Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

106
Chapter 5: Superconductor Types
Table 5.39.
Superconducting state parameters of two alkali metal doped fullerenes [adapted from A. P. Ramirez,
Superconductivity Rev., second issue of Vol. 1, No. 1 (1994)].
K3 C60 Rb3 C60
T c 19.5 29.5
2 (penetration depth--#SR) 4800 4- 200 A 4200 A
2 (optical) 800 4- 500 A 800 4- 500 A
2 (NMR) 6000 A 4600 A
~o (coherence length--Zac) 35 A 30 A
~o (Zac) 29-33 A
Bd(0 ) (#SR) 4.6 mT
B'~I(0) (Zdc) -- -0.28 mT/K
B'd0) (Zdc) -1.2 mT/K -0.7 mT/K
Bc2(0) (Zac) 26 T 34 T
Be2(0) (~ac) 30-38 T
Btc2 (Zac)
-3.73 T/K -3.9 T/K
B'c2 (Zac) --2.14 4-0.08 T/K
B'~2 (p) -1.34 T/K
AC/T c
68 4- 13 mJ/mole K 2
2ko/kBT c
(NMR) 3.0 4.1
2k0/k B T c (optical) 2.98 3.52
2k0/k BT c (#SR) -- 3.6 4- 0.3
2Ao/kuT c
(tunneling) 5.3 4- 0.2 5.2 4- 0.3
dTc/da o
(chemical subst.) 5.4 K/A
dTcdV
(chemical subst.) 0.088 K/A 3
dT~/dP
-7.8 K/GPa -9.7 K/GPa
dT~/dP
-6.3 K/GPa
Table
5.40.
AnB3_ n C60
alkali doped fullerene
compounds, cF252.
Na2 K2 Rb2
Cs 2
Na <2
K 2.5 19.5 27
Rb 2.5 23 29.5
Cs 10.5 24 31
33
47
J. Crystal Chemistry 107
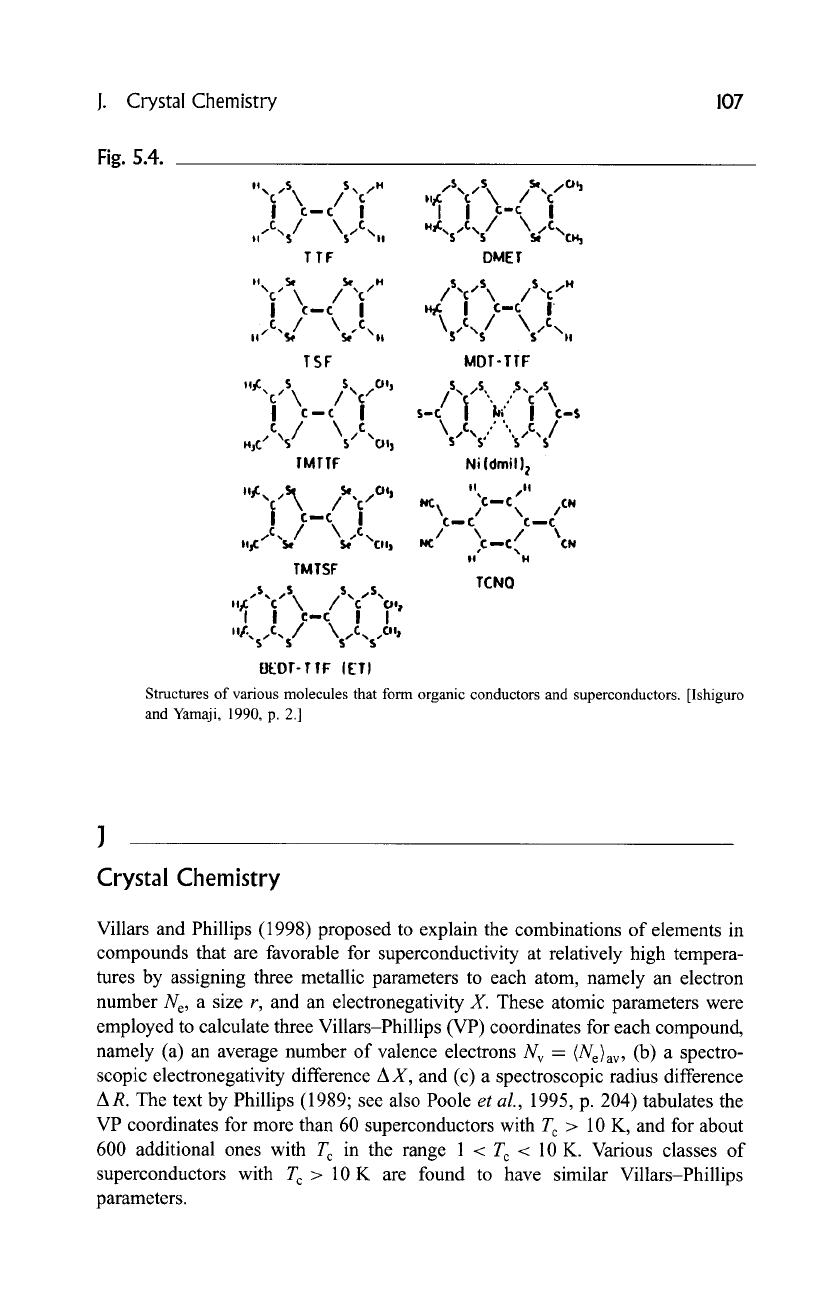
Fig. 5.4.
