Peube J-L. Fundamentals of fluid mechanics and transport phenomena
Подождите немного. Документ загружается.

Thermodynamics of Discrete Systems 33
The natural evolution of the system
S from initial temperatures T
10
and T
20
leads
to the final temperature
2
2010
TT
T
f
, which is identical for the two blocks, and
to the final entropy S of the system:
2const
f
SmCLnT
The variation in entropy 'S between the final and initial instants is thus:
¸
¸
¹
·
¨
¨
©
§
'
2010
2
2010
2010
2
2010
4
1
4 TT
TT
mcLn
TT
TT
mcLnS
It is always positive and independent of the intermediate evolution between the
initial and final instants.
1.4.2.2.2.
Entropy sources
Let dQ
p,q
be the quantity of heat received in time dt by the component p
(
2,1 p
) from the other component (
pq z 2,1
):
0
1,22,1
dQdQ
The heat flow
M
Tp,q
received by the sub-system p is the quantity of heat
dtdQ
qp
,
received per unit time by the component p (
2,1 p
) from the other
component. Thus, we have:
¸
¸
¹
·
¨
¨
©
§
21
2,11,2
2
2,1
1
1111
TTdt
dQ
dt
dQ
Tdt
dQ
Tdt
dS
[1.30]
The rate of entropy creation
dtdS is always positive on account of the fact that
the heat transfer naturally occurs from the hot body to the cold body:
T
1
> T
2
dQ
1,2
< 0 and T
1
< T
2
dQ
1,2
> 0
Let us consider the first situation (T
1
> T
2
). The quantity dQ
2,1
is positive; the
entropy (positive) dS
2
gained by sub-system 2 is greater than the entropy -dS
1
lost by
sub-system 1. This is in accordance with the fact that for irreversible heat transfer,
the entropy gained by a system is greater than the entropy lost by the body which
has provided the heat.
34 Fundamentals of Fluid Mechanics and Transport Phenomena
The higher the degree of the imbalance, the greater the thermal flux, and the
greater the rate of entropy creation.
1.4.2.3.
The insulated system and entropy creation
Let us consider two bodies, each in equilibrium, and which exchange a quantity
X across a wall permeable for this quantity. We define the intensive entropic
parameters:
1
1
1
X
S
Z
w
w
or
2
2
2
X
S
Z
w
w
During a quasi-static transformation where the quantity dX
1,2
= -dX
21
is
exchanged in time dt, we obtain:
21
2,11,2
2
2,1
1
ZZ
dt
dX
dt
dX
Z
dt
dX
Z
dt
dS
[1.31]
As the entropy can only increase, the value of X increases (resp. decreases) in the
sub-system for which the value of Z is greatest (smallest). The transfer of the
quantity X occurs spontaneously from the sub-system with the smallest value of Z to
the sub-system with the greatest value of Z.
As in the preceding section, we see that during an irreversible transfer of an
extensive quantity, the entropy gained by a system is greater than the entropy lost by
the body which has lost this extensive quantity.
Expression [1.31] can be immediately generalized to a system constituted of P
sub-systems characterized by I independent extensive quantities:
.,
11 1
,
,
pq
dt
dX
Z
dt
dS
I
i
P
p
P
q
qip
pi
z
»
»
¼
º
«
«
¬
ª
¦¦ ¦

Thermodynamics of Discrete Systems 35
or, after grouping the opposing fluxes (
p < q, so as to only count this component
once):
qp
dt
dX
ZZ
dt
dS
I
i
P
p
P
q
qip
qipi
»
»
¼
º
«
«
¬
ª
¦¦ ¦
11 1
,
,,
1.4.2.4.
