y:
b
a
ðXYÞ
¼
b
R
X
b
R
Y
¼
d
b
E
X
þ 1 ;000
d
b
E
Y
þ 1 ;000
It is a measure of the partitioning of isotopes between two
or more phases in response to an isotope effect.
Whereas the d value is the result of the whole history of the
sample, the a value is characteristic for, say, an equilibrium
between X and Y or for a process that leads from X to Y. Since
a values are usually very close to 1.0, the e notation is com-
monly used to express isotope fractionations in per mil (%):
b
e
ðXYÞ
¼
b
a
ðXYÞ
1
1;000
Isotope effects
The most important isotope effects arise from differences in: (a)
random mean velocities and (b) vibrational frequencies of
molecules.
a) Random mean velocity effects. In (local) thermodynamic
equilibrium, the mean kinetic energy is equal for all molecules
and determined by temperature alone:
E
kin;j
¼
1
2
m
j
v
j
2
¼
3
2
k
B
T ¼ E
thermal
where m
j
and v
rms;j
¼
ffiffiffiffiffiffiffiffiffiffi
v
2
j
DE
r
are mass and random mean square (rms) velocity of molecule j,
k
B
is Boltzmann’s constant, and T is absolute temperature. At a
given temperature, the rms velocity varies with the mass of the
molecule: light molecules are faster than heavy molecules (Gra-
ham’s law of diffusion):
v
rms;1
=v
rms;2
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffi
m
2
=m
1
p
As a consequence, molecules containing the light isotope
diffuse faster. The ratio of the diffusion coefficients D
v
for
the light (mass m
a
) and the heavy molecule (mass m
b
)ofa
gas that diffuses through air (mass m
c
= 29) is given by
a
ðabÞ
¼
D
b
D
a
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
m
b
þ m
c
m
b
m
c
m
a
m
c
m
a
þ m
c
r
For
13
CO
2
and
12
CO
2
, one obtains
13
a
diff: CO
2
in air
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
45 þ 29
45 29
44 29
44 þ 29
r
0:9956
The relation does not apply, however , for diffusion of gases
through liquids where the ratio of the diffusion coefficients is clo-
ser to one (
13
a
diff. CO
2
in water
0.9993). The variation of the rms
velocity with mass also leads to differences in evaporation.
b) Effects due to molecular vibrations. The zero-point
energy of vibrations in molecules, E
0
, is mass dependent. For
diatomic molecules E
0
= hv/2, where h is Planck’s constant
and v is the (vibration) frequency. The ratio of frequencies v
and v
0
for two diatomic molecules of different isotopic compo-
sition is approximately given by v
0
/v =(µ/µ
0
)
1/2
where µ = m
1
· m
2
/(m
1
+ m
2
) is the reduced mass and m
1
and m
2
are the
masses of the two atoms involved.
Example: compare the frequencies for
16
O
16
O(v, µ = 16 · 16/
(16 + 16) = 8),
17
O
16
O(v
0
, µ
0
=17·16/(17+16) 8.2424), and
18
O
16
O(v
00
, µ
00
=18·16/(18+16) 8.4706): v
0
/v 0.9852,
v
00
/v 0.9718, i.e., the strength of the isotope effect increases with
the mass difference and thus it is not surprising that the resulting
isotope fractionation is also ‘mass-dependent’, as a rule.
Isotope fractionation
The difference in isotopic composition between various com-
pounds or between various phases of a single compound is
called isotope fractionation. It is noted that even the largest iso-
tope effect may not cause fractionation if the reaction goes to
completion, i.e., a quantitative reaction in which the reactant
is completely transformed into the product. However, an isoto-
pic fractionation will always be observed when a reaction has
an isotope effect and the formation of the product is not quan-
titative (Hayes, 1982).
Equilibrium isotope fractionation
Isotope fractionation can occur in equilibrium, for example,
between the various isotopic forms of carbon dioxide, bicarbonate,
and carbonate ions (Zeebe and Wolf-Gladrow, 2001). As a rule:
“The heavy isotope goes preferentially to the chemical compound
in which the element is bound most strongly.” (Bigeleisen, 1965).
For example, in the temperature range between 0 and 25
C, the
480 ISOTOPE FRACTIONATION

d
13
C
CO2
in seawater is 8–10% lower (“isotopically lighter”) than
d
13
C
HCO3
.