",,c,,S\ /S',c/H
I c-r I
~,/c',s/ \s/C\H
TTF
/S,,
,,$~
,,F c \ /s"'c/~
I I ,~.-c I
DMET
il,~ 15r
i\
?':'"
TSF
-sc,C/s \ ;,,c/o's
H,c/~Xs/-- ~S/~'O,,
TMTTF
"~c,c,,s' ~ y,c/o',
I
"F/c
"sJ
\o Ij
TMI"SF
..~,S,c,S\ /S,c,S,o,,
I I c-c, I I
ll/', s..C~ S/ \s..C..s..O la
/S-c/S \ /S,,c/"
~.. I c-c I"
\s,c,,/ \s,,C\.
MOT-Try
S,, /S, ,S~ /S
\s,C,s.. ' ".s,C,s/
Nildmill 2
ii
/ll
"C-" C /ON
NC\ / \
c--c c~c
NC , CN
to H
TCNO
UEDI'- T TF IETI
Structures of various molecules that form organic conductors and superconductors. [Ishiguro
and Yamaji, 1990, p. 2.]
Crystal Chemistry
Villars and Phillips (1998) proposed to explain the combinations of elements in
compounds that are favorable for superconductivity at relatively high tempera-
tures by assigning three metallic parameters to each atom, namely an electron
number Ne, a size r, and an electronegativity X. These atomic parameters were
employed to calculate three Villars-Phillips (VP) coordinates for each compound,
namely (a) an average number of valence electrons N v = (Ne)av , (b) a spectro-
scopic electronegativity difference A X, and (c) a spectroscopic radius difference
A R. The text by Phillips (1989; see also Poole
et al.,
1995, p. 204) tabulates the
VP coordinates for more than 60 superconductors with T c > 10 K, and for about
600 additional ones with Tc in the range 1 < T c < 10 K. Various classes of
superconductors with T c > 10 K are found to have similar Villars-Phillips
parameters.

108
Chapter 5: Superconductor Types
References
R. J. Cava, B. Batlogg, J. J. Krajewski, R. Farrow, L. W. Rupp, Jr., A. E. White, K. Short, W. E Pick and
T. Kometani,
Nature
332, 814 (1988).
M. S. Dresselhaus and G. E Dresselhaus, Eds.,
Science of Fullerenes and Carbon Nanotubes,
Academic Press, Boston, 1996.
T. Ishiguro and K. Yamaji,
Organic Superconductors,
Springer-Verlag, Berlin, 1990.
Landolt-Bornstein, Group III: Condensed Matter,
Vol. 21,
Superconductors,
Subvol. a (1990), b l
(1993), b2 (1994), c 1997), d (in preparation), and e (in preparation), R. Flfikiger and W. Klose, Eds.,
Springer-Verlag, Berlin.
L. E Mattheiss, either
Phys. Rev. B
37, 3749 (1988) or
Phys. Rev. Lett.
60, 2681 (1988).
A. R. Miedema,
J. Phys. (Paris)
F3, 1803 (1973); F4, 129 (1974).
J. C. Phillips,
Physics ofHigh-T c Superconductors,
Academic Press, New York, 1989.
C. P. Poole, Jr., T. Datta and H. A. Farach,
Copper Oxide Superconductors,
Wiley, New York, 1988.
C. P. Poole, Jr. and H. A. Farach, J.
Superconductivity
(1999), in press.
C. E Poole, Jr., H. A. Farach, and R. J. Creswick,
Superconductivity,
Academic Press, New York, 1995.
A. P. Ramirez,
Superconductivity Review, second issue of Vol.
1, 1 (1994).
B. W. Roberts,
J. Phys. Chem. Ref. Data
5, 581 (1976).
A. W. Sleight, J. L. Gillson, and E. Bierstedt,
Solid State Commun.
17, 27 (1975).
P. Villars and J. C. Phillips,
Phys. Rev. B
37, 2345 (1988).
S. V. Vonsovsky, Yu. A. Izumov, and E. Z. Kuramev,
Superconductivity in Transition Metals,
Springer-
Verlag, Berlin, 1982.
Chapter 6
Crystal Structures of
Classical Superconductors
Roman Gladyshevskii
Department of Inorganic Chemistry, L'viv State University, Ukraine
Karin Cenzual
D6partement de Chimie Min6rale, Analytique et Appliqu6e, Universit6 de
Genbve, Switzerland
A. Introduction
a.
b.