Systems with external exchanges and entropy source
Let us first consider the same thermal systems as above (section 1.4.2.2), but
where each of these receives a quantity of heat
dQ
pe
(p = 1,2) in time dt from the
exterior. For the heat exchange we have (Figure 1.6b):
0:with;;
1,22,121,2212,11
dQdQdQdQdQdQdQdQ
ee
The increase in system entropy is:
¸
¸
¹
·
¨
¨
©
§
212
2,1
2
2
1
1
11
TTT
dQ
T
dQ
T
dQ
dS
ee
[1.32]
The external entropy supply terms
dt
dQ
T
e1
1
1
and
dt
dQ
T
e2
2
1
can now take any
sign. The term in [1.32] which corresponds to irreversible internal heat exchange,
which is always positive, constitutes an entropy source. We thus have:
dS
T
dQ
p
p
pe
d
¦
2,1
[1.33]
Suppose, in addition, that the external
sources of heat are in thermostatic
equilibrium
, such that their temperatures T
1S
and T
2S
are defined. These transfers are
natural internal evolutions for any ensemble constituted of a sub-system and a
corresponding external source. They are, as before, entropy generators; we can
therefore write:
0
11
2,12,12,1
t
¸
¸
¹
·
¨
¨
©
§
¦¦¦
pSp
p
p
pe
p
pS
pe
p
p
pe
TTT
dQ
T
dQ
T
dQ
[1.34]
36 Fundamentals of Fluid Mechanics and Transport Phenomena
By then evaluating the entropy lost by the external sources we obtain the
Clausius inequality:
dS
T
dQ
T
dQ
p
p
pe
p
pS
pe
dd
¦¦
2,12,1
[1.35]
Similarly, for internal and external exchanges of an extensive quantity
X, we
obtain, using the same notations as above:
12
12
12 12
positive term
,
ee
dX
dS dX dX
Z
ZZZ
dt dt dt dt
The term
21
2,1
ZZ
dt
dX
, which is always positive, is the entropy source
associated with internal exchanges.
The rate of entropy creation is proportional to
the intensity
dt
dX
2,1
of the internal exchanges and to the imbalance
21
ZZ
between the two sub-systems.
In general, the internal production of entropy
dtdS
int
(entropy source) is
associated with the evolution of all of the extensive quantities in this system, which
is made up of two sub-systems:
IiZZ
dt
dX
dt
dS
I
i
ii
i
,....2,1
1
21
2,1
int
¦
[1.36]
Finally, let us recall that the entropic intensive quantities Zi are related to the
energetic intensive quantities ([1.12]:
ii
TZY ) and that it is possible to express
the entropy source as a function of any other quantities.
The reasoning used above leads to an expression for entropy sources
dt
dS
int
in a
system made up of
P sub-systems characterized by I independent extensive
quantities:
,
int
,,
111
()
IPP
ip q
ip ip
ipq
dx
ds
ZZ pq
dt dt
¯
¡°
¡°
¢±
[1.37]

Thermodynamics of Discrete Systems 37
Expression [1.37] only applies to internal entropy creation in the system
considered. Entropy production is also associated with the transfer of extensive
quantities from sources external to the system; this entropy production can be
evaluated in the same way. For thermal transfers between external temperature
sources
T
pS
(p = 1,…,P) and the system, we have, as above, the Clausius inequality:
dS
T
dQ
T
dQ
P
p
p
pe
P
p
pS
pe
dd
¦¦
11
1.4.2.5.
The average intensive quantity
1.4.2.5.1. Definition
An out-of-equilibrium system is characterized by a collection of intensive
quantities whose values differ according to the sub-systems considered. It may be
useful to characterize the system by a global intensive variable, which is an “average
value” of the intensive variables of the sub-systems. In order to define this average
value, we will refer to an “equivalent” equilibrium state of the system.
Consider an out-of-equilibrium system
S made up of P sub-systems S
p
each of
which is in instantaneous equilibrium (quasi-static transformations). For each of
these, we can define the intensive entropic quantities
Z
ip
associated with their N
extensive quantities
X
ip
. The total amount of extensive quantity X
i
contained in the
system
S is the sum
¦
P
p
ip
X
1
of the extensive quantities of each sub-system.
It is clear that the system
S cannot be described by any intensive quantity
associated with
X
i
. We can however associate system S with an average intensive
quantity
Y
m
or Z
m
at any given instant t, defined as the intensive quantity which the
system S would attain following a natural evolution during which values X
i
should
be constant (without any external contribution). Let us consider as an example the
variables
Z
m
.
Suppose that during the transformations undergone by the system, certain
extensive quantities
);,...,1(
pIiX
ip
of the sub-systems are exchanged, while
the other
IN extensive quantities remain constant in each of the sub-systems (for
example mass, number of moles, volume, etc.). All intensive quantities of all sub-
systems vary during the exchange of extensive quantities. In the final state of the
previously defined system,
intensive quantities Z
ip
corresponding to exchanged
extensive quantities have the uniform value Z
im
for all sub-systems:
38 Fundamentals of Fluid Mechanics and Transport Phenomena
In each sub-system
p, every intensive quantity Z
ip
corresponding to the extensive
exchanged quantities X
ip
can be expressed as a function of the N extensive quantities
(
I extensive quantities X
ip
exchanged with other sub-systems and N – I other
extensive quantities
X
jp
which are constant for the sub-system).