The fractionation factors for equilibrium fractionation invol-
ving three isotopes with masses m
1
< m
2
< m
3
usually scale such
thata
2=1
¼ a
b
3=1
,whereb =(1/m
1
– 1/m
2
)/(1/m
1
– 1/m
3
)(Young
et al., 2002). For the three stable oxygen isotopes:
17
a =(
18
a)
0.529
.
Nonequilibrium (“kinetic”) isotope fractionation
Nonequilibrium effects are associated with incomplete or uni-
directional processes such as evaporation, kinetic isotope
effects in chemical reactions, diffusion, or metabolic effects.
Kinetic isotope effects in chemical reactions occur when reac-
tion rates for compounds containing light or heavy isotopes
are different, which is almost always the case and thus isotope
fractionation can be expected. If the reservoir of reactants is
finite, the isotope effect associated with the reaction will not
only yield a product of different isotopic composition but will
lead to a change in the isotopic composition of the reservoir
as well (Rayleigh distillation; Bigeleisen and Wolfsberg,
1958). Kinetic isotope effects can quantitatively be understood
on the basis of the ‘transition state theory’ (Bigeleisen and
Wolfsberg, 1958). The fractionation factors for kinetic fractio-
nation involving three isotopes with masses m
1
< m
2
< m
3
often scale such that a
2=1
¼ a
b
3=1
, where b =l
n
(m
1
/m
2
)/l
n
(m
1
/-
m
3
) (Young et al., 2002). For the three stable oxygen isotopes:
17
a =(
18
a)
0.515
.
“Mass-independent” fractionation
A mass-independent fractionation of stable oxygen isotopes
was reported (for the first time) in 1973 in meteorites and in
1983 in laboratory studies (Thiemens and Heidenreich, 1983).
These studies show differences in the isotopic compositions
for certain compounds that contain
16
O only (Substance X)
and Compounds X and compound Y with fractionation
factors
17
a and
18
a (between X and Y) that are almost equal,
i.e.,
17
a
18
a. These fractionation processes are called ‘mass-
independent’. They may occur at low pressures and, for exam-
ple, play an essential role for stratospheric ozone. The resulting
unusual composition of atmospheric oxygen has been used in
the so-called ‘triple-isotope method’ to derive estimates of
the biosphere productivity (Luz et al., 1999). A theoretical
explanation based on an extension of the Rice, Ramsperger,
Kassel, Marcus (RRKM) theory has been developed only
recently (Gao and Marcus, 2001). A mass-independent isotope
effect has also been observed during thermal decomposition of
carbonates in vacuo (Miller et al., 2002).
Dieter A. Wolf-Gladrow and Richard E. Zeebe
Bibliography
Bigeleisen, J., 1965. Chemistry of isotopes. Science, 147, 463–471.
Bigeleisen, J. and M.G. Mayer. Calculation of equilibrium constants for
isotopic exchange reactions. J. Chem. Phus., 15: 261–267, 1947.
Bigeleisen, J., and Wolfsberg, M., 1958. Theoretical and experimental
aspects of isotope effects in chemical kinetics. Adv. Chem. Phys., 1,
15–76.
Criss, R.E., 1999. Principles of Stable Isotope Distribution. New York:
Oxford University Press, 264pp.
Gao, Y.Q., and Marcus, R.A., 2001. Strange and unconventional isotope
effects in ozone formation. Science, 293, 259–263.
Hayes, J.M., 1982. Fractionation et al.: An introduction to isotopic
measurement and terminology. Spectra, 8(4), 3–8.
Hoefs, J., 1997. Stable Isotope Geochemistry, (4th edn.). Heidelberg:
Springer.
Luz, B., et al., 1999. Triple-isotope composition of atmospheric oxygen as
a tracer of biosphere productivity. Nature, 400, 547–550.
Miller, et al., 2002. Mass-independent fractionation of oxygen isotopes
during thermal decomposition of carbonates. Proc. Natl. Acad. Sci.,
99(17), 10988–10993.
Thiemens, M.H., and Heidenreich, J.E. III., 1983. The mass-independent
fractionation of oxygen: A novel isotope effect and its possible cosmo-
chemical implications. Science, 219, 1073–1075.
Urey, H.C., 1947. The thermodynamic properties of isotopic substances.
J. Chem. Soc., 562–581.
Young, et al., 2002. Kinetic and equilibrium mass-dependent isotope
fractionation laws in nature and their geochemical and cosmochemical
significance. Goechim. Cosmochim. Acta, 66(6), 1095–1104.
Zeebe, R.E., and Wolf-Gladrow, D., 2001. CO
2
in Seawater: Equilibrium,
Kinetics, Isotopes. Amsterdam: Elsevier.
Cross-references
Carbon Isotopes, Stable
Oxygen Isotopes
Paleotemperatures and Proxy Reconstructions
Stable Isotope Analysis
Strontium Isotopes
Sulfur Isotopes
ISOTOPE FRACTIONATION 481

K
KAMES
A kame is an ice contact glaciofluvial landform. It is composed
of sediment deposited by water in contact with glacier ice
either at the side of, or on the surface of a glacier. The sediment
fills of supraglacial streams and lakes, ice marginal channels
and ice-dammed lakes often form flat-topped hills or terraces
after the ice has disappeared. The term kame is a Scottish word
to describe such flat-topped hills. Kames are closely related to
eskers, and transitional forms between the two occur. Generally
kames are less continuous and are associated with supraglacial
or ice-marginal water, whereas eskers are more continuous and
are associated with subglacial water. The presence of flat or
gently sloping surfaces, representing fluvial surfaces, is some-
times said to be a diagnostic characteristic of kames. However,
postglacial collapse following the loss of ice support can ser-
iously disturb kame morphology.
Kames generally consist of well-sorted, stratified sediments.
If deposited by glacial streams they are dominated by sands
and gravels. If deposited in lakes they are characterized by clay,
silt and fine sand. Kames can occur as individual hills, as pla-
teau areas or as terraces. At the smallest end of the spectrum of
kame forms are supraglacial crevasse fillings. Where many
kames occur together within an area the term kame field is
sometimes used. Kames are often associated with kettle holes
to produce “kame and kettle” topography. Kame landscapes
tend to reflect environments where ice retreat was accompanied
by abundant meltwater and sediment supply.
The term “kame” is often applied to mean “ice contact” in
compound terms such as kame terrace, kame delta or kame
plateau. Terraces generally form where streams flow along
margins of glaciers, and therefore they lie parallel to the ice mar-
gin where the margin lies on a reverse slope. So-called “flights”
of terraces at different levels may be created as a glacier surface
reduces in elevation during ice retreat. Kame deltas, also known
as delta moraines or simply ice-contact deltas, form where gla-
cial streams emerge from the glacier into water.
As they are created in ice-contact situations kames are prone
to collapse as ice retreats. Their sedimentary architecture
therefore reflects not only the processes of deposition but also
the processes of structural collapse. Supraglacially-derived sedi-
ments are likely to be more disturbed than ice marginal deposits.
Survival of extensive kames indicates that an area was domi-
nated by down-wasting and meltout of ice after any phase of
extensive proglacial fluvial erosion, which would have removed
the kames. Benn and Evans (1998), Bennett and Glasser (1996)
and Hambrey (1994) provide useful reviews of this topic.
Peter G. Knight
Bibliography
Benn, D.I., and Evans, D.J.A., 1998. Glaciers and Glaciation, London,
UK: Arnold, 734pp.
Bennett, M.R., and Glasser, N.F., 1996. Glacial Geology. Chichester, UK:
Wiley, 364pp.
Hambrey, M.J., 1994. Glacial Environments, London, UK: UCL Press,
296pp.
Cross-references
Eskers
Glacial Geomorphology
Glacial Sediments
Glaciofluvial Sediments
Kettles
KETTLES
A kettle, or kettle hole, is an enclosed depression within glacial
sediments, caused by the melting of ice that was buried within
the sediments. The ice may be a remnant of the glacier margin
detached by ablation, or an ice block deposited from a glacial
stream. Kettles are characteristic of proglacial outwash plains
and areas of stagnant ice. They very often fill with water to
form kettle lakes. Kettles vary in size and may be just a few
meters or several hundred meters in diameter.
Some kettles form from the melting of completely buried ice
blocks, others from the melting of partially covered ice blocks,

and the morphology of the holes created reflects their origin. Where
ice is completely buried, as may be the case when stagnant glacier
ice is blanketed by supraglacial meltout sediment or where ice
blocks in a proglacial flood are deeply buried in glacifluvial depos-
its, kettles form by subsidence and may initially be very steep sided.
Where ice blocks are partially exposed, as may be the case where
ice blocks are deposited on a sandur plane during a short proglacial
flood, the debris content of the ice block and the depth of burial
have a significant impact on the form of the kettle. Debris released
from the melting ice block may create a ridge around the edge of the
kettle or may completely fill the kettle with debris, creating a
mound r ather than a hollow (Maizels, 1992) . D i ff er en t f or m s t h a t
have been described include“rimmed,”“crater,” and “till-fill”
kettles. Where an outwash plain is marked by a large number of ket-
tles the term “pitted plain” is sometimes used. Many ice blocks are
deposited on outwash plains during glacial floods, and the distribu-
tion of kettles on an outwash plain can be used to reconstruct the
routing and magnitude of flow during flood events.
Kettles may be completely filled with sediment if deposition
occurs after the ice has completely melted, but will still be recog-
nizable in the sedimentary record. The kettle will be represented
by a downfaulted block, and the amount of downfaulting at dif-
ferent levels in the profile will indicate the depth above which
sediment was deposited after the ice completely melted. Benn
and Evans (1998) and Bennett and Glasser (1996) provide useful
reviews of this topic.
Peter G. Knight
Bibliography
Benn, D.I., and Evans, D.J.A., 1998. Glaciers and Glaciation, London,
UK: Arnold, 734pp.
Bennett, M.R., and Glasser, N.F., 1996. Glacial Geology, Chichester, UK:
Wiley, 364pp.
Maizels, J., 1992. Boulder ring structures produced during Jökulhlaup
flows. Geogr. Ann., 74A,21–33.
Cross-references
Glacial Geomorphology
Glacial Sediments
Kames
Outwash Plains
484 KETTLES

L
LACUSTRINE SEDIMENTS
Introduction
Concerns about global change and its potential effects on
human activities, including economic, social, and political
affairs, are exerting great pressure on science to produce mod-
els and predictions for future climatic variability. In this regard,
the key to the future is a proper understanding of the past. In
principle, data sources for past climatic variations are instru-
mental meteorological and historical data. However, meteor-
ological time series are rather short and thus limited for
interpretation (Bradley, 1999). Historical data are available for
the last couple of centuries, prior to the instrumental record,
and these enable a more or less accurate determination of nat-
ural climatic and anthropogenic changes. These data, however,
become scarce and unreliable for longer timescales and thus
need to be complemented by information from indirect archives
for a better interpretation of the past. On the continents, lacus-
trine sediments provide an excellent archive for the extension
of instrumental and historical data of environmental and cli-
matic changes on a high-resolution decadal or even annual
timescale.
Lacustrine sediments contain a wide variety of information
indirectly reflecting environmental conditions. They can be
analyzed by sedimentological, geochemical, geophysical, and
biological methods combined within the field of paleolimno-
logy (Berglund, 1986; Last and Smol, 2001; Cohen, 2003).
The obtained data are generally called “paleoclimate proxy
data” or “proxies” and are used to evaluate the variability of
past climatic conditions as well as human influences on the
lake system and on the catchment basin. This is possible
because lacustrine sediments record many environmental pro-
cesses that occur in the water column and in the drainage area
of the lake.
Due to this availability of multi-proxy data sets in combina-
tion with high resolution and accurate dating, lacustrine sedi-
ments often provide a more comprehensive environmental
interpretation than other continuous continental records like
tree rings, peat bogs, and speleothems. Discontinuous records
like fluvial, colluvial, and loess deposits or soils are prone to
hiatuses and therefore are only suitable for environmental
reconstructions under special circumstances, for short time
intervals, or for certain scientific questions.
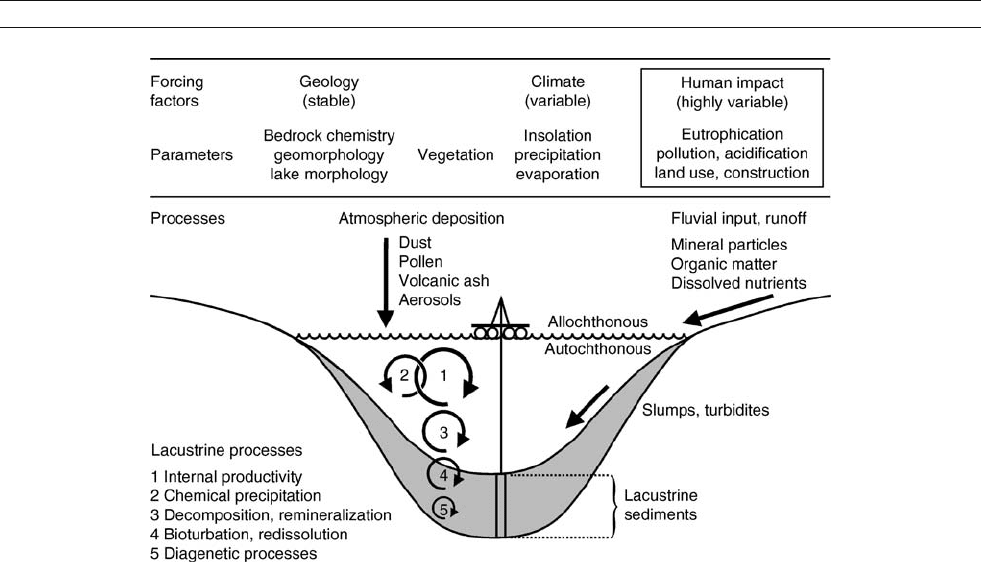
The formation of lacustrine sediments is controlled by atmo-
spheric processes (climate) but also by the geology of the
catchment basin, which is connected to the lake system through
(climatically-controlled) hydrology (Figure L1). Climate and
geology also control the formation of soils and plant cover in
the catchment as well as water chemistry and plankton commu-
nities in the lake (Wetzel, 2001). As geology does not change
significantly through the life span of most lakes, variations
recorded in the sedimentary record are almost certainly related
to climate variability, which itself is controlled by solar forcing.
However, in the most recent past anthropogenic influences
have become increasingly important.
Occurrence
Lakes are standing bodies of water that accumulate sediments
from the surrounding environment and from their water col-
umn. Lakes are relatively young features (10
4
years) that
have predominantly been formed during the last cold period
of the Pleistocene by glacial activities (Hutchinson, 1957;
Gierlowski-Kordesch and Kelts, 1994). Sediments deposited
in these lakes generally provide excellent archives for a large
variety of Late Glacial and Holocene paleoenvironmental
investigations. Lakes can also be found in volcanic regions
(crater lakes). In some rare occurrences, lakes of tectonic origin
(e.g., along the Great African Rift System) or impact crater
lakes exist, all of which may contain much longer sediment
records than glacial lakes, covering several glacial/interglacial
cycles or even the entire Quaternary (up to 10
6
years).
Formation of lacustrine sediments
The formation of lacustrine sediments depends on climatic fac-
tors and therefore, under natural conditions, the dominating
regional climate controls the type of lacustrine sedimentation
(clastic, biogenic, or evaporitic). Lacustrine sediments are the
result of three different mechanisms (Håkanson and Jansson,
1983):
The flux of mineral particles through fluvial input from the
catchment area or by atmospheric deposition forms clastic
sediments, which to a certain extent may contain organic
particles, e.g., leaves or pieces of wood carried into the lake
by runoff.
Biological productivity in the lake generates organic or bio-
genic sediments as the dead organic matter sinks and accu-
mulates at the lake bottom.
Chemical precipitation of minerals from the water column
forms evaporitic sediments.
In general, sediment components can be classified into two
groups: those produced in the lake (autochthonous compo-
nents) and those formed in the catchment basin (allochthonous
components), which are transported to the lake by overland
flow, rivers and streams, or by eolian activities. Although there
are clear constraints for the environmental conditions necessary
for the formation of clastic, organic, or evaporitic sediments,
lacustrine sediments usually are the result of a combination of
these processes.
Clastic lacustrine sediments
Clastic (detrital) sediments predominate under cold climatic
conditions, e.g., at high latitudes or in alpine regions. Such
clastic or minerogenic sediments are typical of proglacial lakes.
Intensive physical weathering and the lack of a dense vegeta-
tion cover provide high amounts of minerogenic detritus, which
can easily be eroded and transported to the lake. In general, the
sediment transfer from the catchment area into the lake is cor-
related with the amount of runoff. In regions with a continental
climate, runoff is governed by the melting of snow and ice
through solar insolation during the summer. Under oceanic
climatic conditions, runoff is controlled either by the melting
of snow and ice through advective heat transport associated
with increased rainfall or solely by precipitation. Lakes with
clastic deposits are generally poor in nutrients (oligotrophic)
inhibiting high organic productivity.
Organic lacustrine sediments
Under temperate or warm, humid climatic conditions, the catch-
ment area is vegetated, reducing the availability and the
transport capability of clastic material into the lake. Chemical
weathering prevails and releases nutrients from the bedrock
that are incorporated into plant organic matter, buffered in soils,
or washed out and transported as dissolved ions into the lake.
This results in higher nutrient levels in the lake (mesotrophic
to eutrophic conditions) and the related increase of organic pro-
ductivity leads to the formation of organic or biogenic sedi-
ments. Algal blooms start with diatoms in spring, are
followed by green and blue-green algae during summer, and
are sometimes terminated by a second diatom bloom in fall.
While green and blue-green algae are easily decomposed dur-
ing deposition, siliceous diatom frustules are much more resis-
tant to dissolution and are generally well preserved in the
sedimentary record. In addition to this autochthonous sedimen-
tary component, an allochthonous component may occur dur-
ing winter: as the plant cover is reduced and thus soils
become more susceptible to erosion, runoff increases and trans-
ports minerogenic as well as organic debris of littoral and ter-
restrial origin to the lake. The latter two components are
washed into the central part of the lake basin during runoff
events related to fall or winter rains.
If carbonates are present in the catchment area, autochtho-
nous calcite precipitation adds an additional component to the
organic sediment fraction. Carbonaceous rocks are dissolved
and the solutes are transferred into the lake. In these hard water
Figure L1 Simplified illustration of forcing factors and parameters as well as processes that lead to the formation of lacustrine sediments.
486 LACUSTRINE SEDIMENTS

lakes, calcite crystals are produced by autochthonous biochem-
ical precipitation: Ca
2+
and HCO
3
in the lake water stay in
solution only until the saturation point is reached. This is partly
achieved by the seasonal temperature increase in the surface
water layer during the summer insolation maximum. As CaCO
3
is less soluble with increasing temperature, calcite may precipi-
tate due to this effect alone. However, even more important is
the temperature-related increase of photosynthetic activities in
the water column through phytoplankton blooms. As a result
of enhanced photosynthesis, CO
2
is withdrawn from the lake
water and thus the pH rises up to pH 9 with a consequent
reduction in CaCO
3
solubility. This combination of temperature
increase and phytoplankton activity contributes to additional
precipitation of calcite crystals. Under such conditions, the
shells of calcifying organisms (e.g., ostracods, mollusks) may
also be a significant sediment constituent.
Evaporitic lacustrine sediments
Under arid or semiarid climatic conditions, evaporitic sedi-
ments are formed as the salinity and the pH of a lake increase
through enhanced evaporation of the lake water. This results
in the saturation of specific mineral compounds (salts), which
then precipitate out of the lake’s water column. In addition
to calcite, which can precipitate either biogeochemically in
mid-latitudes or physicochemically under semi-arid climatic
conditions, evaporitic sediments are also composed of calcium
sulfate (gypsum) and sodium chloride (halite), both precipitat-
ing only under arid climates.
Modification of lacustrine sedimentation
Several processes may alter lacustrine sedimentary records:
Especially in deep lakes with steep sidewalls, turbidity cur-
rents may transport large amounts of sediment from the lake-
shores into the central basin. Turbidites may be associated
with erosion at their base, which causes a hiatus in the sedi-
mentary record.
In shallow parts of lake basins or if strong winds induce
deep-reaching water movements, currents can cause resus-
pension of fine-grained sediments and redeposition some-
where else.
Bioturbation caused by the burrowing activities of benthic
fauna mixes the uppermost sediment layers. The effects of
bioturbation are reduced if anoxic conditions prevent the
presence of higher organisms at the lake floor. For example,
organic rich muds (sapropels) cause anoxic conditions near
the sediment/water interface due to oxygen consumption
during the decomposition of organic matter.
In the course of early diagenesis the growth of iron- and
manganese-minerals like vivianite, siderite, pyrite, or rhodo-
chrosite may also disturb the original layering of the soft
sediments.
If one or more of these processes are or have been present in a
lake, they can introduce hiatuses and mixing into the record
and make high-resolution investigations difficult or impossible.
Sedimentation rates
The rapid reaction of the local hydrological regime to environ-
mental change is coupled with changes in sedimentation rates
(mm yr
1
) and accumulation rates (g cm
2
yr
1
). Lacustrine sedi-
mentation rates are in the range of 0.3 mm to several mm per
year, which is up to three magnitudes higher than in marine
sediment records. As a consequence, this high sedimentation rate
reduces the length of the time window that might be studied to ca.
10
4
years, whereas marine records reach up to 10
8
years.