C.
d.
e.
B. Elements 120
a. Close-Packed Element Structures
b. Other Element Structures 123
C. Intermetallic Compounds 126
a. Close-Packed Structures 126
b.
C.
d.
e.
f.
g.
h.
110
Preliminary Remarks 110
Structure Types and Structural Relationships 111
Atom Coordinations 115
Definitions and Conventions 116
Strukturbericht Notation for Structure Types 119
120
CsC1 Type and Related Structures 127
Laves Phases 129
A15 Phases 130
Other Tetrahedrally Close-Packed and Related Structures 131
CaCu5 Type and Related Structures 133
Structures with Atoms in Square-Antiprismatic Coordination 134
Structures with Atoms in Trigonal Prismatic Coordination 136
ISBN: 0-12-561460-8
$30.00
HANDBOOK OF SUPERCONDUCTIVITY
Copyright 9 2000 by Academic Press.
All rights of reproduction in any form reserved.
109

110 Chapter
6:
Crystal Structures of Classical Superconductors
i.
Yb3Rh4Snl3
Type and Related Structures 140
j. Other Structures of Intermetallic Compounds 141
D. Interstitial Carbides, Nitrides, Oxides, and Hydrides 142
a. NaC1 Type 142
b. Other Structures with Close-Packed Metal Atom Layers
c. Other Interstitial Structures 145
E. Borides, Carbides, and Borocarbides with Nonmetal Polymers
a. Layered Carbohalides 146
b. CeCo4B 4 Type and Stacking Variants 148
c. Other Structures with Nonmetal Atom Dumbbells 150
d. LuNi2B2C Type and Members of the Same Structure Series
e. Structures with Nonmetal Polymers 155
F. Chalcogenides 157
a. Perovskite, Bronze, and Spinel 157
b. Layered Structures and Intercalation Compounds 158
c. Chevrel Phases and Related Structures 160
d. Other Chalcogenide Structures 163
G. Heavy-Electron Compounds 164
H. Organic Compounds 165
a. Fullerides 165
b. ET and Other Charge-Transfer Salts 167
I. Crystallographic Data Sets 170
a. Criteria for Selection 170
b. Presentation and Notation 171
J. Tabulated Data 173
a. Element Structure Types 173
b. Binary Structure Types 177
c. Ternary Structure Types 207
d. Quaternary Structure Types 233
e. Charge-Transfer Salts 235
References 236
144
146
152
A
Introduction
a. Preliminary Remarks
Classical superconductors cover a large spectrum of chemically different
compounds, ranging from alloys to chalcogenides and organic compounds, and
crystallize with very different structures. The highest superconducting transition
temperatures are observed among representatives of structure types such as the
K3C60 type with C60 fullerene "balls," the simple cubic Cr3Si (A15) and NaC1
types, the Pu2C 3 type with C 2 dumbbells, the layered borocarbide type

A. Introduction 111
LuNi2B2C , the Chevrel phases with octahedral metal atom clusters, and the
perovskite type, precursor of the high-T c superconducting oxides. When the limit
in Tc is decreased, superconducting representatives are found for a relatively large
number of different structure types.
The present overviews have, with a few exceptions, been restricted to
structure types for which at least one compound has been reported with a critical
temperature above the boiling point of helium, 4.2 K. The exceptions concern
closely related structures or compounds, as well as heavy-electron compounds.
Structure types represented by materials that become superconducting only under
high pressure, after irradiation, or in thin films have not been taken into
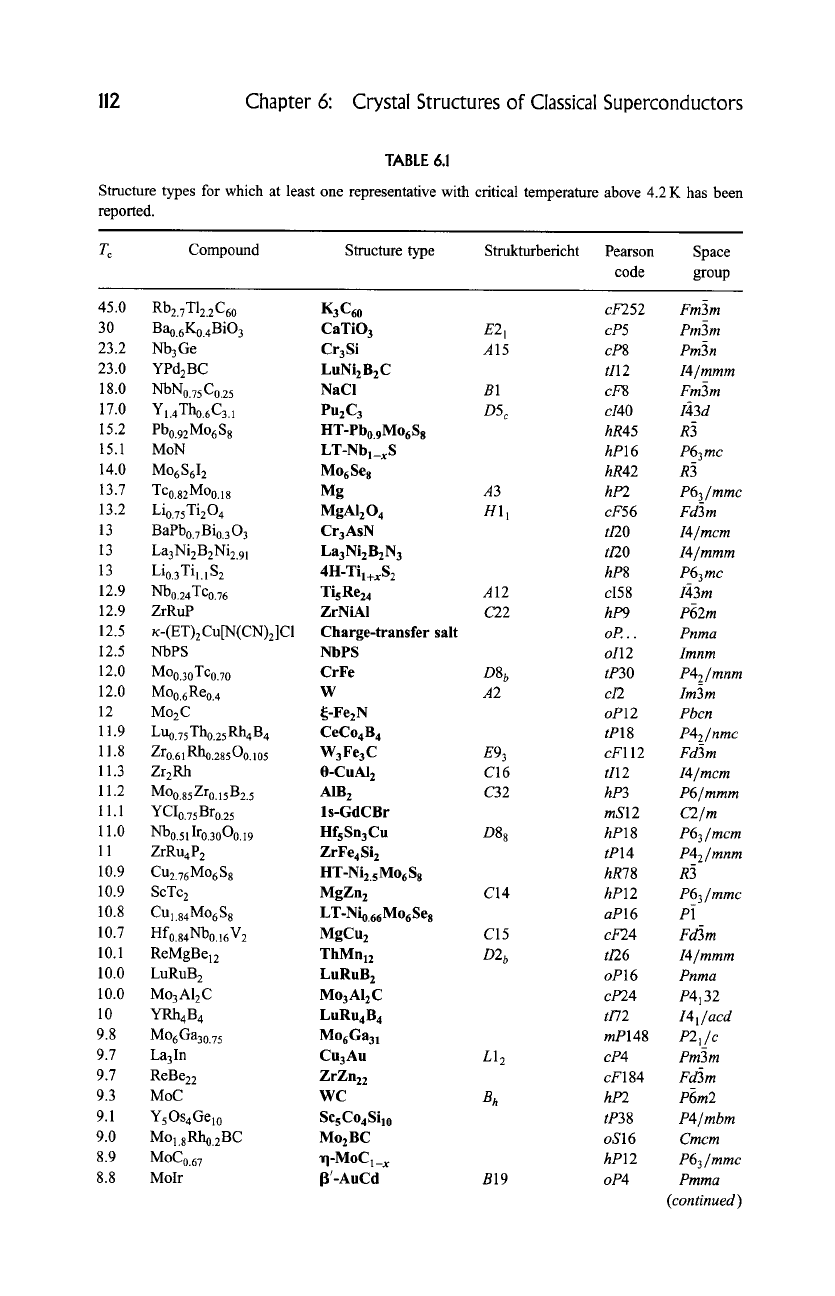
account. The more than 100 structure types presented here are listed in Table
6.1, ordered according to the highest temperature reported for an isotypic
compound.
In the text the structures have been grouped at a first level according to the
chemical family within which superconducting representatives are found. The
chapter has thus been subdivided roughly into structures found among super-
conducting elements, intermetallics, interstitial compounds, borides and carbides,
chalcogenides, and organic compounds. This classification is, however, not
absolute, since the same structure type is sometimes adopted by different classes
of compounds. Within each section, particular structural features, such as the
substructure formed by one of the elements or selected coordination polyhedra,
have been emphasized. Also, this subdivision is only approximate, since a
structure type may contain both a particular substructure and a defined coordina-
tion.
Almost one century has passed since the discovery of superconductivity,
and a huge amount of literature on superconductors has been published. The
literature search for the preparation of this chapter was simplified by the existence
of lists of superconducting compounds, such as those given in the works by B. T.
Matthias
et al.
(1963), S. V. Vonsovsky
et al.
(1982), B. W. Roberts (1976), E. M.
Savitsky
et al.
(1985), and L. I. Berger and R. W. Roberts (1997). A certain
number of books and review articles on particular classes of superconductors
were also consulted. Publications with structural data were often found via
TYPIX
(Parth6
et al.,
1993/94; Cenzual
et al.,
1995),
Pearson's Handbook
(Villars,
1997), or the
Inorganic Crystal Structures Database
(Kirchhoff
et al.,
1991);
however, the original papers were always examined. No claim is made on
completeness; however, we hope that few structure types responding to the
criteria defined here have been overlooked.
b. Structure Types and Structural Relationships
Ignoring the chemical nature of the constituents, a particular geometric arrange-
ment of atoms is generally referred to as a
structure type.
Following recommen-
dations of the International Union of Crystallography, to be considered as such,
isotypic
compounds should crystallize with the same space group and comparable
112
Chapter 6: Crystal Structures of Classical Superconductors
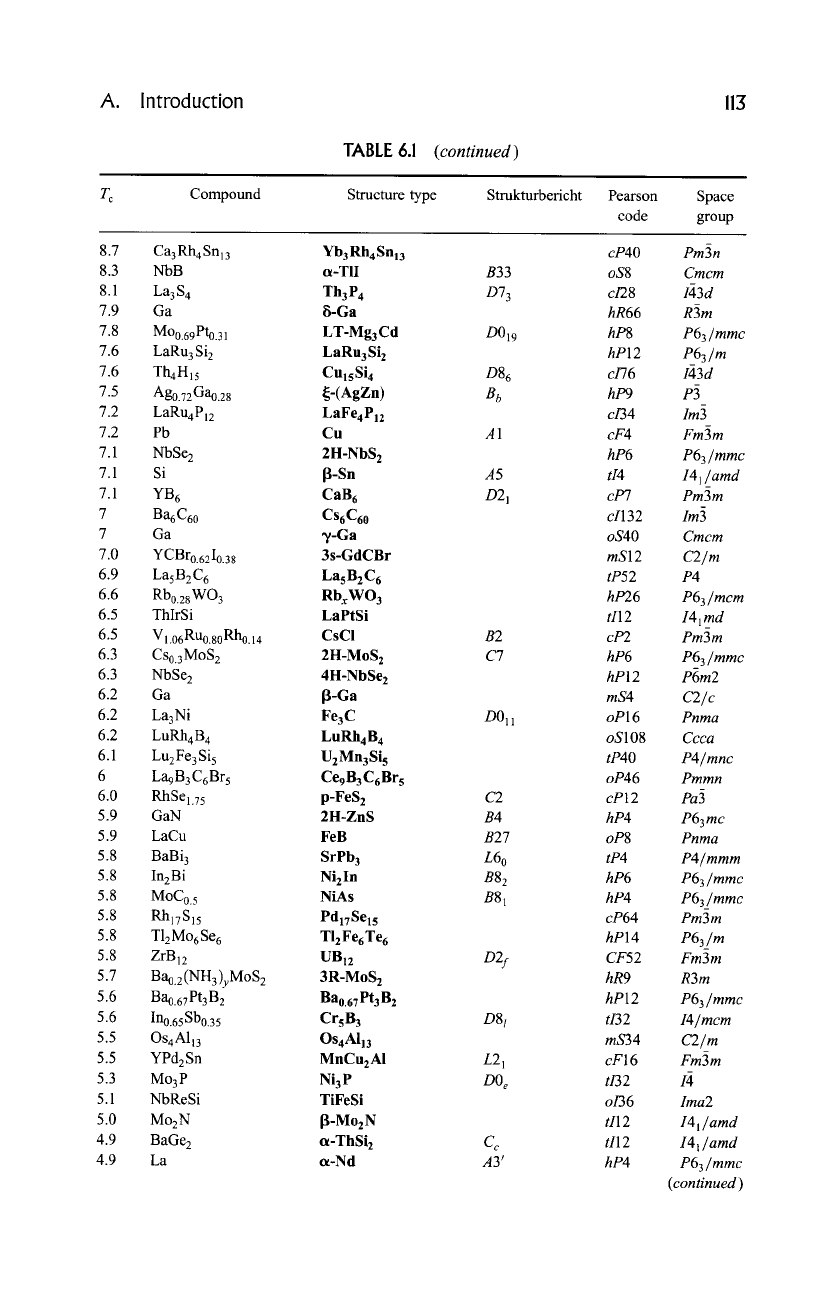
TABLE 6.1
Structure types for which at least one representative with critical temperature above 4.2 K has been
reported.
T c Compound Structure type Stmkturbericht Pearson Space
code group
45.0 Rb2.7T12.2C60
K3C6o
cF252 Fm~3m
30 Bao.6Ko.4BiO 3 CaTiO 3 E21 cP5 Pm~3m
23.2 Nb3Ge Cr3Si A15 cP8 Pm3n
23.0 YPd2BC LuNi2B2C tI12 I4/mmm
18.0 NbNo.75 Co.25 NaCI B1 cF8 Fm3m
17.0 YI.aTho.6C3.1 PuzC3 D5 c ci40 I2~3d
15.2 Pbo.92Mo6S 8 I-IT-Pbo.9Mo6S 8 hR45 R~3
15.1 MoN LT-Nbl_xS hP16 P63 mc
_
14.0 Mo6S6I 2 Mo6Se 8 hR42 R3
13.7 Tco.82Moo.18 Mg A3 hP2 P63/mmc
_
13.2 Lio.75Ti20 4 MgAI20 4 H11 cF56 Fd3m
13 BaPbo.7Bio.30 3 Cr3AsN tI20 14/mcm
13 La3Ni2B2Ni2.91 La3Ni2B2N 3 tI20 I4/mmm
13 Lio.3 Til.1 $2 4H-Til+xS2 hP8 P63mc
_
12.9 Nbo.24Tco.76 TisRe24 A12 ci58 I43m
12.9 ZrRuP ZrNiAI C22 hP9 P62m
12.5 x-(ET)2Cu[N(CN)2]C1 Charge-transfer salt oP... Pnma
12.5 NbPS NbPS o112 Imnm
12.0 Moo.3oTco.7o CrFe D8 b tP30 P42/mnm
12.0 Moo.6Reo.4 W A2 ci2 Im3m
12 Mo2C ~-Fe2N oP12 Pbcn
11.9 Luo.75 Tho.25RhaB 4 CeCo4B 4 tP18 P42/nmc
_
11.8
Zro.61Rho.28500.105 W3Fe3C
E93 cF112 Fd3m
11.3 Zr2Rh
0-CuAl 2
C16 tI12 I4/mcm
11.2 Moo.ssZro.15B2.5 AIB 2 C32 hP3 P6/mmm
11.1 YCIo.75Bro.25 ls-GdCBr mS12 C2/m
11.0
Nb0.51 Iro.3oOo.19
HfsSn3 Cu D88 hP18 P63/mcm
11 ZrRu4P 2 ZrFe4 Si 2 tP14 P42/mnm
10.9 Cu2.76Mo6 $8 HT-Ni2.sMo6S s hR78 R3
10.9 ScTc 2 MgZn 2 C14 hP12 P63/mmc
10.8 CUl.84Mo6 S 8 LT-Nio.66M06 Se s aP16 P1
10.7 Hfo.84Nbo. 16V 2 MgCu 2 C15 cF24 Fd3m
10.1 ReMgBe u ThMnl2 D2 b ti26 I4/mmm
10.0 LuRuB 2 LuRuB 2 oP16 Pnma
10.0 Mo3A12C Mo3AI2C cP24 P4132
10 YRh4B 4
LuRu4B 4
ti72 I41/acd
9.8 Mo6Ga3o.75 Mo6Ga31 raP148 P21/c
_
9.7 La3In Cu3Au L12 cP4 Pm3m
9.7 ReBe22 ZrZn22 cF184 Fd3m
9.3 MoC WC B h hP2 P6m2
9.1 YsOs4Gelo SesCo4Silo tP38 P4/mbm
9.0 MOl.8RI~.2BC Mo2BC oS16 Cmcm
8.9 MoCo.67 II-MoCI_ x hP12 P63/mmc
8.8 MoIr 13'-AuCd B19 oP4 Pmma
(continued)
A. Introduction 113
TABLE 6.1
(continued)
T c Compound Structure type
Strukturbericht Pearson Space
code group
8.7 Ca3Rh4Snl3
8.3 NbB
8.1 La3S 4
7.9 Ga
7.8 Moo.69Pto.31
7.6 LaRuaSi 2
7.6 Th4H15
7.5 Ago.72Gao.28
7.2 LaRu4P12
7.2 Pb
7.1 NbSe 2
7.1 Si
7.1 YB 6
7 Ba6C60
7 Ga
7.0 YCBro.62Io.38
6.9 LasB2C 6
6.6 Rbo.28WO3
6.5 ThlrSi
6.5
VI.o6Ruo.8oRho.14
6.3 CSo.3MoS 2
6.3 NbSe 2
6.2 Ga
6.2 La3Ni
6.2 LuRh4B 4
6.1 Lu2Fe3Si 5
6 La9B3C6Br 5
6.0 RhSel.75
5.9 GaN
5.9 LaCu
5.8 BaBi 3
5.8 In2Bi
5.8 MoCo. 5
5.8 Rhl7S15
5.8 T12Mo6Se 6
5.8 ZrBl2
5.7 Bao.2 (NH3)yMOS 2
5.6 Bao.67PtaB 2
5.6 Ino.65 Sbo.35
5.5 OsnAl13
5.5 YPd2Sn
5.3 Mo3P
5.1 NbReSi
5.0 Mo2 N
4.9 BaGe 2
4.9 La
Yb3 Rh4
Snl 3 cP40
t~-TlI B33 oS8
Th3P 4
D73 c/28
~-Ga hR66
LT-Mg3Cd D019 hP8
LaRuaSi2 hP12
Cul5Si 4 D86 c/76
~-(AgZn) B b hP9
LaFe4P12 c/34
Cu A 1 cF4
2H-NbS2 hP6
13-Sn A5 tI4
CaB 6 D21 cP7
Cs6C60
c/132
~/-Ga oS40
3s-GdCBr mS12
LasB2C6 tP52
RbxWO 3 hP26
LaPtSi tI12
CsCI B2 cP2
2H-MoS 2 C7 hP6
4H-NbSe2 hP12
[~-Ga mS4
Fe3C D011 oP16
LuRh4B4
oS108
U2Mn3Sis tP40
Ce9B3C6Brs oP46
p-FeS 2 C2 cP12
2H-ZnS B4 hP4
FeB B27 oP8
SrPb3 L6 o tP4
Ni2In B82 hP6
NiAs B81 hP4
Pd17Sels cP64
Tl2Fe6Te6 hP14
UB12 D2f CF52
3R-MoS2 hR9
Bao.67Pt3B2 hP12
CrsB3 D8 t t/32
Os4Al13 mS34
MnCu2AI L21 cF16
Ni3P DO e t132
TiFeSi 0/36
[3-Mo2N tI12
t~-ThSi 2 C c tI12
ct-Nd A3' hP4
Pm3n
Cmcm
I2~3d
R3m
P63/mmc
P63/m
fi3d
P~
Im~
Fm3m
P63/mmc
I41/amd
Pm3m
Im~
Cmcm
C2/m
P4
P63/mcm
141 md
_
Pm3m
P63/mmc
_
P6m2
C2/c
Pnma
Ccca
P4/mnc
Pmmn
l~a~
P63mc
Pnma
P4/mmm
P63/mmc
P63/mmc
_
Pm3m
P63/m
_
Fm3m
R3m
P63/mmc
I4/mcm
C2/m
Fm3m
Ima2
I41/amd
I41/amd
P63/mmc
(continued)
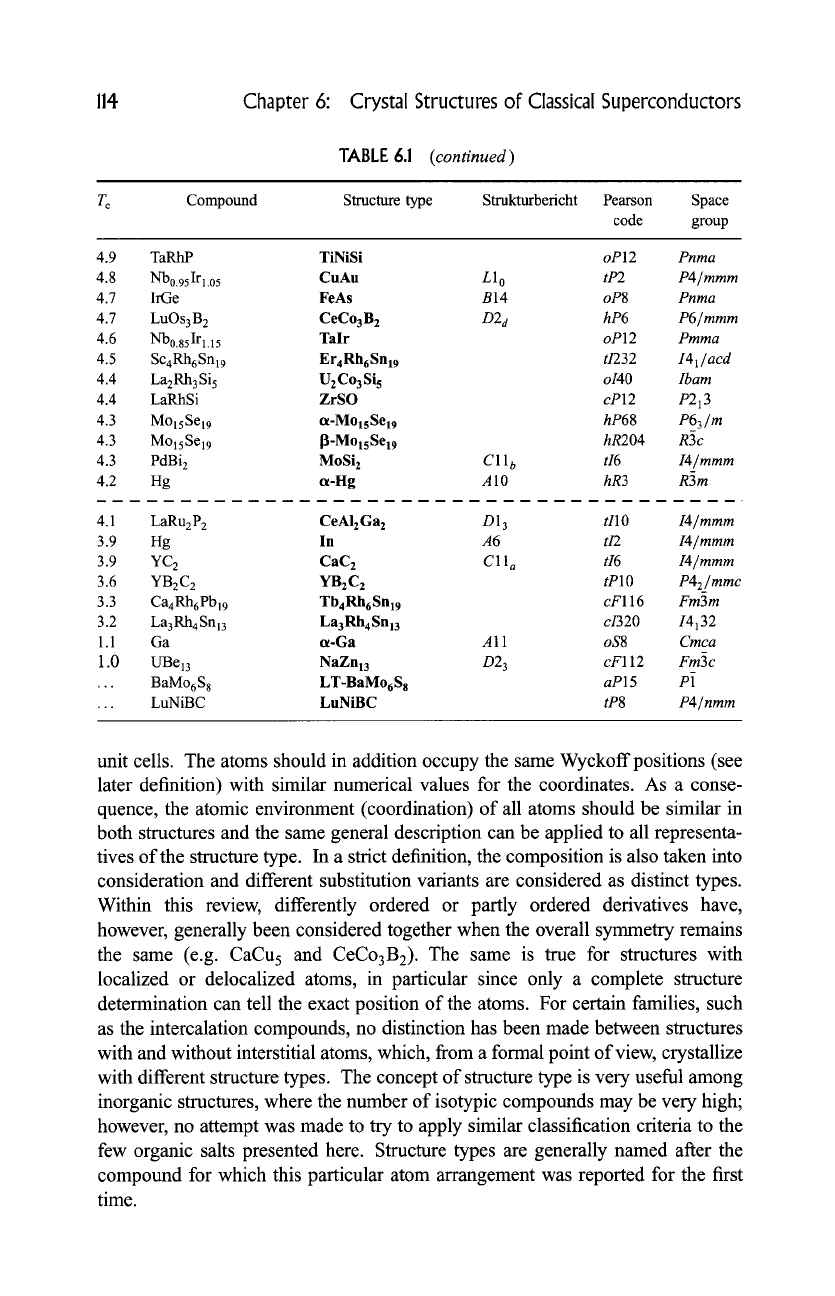
114 Chapter 6: Crystal Structures of Classical Superconductors
TABLE 6.1
(continued)
T c Compound Structure type Strukturbericht Pearson Space
code group
4.9 TaRhP TiNiSi
oP12 Pnma
4.8 Nb0.95Irl.05 CuAu L10
tP2 P4/mmm
4.7 IrGe FeAs B14
oP8 Pnma
4.7 LuOs3B 2 CeCo3B 2
D2 d hP6 P6/mmm
4.6 Nb0.85Irl.15 Talr
oP12 Pmma
4.5 ScaRh6Snl9 Er4RhrSnl9 t/232
I41/acd
4.4 La2Rh3Si 5 U2Co3Si s
o140 Ibam
4.4 LaRhSi ZrSO
cP12
P213
4.3 MolsSel9 ot-MolsSel9
hP68 P63/m
4.3 MolsSe19 I~-MolsSel9 hR204
R3c
4.3 PdBi 2 MoSi 2
C11 b tI6 I4/mmm
_
4.2 Hg ot-Hg A 10
hR3 R3m
4.1 LaRu2P 2 CeAI2Ga 2 D13
tllO I4/mmm
3.9 Hg In
A6 tI2 I4/mmm
3.9 YC 2
CaC 2 C11 a tI6 I4/mmm
3.6 YB2C2 YB2C2
tPlO
P42/mmc
3.3 Ca 4Rh6Pbl9 Tb4RhrSnl9
cF116 Fm3m
3.2 La3RhaSnl3 La3Rh4Snl3 c/320 14132
1.1
Ga ot-Ga A 11
oS8 Cmca
1.0 UBel3 NaZn13 D23
cF112 Fm3c
... BaMo6S 8 LT-BaMorS a
aP15 P1
... LuNiBC LuNiBC
tP8 P4/nmm
unit cells. The atoms should in addition occupy the same Wyckoff positions (see
later definition) with similar numerical values for the coordinates. As a conse-
quence, the atomic environment (coordination) of all atoms should be similar in
both structures and the same general description can be applied to all representa-
tives of the structure type. In a strict definition, the composition is also taken into
consideration and different substitution variants are considered as distinct types.
Within this review, differently ordered or partly ordered derivatives have,
however, generally been considered together when the overall symmetry remains
the same (e.g. CaCu 5 and CeCo3B2). The same is true for structures with
localized or delocalized atoms, in particular since only a complete structure
determination can tell the exact position of the atoms. For certain families, such
as the intercalation compounds, no distinction has been made between structures
with and without interstitial atoms, which, from a formal point of view, crystallize
with different structure types. The concept of structure type is very useful among
inorganic structures, where the number of isotypic compounds may be very high;
however, no attempt was made to try to apply similar classification criteria to the
few organic salts presented here. Structure types are generally named after the
compound for which this particular atom arrangement was reported for the first
time.

A. Introduction 115
Some 10,000 structure types are known for inorganic compounds and the
comparison of different types often reveals similar geometrical features. Struc-
ture types may, for instance, be related to each other by simple relations such as
deformation, substitution, or filling up of vacancies. A deformation may cause a
lowering of the symmetry; for example, the orthorhombic FeAs type is a
deformation derivative of the hexagonal NiAs type. Partial substitution of one
element by another in a particular ratio commonly leads to an ordered atom
distribution. The resulting structure may respect the original symmetry, as is the
case for c~-Mn and TisRe24, or break some of the symmetry elements present in
the parent type. For example, on replacing half of the nonmetal atoms in a-ThSi 2
to obtain the LaPtSi type, one of the mirror planes is lost. A particular case of
substitution occurs when a pair of atoms replaces one atom. An example of such
a relationship is found between
Sc4C 3
with single carbon atoms (Th3P 4 antitype)
and Pu2C 3 with carbon atom dumbbells. Several structure types present vacan-
cies large enough to host additional atoms. In the Chevrel phases, for example,
the large cavities in the Mo6Se 8 type are occupied by cations. The super-
conducting nitrides and carbides crystallizing with NaCl-type structures are
generally considered as interstitial compounds, whereas the layered structures
found with chalcogenides are known to form a large number of intercalation
derivatives, sometimes hosting very thick layers of molecules between the
chalcogenide slabs. Structural units with common geometric features are often
recognized in different structures; for example, CaTiO 3 and LaFe4P12 both
contain frameworks of corner-sharing octahedra. When two structures can be
decomposed into the same kind of subunit, arranged in different ways, they are
considered as stacking variants. The types FeB and a-TlI represent two different
ways to stack a several-angstrom thick structural slab of interconnected trigonal
prisms. Two or more kinds of subunit may be present in different proportions in
several structures, and a series of intergrowth structures may be considered. This
is the case for some of the superconducting borocarbides and boronitrides, which
are conveniently decomposed into two different kinds of slab. Finally, larger
units may be packed in the same way as smaller units; for example, in one of the
fulleride structures fullerene molecules and alkaline-earth cations adopt the same
arrangement as the single atoms in intermetallic Cr3Si.
c. Atom Coordinations
The stability of a structure is deduced from its overall band structure, but at the
very local level, each individual atom in a structure will try to accommodate itself
to satisfy a series of criteria. These criteria represent a combination of constraints
due to covalent bonding, local electroneutrality, and space-filling. An atom
connected to the surrounding atoms by covalent bonds will have a well-defined
coordination that can suffer relatively little distortion. On the other extreme, a
highly ionized atom has less demand on the shape of the cavity in which it is