),...,1;,...,1;,...,1(, PpINjIiXXZZ
jpipipip
By solving the preceding system of
I equations for each sub-system p, with
respect to the extensive quantities
X
ip
, we obtain:
),...,1;,...,1;,...,1(, PpINjIiXZXX
jpipipip
The quantities
X
i
for the system S can be immediately obtained:
);,...,1;,...,1(,
1
INjIiXZXX
P
p
jpipipi
¦
The equilibrium conditions for the system can be written
);,...,1( pIiZZ
imip
pIZZ
.
Considering the quantities
X
i
to be constant, we obtain a system of I equations
from which we can evaluate the
I average intensive quantities Z
im
which
characterize the out-of-equilibrium system:
1
, ( 1,... ; 1,..., 1)
P
iipimjp
p
XXZXiIjN
[1.38]
We laid down that certain extensive variables were not exchanged between the
sub-systems. The problem can be easily discussed in the same manner with different
conditions: for example, by fixing the uniform value to certain intensive quantities
in the whole system (section 1.4.2.5.2, example 2), or by choosing different
conditions according to certain ensembles of sub-systems.
1.4.2.5.2.
Examples
We will consider two examples in order to illustrate the preceding procedure,
which we will encounter again in the study of fluid mechanics:
1)
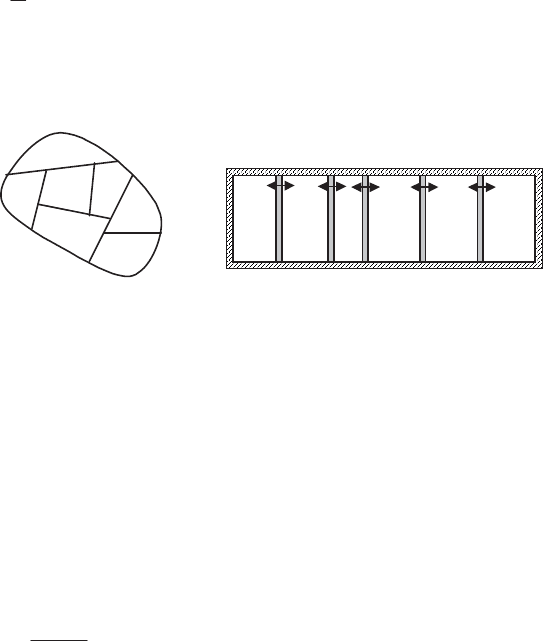
A thermal system – consider a system S
1
(Figure 1.7a) whose P sub-systems
exchange heat via a constant-volume process, such that the temperature
T
p
is the
only variable intensive quantity of any of the sub-systems during the process. The
energy
E
p
of each sub-system can be expressed as a function of its temperature and
Thermodynamics of Discrete Systems 39
its specific heat capacity
*
p
, which is assumed to be constant for the sake of
simplicity:
ppp
TE
*
The energy
E of the system and its average temperature T
m
can be obtained:
¦¦¦
* **
¸
¸
¹
·
¨
¨
©
§
* *
P
p
pmm
P
p
p
P
p
pp
TTTE
111
:with;
The average temperature of the out-of-equilibrium system can thus be written:
¦
*
*
p
ppm
TT
1
In this particular case, the average temperature is the average of the temperatures
weighted by the specific heat capacities.
T
p
T
2
T
1
(a) System
S
1
p
1
T
1
p
2
T
2
p
p
T
p
(b) System
S
2
Figure 1.7.
Examples of out-of-equilibrium system:
(a) incompressible thermal system S
1
; (b) compressible thermal system S
2
2)
A thermo-compressible system – consider now a system S
2
(Figure 1.7b),
which is comprised of sub-systems of variable volumes
V
i
, containing a perfect
gas and susceptible to exchange heat between each other. The volumes
V
i
are
separated by pistons which may be subject to fluid friction. The equations of
state for a perfect gas (section 1.3.2.5) give the expression sought for the
extensive quantities:
( : heat capacity at constant volume)
pp
ppvppvp
p
nRT
VET
p
**
40 Fundamentals of Fluid Mechanics and Transport Phenomena
The global volume and internal energy balances for a natural transformation
allow the average pressure and temperature to be defined:
11 1
11
;
with: and:
PP P
pp
m
p
vp p v m
pp p
pm
PP
pvvp
pp
nRT
nRT
VV E TT
pp
nn
**
**
We obtain the following expressions for the average temperature and pressure:
¦¦
*
*
P
p
p
pp
mm
P
p
pvp
v
m
p
Tn
nTp
TT
11
111
The average values of the intensive quantities are no longer simply weighted
arithmetic averages.
1.4.2.5.3.
Some comments
1) The “equivalent” system used to define the average intensive quantities is in
thermostatic equilibrium. It thus behaves according to a general state equation and
may therefore constitute a reduced representation of the out-of-equilibrium system,
which is clearly incomplete for a detailed description of the sub-systems. With the
exception of entropy, this is coherent with the laws of thermostatics (we can also say
that this is a “consistent” representation).