Therefore, lacustrine sediments facilitate stratigraphic studies
with centennial to decadal resolution. If annually laminated
(varved) sediments are preserved, annual to even seasonal time
resolution is possible (Zolitschka, 2003). Thus, different kinds
of abrupt changes can be detected by the wide variety of applic-
able sedimentological, geochemical, geophysical, and biological
analytical methods (Last and Smol, 2001). Lately, multi-proxy
investigations of lake sediments became more and more impor-
tant because they provide several independent lines of evidence
for a common observation – environmental change as the re-
sponse to climate change and human impact – and therefore
improve the reliability of the interpretation.
Dating
Time control is one of the most important issues in the study of
lacustrine sediments. Without an accurate timescale a compari-
son of the sample proxy data with historical and archaeological
data and with other lacustrine archives as well as with other
proxy records on a local, regional, or global scale is impossible.
Among all sedimentary records, lake sediments provide the best
time control, as different dating methods can be applied to the
same cores. Usually, radiometric dating methods are carried
out depending on the decay rates of radiogenic isotopes, e.g.,
14
C dating for the last ca. 50,000 years and
137
Cs and
210
Pb for
the last 50 and 200 years, respectively. Radiometric measure-
ments are related to time by the half-life of the isotope and
through models of production and deposition of radiogenic iso-
topes. The time is provided in isotopic years and needs to be
calibrated into calendar years by comparison with monitoring
data of radioactive fallout (
137
Cs), by using depositional models
(
210
Pb), or with the help of dendrochronology (
14
C).
In many regions, the annual climatic cycle represents the
strongest observed cyclicity. Under permanent or seasonal
anoxic conditions near the lake bottom, this cyclicity can be
preserved as annually laminated or varved sediments. However,
varved sediments are not the rule because processes like biotur-
bation in well-oxygenated lakes disturb this cyclical layering.
Incremental dating can be applied (Zolitschka, 2003) to well-
preserved annually laminated sediments. This method is based
on cyclical accumulation of biological or lithological material
with time, leading to the formation of organic (biogenic) or
clastic varves. The major control mechanisms of this cyclicity
are the seasonal climatic variations that produce an “internal
clock.” Such a varve chronology provides either an absolute
chronology in calendar years, if the varves continue to the pre-
sent, or a floating chronology, if the record is not annually
laminated to the top of the sequence.
In addition to these “absolute” dating methods, stratigraphic
methods are often applied to provide further time control and to
facilitate correlation between neighboring sites of investigation.
Most common is the biostratigraphic method of pollen analysis
(palynology) where distinct changes in the regional pattern
of vegetation are used. For a larger regional scale, paleomagnetic
studies are also sensible and might even reveal global variations
of inclination and declination changes of the Earth’s magnetic
field. For very long records, reversals or event-like excursions
of the polarity of the Earth’s magnetic field can be used as a stra-
tigraphic tool (magnetostratigraphy). If a lake is situated in a vol-
canic region, tephra layers are another possibility to elaborate an
independent time-frame through tephrochronology. Lacustrine
LACUSTRINE SEDIMENTS 487

sediments have the advantage that one record provides several
different dating methods, which allows significant improvement
of the overall dating accuracy.
Proxy data recorded in lacustrin e sediments
Since meteorological parameters are not directly recorded
in lacustrine sediments, indirect information has to be used
for climatic reconstruction. These so-called proxy data are
either quantitative or descriptive information about sedimento-
logical, geophysical, geochemical, or biological processes that
are dominantly controlled by climatic conditions and thus pro-
vide a good approximation of climatic variability. Three major
groups of proxy data are used with lake sediments to recon-
struct past climatic and related environmental changes:
Biological proxies (e.g., pollen, diatoms, chironomids) are
calibrated using regional training sets, which are statistically
transformed into quantitatively reconstructed values of tem-
perature, pH, salinity, or total phosphorus concentration.
Physicochemical proxies (e.g., isotopes) can be analyzed
relatively fast and easily compared with biological proxies.
Carbon isotopes of bulk organic matter provide information
about changes in the trophic condition of a lake while oxy-
gen isotopes of authigenic carbonates or calcareous skele-
tons of microfossils like ostracods provide quantitative
information that can be linked to temperature and precipita-
tion on a global scale. Furthermore, as water residence times
in lakes are rather short, isotope records provide data about
climatic changes almost without any time lags whereas lag
effects of decades or more are typical for biological systems.
Sedimentary proxies (e.g., flux rates, grain sizes, elemental
composition, magnetic properties, clay minerals) provide
qualitative information about major environmental changes
in the lake and the catchment basin. These data are usually
more complex than biological proxies and therefore much
more difficult to interpret.
If several different proxy data are used for a combined interpre-
tation (multi-proxy approach), the overall significance of the
results is improved considerably.
Modern calibration
The key to an improved interpretation of lacustrine sediment
records is a proper understanding of present-day processes
leading to the formation of such deposits. The primary climatic
signal can be modified and transformed in several ways. For
this reason it is necessary to establish correlations between run-
off events, sediment sources, and sediment deposition as well
as between climatic parameters and biological sedimentary
proxies, e.g., vegetation (pollen), diatoms, or chironomids. Cor-
relations need also to be established between climatic parameters
and stable isotopes or between sediment texture, structure, and
composition. The period of monitoring should ideally exceed sev-
eral years to obtain site-specific calibration data sets and transfer
functions valid for larger regions. These can then be used to recon-
struct quantitative variations of climatic variables like tempera-
ture or precipitation, which are crucial for the evaluation and
refining of global to regional climate models.
Conclusions
Evidence of past and present climate variability as well as more
recent anthropogenic influences on lakes and their catchment
basins are recorded in lacustrine sediments. The major difficulties
in employing these natural archives for paleoenvironmental and
paleoclimatic reconstructions are in establishing a precise and reli-
able timescale and interpreting the record properly. Ideally, both
obstacles can be circumvented if lake sediments are studied,
obtaining multi-proxy data on multiple-dated timescales. Success-
fully applied, lacustrine sedimentary archives provide a unique
opportunity to study the past dynamics of environmental systems
on local, regional, and even global scales. This geological perspec-
tive is necessary in order to generate realistic projections of future
environmental changes that might, at least in part, result from
human activities.
Bernd Zolitschka and Dirk Enters
Bibliography
Berglund, B.E. (ed.), 1986. Handbook of Holocene Palaeoecology and
Palaeohydrology. Chichester & New York: Wiley, 869pp.
Bradley, R.S., 1999. Paleoclimatology – Reconstructing Climates of the
Quaternary. San Diego, CA: Harcourt Academic Press. International
Geophysics Series, 64, 613pp.
Cohen, A.S., 2003. Paleolimnology: The History and Evolution of Lake
Systems. Oxford: Oxford University Press, 528pp.
Gierlowski-Kordesch, E., and Kelts, K.(eds.), 1994. Global Geological Record
of Lake Basins. Cambridge, UK: Cambridge University Press, 427pp.
Håkanson, L., and Jansson, M., 1983. Principles of Lake Sedimentology.
Berlin, Heidelberg: Springer-Verlag, 316pp.
Hutchinson, G.E., 1957. A Treatise on Limnology. I. Geography, Physics
and Chemistry. New York: Wiley.
Last, W.M., and Smol, J.P. (eds.), 2001. Tracking Environmental Change
Using Lake Sediments. Developments in Paleoenvironmental Research,
vol. 1–4. Dordrecht: Kluwer.
Wetzel, R.G., 2001. Limnology: Lake and River Ecosystems. San Diego,
CA: Academic Press, 1006pp.
Zolitschka, B., 2003. Dating based on freshwater and marine laminated
sediments. In Mackay, A., Battarbee, R., Birks, J., and Oldfield, F.
(eds.), Global Change in the Holocene: Approaches to Reconstructing
Fine-resolution Climate Change. London: Edward Arnold, 92–106.
Cross-references
Carbon Isotopes, Stable
Climate Forcing
Climate Variability and Change, Last 1,000 Years
Continental Sediments
Cyclic Sedimentation (Cyclothems)
Dating, Biostratigraphic Methods
Dating, Magnetostratigraphy
Dating, Radiometric Methods
Diatoms
Evaporites
Geochemical Proxies (Non-Isotopic)
Holocene Climates
Lake Level Fluctuations
Mineral Indicators of Past Climates
Ostracodes
Oxygen Isotopes
Paleoclimate Proxies, an Introduction
Paleohydrology
Paleolimnology
Palynology
Pleistocene Climates
Pollen Analysis
Proglacial Lacustrine Sediments
Quaternary Climate Transitions and Cycles
Radiocarbon Dating
Sapropels
Sedimentary Indicators of Climate Change
Stable Isotope Analysis
Tephrochronology
Transfer Functions
Varved Sediments
488 LACUSTRINE SEDIMENTS