2) The average intensive quantities are only weighted arithmetic averages if the
expressions for the extensive quantities
X
ip
are linear functions of X
jp
and Y
ip
. The
reader can verify that the state equation for the equivalent complete system
comprises the same linear properties.
3) In the examples of the last section we have considered two systems
constituted of sub-systems with identical properties. The study of more complex
systems, comprising combinations of sub-systems with different structures, can be
effected in the same way.
4) The procedure for definition of average intensive quantities can also be
applied to systems whose intensive quantities may be subjected to certain
constraints. Let us reconsider the example of Figure 1.7a, in which the sub-systems
are all at constant pressure equal to that of the atmosphere, rather than being at
constant volume. We suppose nonetheless that the external walls of the system,
which are in contact with the atmosphere, are adiabatic. The conservation of internal
energy can be written by taking into account the work done by the atmospheric
Thermodynamics of Discrete Systems 41
pressure on the system. The reader can verify that this amounts to writing the
conservation of enthalpy of the sub-systems.
1.4.2.6.
Phenomenological laws
1.4.2.6.1. Introduction
In general, all causes of the same tensor nature act on all of the corresponding
effects: we thus have a
coupling of irreversible phenomena; the example of a
thermocouple is well known. Phenomena of different tensorial order do not mutually
interact (Curie’s principle). The interested reader should refer to the specialized
literature ([DEG 62], [BYU 02], [PRI 68]).
Consider two sub-systems
p and q between which scalar quantities X can be
exchanged. This exchange between the two sub-systems is assumed to be
independent of other sub-systems, which is the case when exchanges only occur via
direct contact, action at a distance not being possible. The irreversible evolution
which occurs takes the form of a flux,
dtdX
qip,
between the two sub-systems, and
is caused by the existence of an imbalance characterized by the different values
Z
jp
and
Z
jq
of all the intensive entropic quantities of the two sub-systems (j = 1,..., I).
We have a
relation between the causes Z
jp
and Z
iq
and the effects (the fluxes)
whose general form can be written as:
IjiZZF
dt
dX
jqjpi
qip
,...,1,,
,
The principle of action and reaction and the condition of zero flux at thermostatic
equilibrium
)(
jejqjp
ZZZ
can be represented by the relations:
0,;,,
jejeijpjqijqjpi
ZZFZZFZZF
From this we can deduce a property of the derivatives of
F
i
which will be used in
the following section:
jpjq
jq
i
jqjp
jp
i
ZZ
Z
F
ZZ
Z
F
,,
w
w
w
w
1.4.2.6.2.
Linear thermodynamics
Provided the degree of thermodynamic imbalance is reasonably small, it is
possible to perform a limited Taylor expansion of the functions
jqjpi
ZZF ,
in the
42 Fundamentals of Fluid Mechanics and Transport Phenomena
vicinity of the point ,( 1,..., )
ie
Zie I , of thermodynamic equilibrium. Taking into
account the above equality we have:
IjiZZoZZ
Z
F
ZZZZF
jqjpieie
jp
i
jqjpjqjpi
,...,1,,,,
w
w
In the preceding relations,
ieie
jp
i
iij
ZZ
Z
F
ZL ,
0
w
w
is a square matrix of
order
I, the properties of which cannot be obtained via thermodynamic
considerations. Only an experiment or a suitably adapted theory can provide these
laws, which we here qualify as
phenomenological laws.
Statistical reasoning shows that this matrix is often symmetric (Onsager
relations).
This symmetry is no longer maintained if other intensive variables are
used in place of intensive entropic variables.
As long as the preceding approximation is valid, we say that we are dealing with
linear thermodynamics of irreversible phenomena. We thus have, for each
thermodynamic flux of an extensive quantity:
jqjpieij
qip
ZZZL
dt
dX
,
[1.39]
Internal entropy source [1.36], which exists at the interface of the two sub-
systems considered, becomes:
njiZZZZZL
dt
dS
jj
i
iieij
j
,....2,1,
2121
int
¦¦
[1.40]
This internal entropy source is thus a
positive quadratic form whose principal
diagonal elements are all positive (
0!
eii
ZL ).
We can also perform a change of variables which involves expressing the
intensive entropic variables as a function of other thermodynamic variables, for
example the
energetic intensive variables. Consider two sub-systems 1 and 2. The
differences
21 ii
ZZ are generally linear functions (to second order excepted) of
the differences between the new variables which are chosen.
For example, with the intensive entropic variable
T1 which is associated with
energy, we obtain:
