Poole Ch.P., Jr. Handbook of Superconductivity
Подождите немного. Документ загружается.

166
Chapter 6: Crystal Structures of Classical Superconductors
sites are occupied by single atoms. The resulting arrangement of metal atoms and
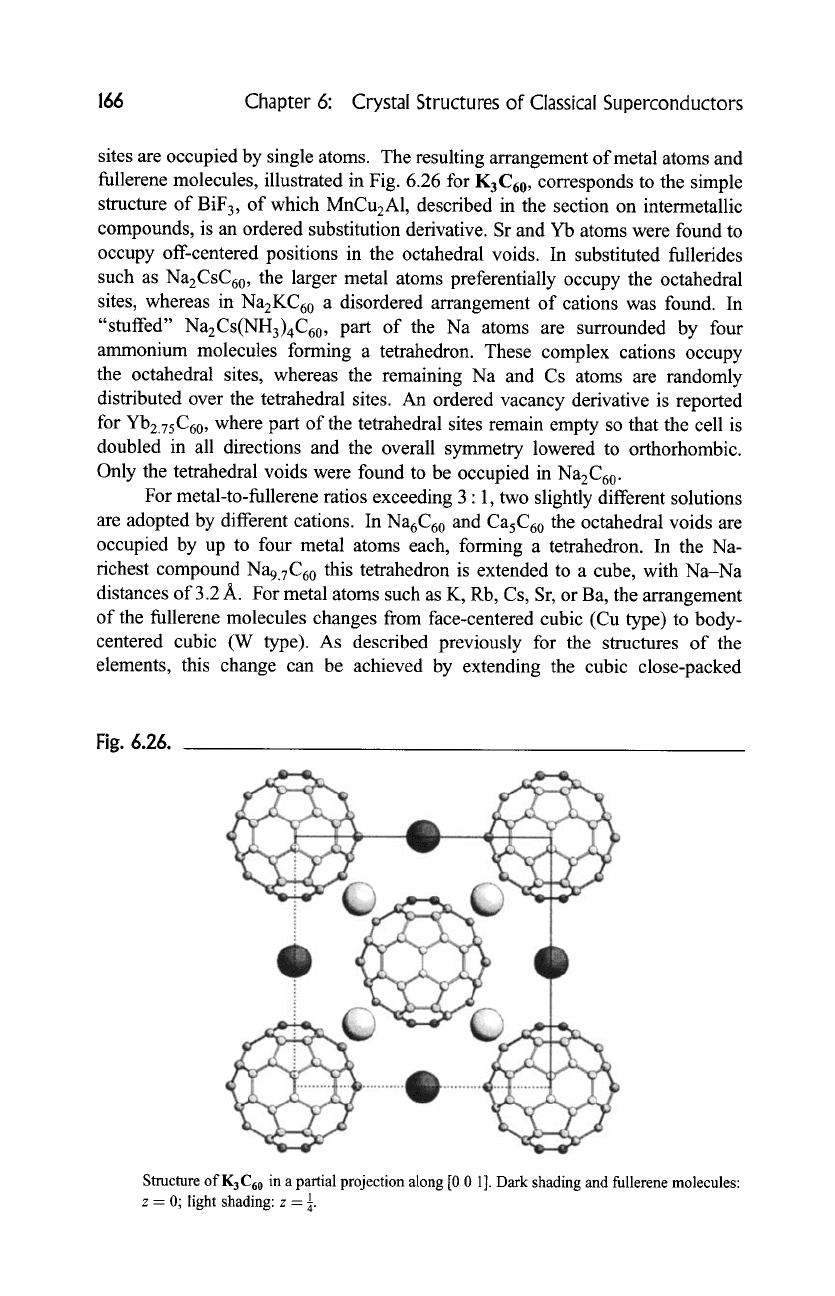
fullerene molecules, illustrated in Fig. 6.26 for K3C60, corresponds to the simple
structure of BiF3, of which MnCu2A1, described in the section on intermetallic
compounds, is an ordered substitution derivative. Sr and Yb atoms were found to
occupy off-centered positions in the octahedral voids. In substituted fullerides
such as Na2CsC60 , the larger metal atoms preferentially occupy the octahedral
sites, whereas in Na2KC60 a disordered arrangement of cations was found. In
"stuffed" Na2Cs(NH3)4C60 , part of the Na atoms are surrounded by four
ammonium molecules forming a tetrahedron. These complex cations occupy
the octahedral sites, whereas the remaining Na and Cs atoms are randomly
distributed over the tetrahedral sites. An ordered vacancy derivative is reported
for
Yb2.75C60 ,
where part of the tetrahedral sites remain empty so that the cell is
doubled in all directions and the overall symmetry lowered to orthorhombic.
Only the tetrahedral voids were found to be occupied in Na2C60.
For metal-to-fullerene ratios exceeding 3 : 1, two slightly different solutions
are adopted by different cations. In Na6C60 and CasC60 the octahedral voids are
occupied by up to four metal atoms each, forming a tetrahedron. In the Na-
richest compound Na9.7C60 this tetrahedron is extended to a cube, with Na-Na
distances of 3.2 A. For metal atoms such as K, Rb, Cs, Sr, or Ba, the arrangement
of the fullerene molecules changes from face-centered cubic (Cu type) to body-
centered cubic (W type). As described previously for the structures of the
elements, this change can be achieved by extending the cubic close-packed
Fig. 6.26.
Structure of K 3 C60 in a partial projection along [0 0 1]. Dark shading and fullerene molecules:
z = 0; light shading: z -- 88

H. Organic Compounds
167
structure in the plane perpendicular to one of its 4-fold axes. Such packing is less
dense and contains six tetrahedral voids per molecule. In the cubic body-centered
structure of Cs6C60 all these voids are occupied by metal atoms, which form a
pattern where all faces of the cubic cell contain a square of atoms. At the metal-
to-molecule ratio 4: 1, an ordered arrangement of metal atoms and vacancies is
found, with a square of metal atoms on two and a pair of atoms on the other four
faces of the cell. The symmetry of this structure is tetragonal and the
c/a
ratio
slightly lower than unity. For the ratio 3 : 1, one-half of the tetrahedral voids are
empty and all faces of the cell contain two metal atoms. The structure is cubic
primitive and the arrangement of metal atoms and fullerene molecules identical to
the one formed by the atoms in the Cr3Si (A 15) type.
Different kinds of rotational disorder are reported for the fullerene
molecules in different compounds, the exact situation being not always clear. The
rotational disorder in Li2CsC60 is considered to be spherical. K3C60 presents a
merohedral disorder, where the C60 molecules are randomly distributed over two
orientations. In both orientations, related by a 90 ~ rotation, eight of the 20
hexagonal faces are perpendicular to 3-fold axes. In LT-Na2CsC60 and
Na2RbC60 a preferred orientation is adopted, where the molecules are rotated
by 98 ~ around the body diagonals. The ordered atom arrangement is described in
Pa3,
the same space group as found for the LT-modification of C60. In the "A 15-
type" structure the molecules located at 0 0 0 and 89 1 i are rotated by 90 ~ with
respect to each other.
The alkaline metal atoms fully donate their electrons to the C60 unit.
Superconductivity is observed for a metal-to-fullerene ratio close to 3:1, with
critical temperatures near 30K measured for Na2Cs(NH3)4C60, Rb3C60,
Rb2CsC60 , and Cs2RbC60. A maximum value of 45.0 K is reported for nominal
Rb2.TT12.2C60.
For the alkaline-earth metals the charge transfer is not complete
and superconductivity is found for a higher metal-to-fullerene ratio, e.g., Ca5C60
(8.4 K), Sr6C60 (4 K) and Ba6C60 (7 K). T c increases monotonically with increas-
ing cell parameter for both the f.c.c, and the b.c.c, packing.
b. ET and Other Charge-Transfer Salts
A large number of crystal structures of charge-transfer salts containing the
molecule bis(ethylenedithio)tetrathiafulvalene, abbreviated BEDT-TTF or simply
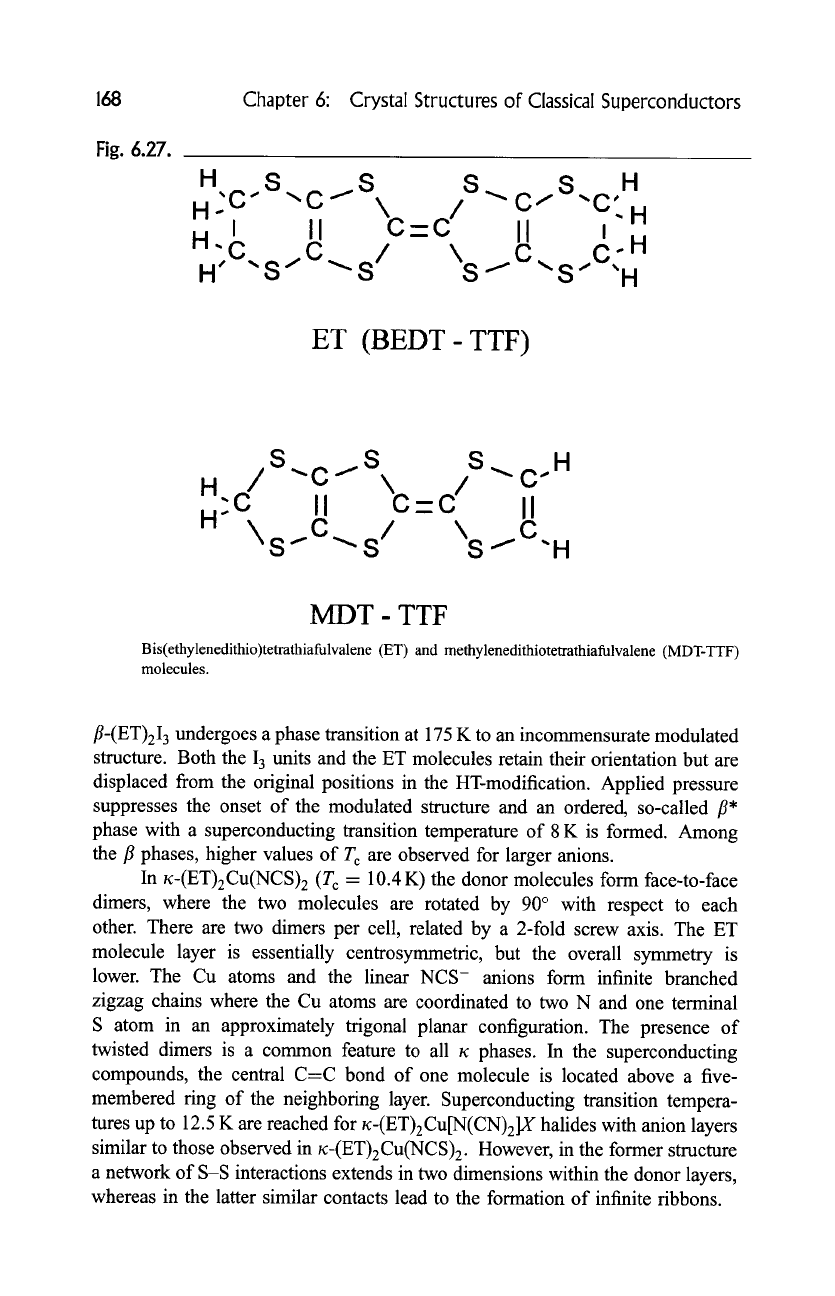
ET, have been determined. The ET molecule, shown in Fig. 6.27, is rather fiat
because of the presence of an extended n-electron system, but deviations are
always observed, in particular in the ethylene end groups. ET salts contain slabs
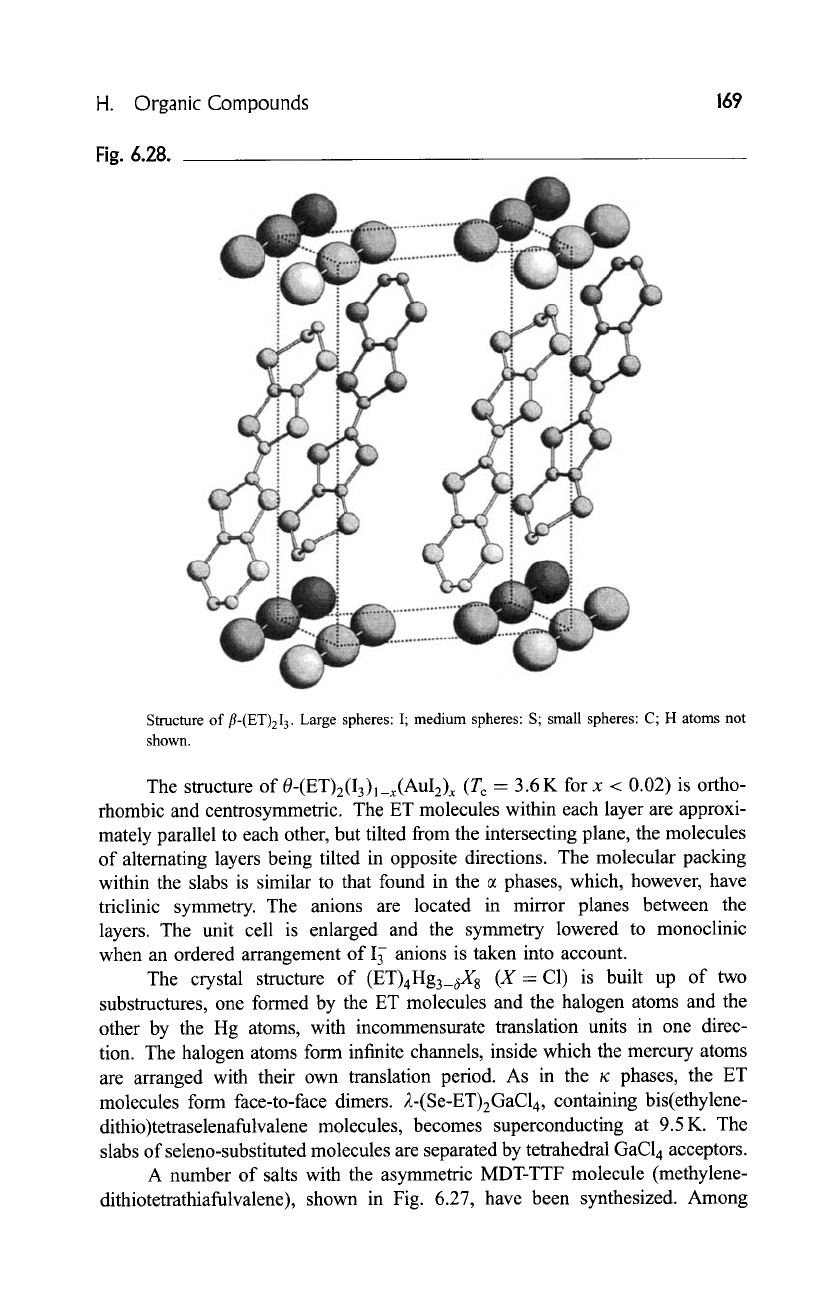
of ET molecules separated by anion layers, as illustrated by the structure of
fl-(ET)2I 3 in Fig. 6.28.
The structures of the fl-(ET)2X 3 family of compounds are triclinic with one
donor-molecule layer per translation unit. All molecules are parallel and the
linear anions, which can be 13, IBr 2, or AuI 2, are located at inversion centers.
168
Fig. 6.27.
Chapter 6:
Crystal
Structures of Classical Superconductors
H,c.S..c~S S~ S H
H-I II \C--C / C / "C'H
II ~ .H
H "C C / \ C ,,C,H
H" "S" ~S S ~
"S
ET (BEDT- TTF)
H c /--C.H
H" \ S / \S
S" ~ ~ "H
MDT- TTF
Bis(ethylenedithio)tetrathiafulvalene (ET) and methylenedithiotetrathiafulvalene (MDT-TTF)
molecules.
fl-(ET)2I 3 undergoes a phase transition at 175 K to an incommensurate modulated
structure. Both the 13 units and the ET molecules retain their orientation but are
displaced from the original positions in the HT-modification. Applied pressure
suppresses the onset of the modulated structure and an ordered, so-called r*
phase with a superconducting transition temperature of 8 K is formed. Among
the fl phases, higher values of T c are observed for larger anions.
In tc-(ET)2Cu(NCS)2 (T c = 10.4 K) the donor molecules form face-to-face
dimers, where the two molecules are rotated by 90 ~ with respect to each
other. There are two dimers per cell, related by a 2-fold screw axis. The ET
molecule layer is essentially centrosymmetric, but the overall symmetry is
lower. The Cu atoms and the linear NCS- anions form infinite branched
zigzag chains where the Cu atoms are coordinated to two N and one terminal
S atom in an approximately trigonal planar configuration. The presence of
twisted dimers is a common feature to all tc phases. In the superconducting
compounds, the central C-C bond of one molecule is located above a five-
membered ring of the neighboring layer. Superconducting transition tempera-
tures up to 12.5 K are reached for tc-(ET)ECu[N(CN)2]X halides with anion layers
similar to those observed in tc-(ET)ECu(NCS)2. However, in the former structure
a network of S-S interactions extends in two dimensions within the donor layers,
whereas in the latter similar contacts lead to the formation of infinite ribbons.
H.
Organic
Compounds
169
Fig. 6.28.
Structure of
fl-(ET)2I 3.
Large spheres: I; medium spheres: S; small spheres:
C; H
atoms not
shown.
The structure of
O-(ET)2(I3)l_x(AuI2)x
(T c -- 3.6 K for x < 0.02) is ortho-
rhombic and centrosymmetric. The ET molecules within each layer are approxi-
mately parallel to each other, but tilted from the intersecting plane, the molecules
of alternating layers being tilted in opposite directions. The molecular packing
within the slabs is similar to that found in the e phases, which, however, have
triclinic symmetry. The anions are located in mirror planes between the
layers. The unit cell is enlarged and the symmetry lowered to monoclinic
when an ordered arrangement of 13 anions is taken into account.
The crystal structure of (ET)4Hg3_6X 8 (X--C1) is built up of two
substructures, one formed by the ET molecules and the halogen atoms and the
other by the Hg atoms, with incommensurate translation units in one direc-
tion. The halogen atoms form infinite channels, inside which the mercury atoms
are arranged with their own translation period. As in the K phases, the ET
molecules form face-to-face dimers. 2-(Se-ET)2GaC14, containing bis(ethylene-
dithio)tetraselenafulvalene molecules, becomes superconducting at 9.5K. The
slabs of seleno-substituted molecules are separated by tetrahedral GaC14 acceptors.
A number of salts with the asymmetric MDT-TTF molecule (methylene-
dithiotetrathiafulvalene), shown in Fig. 6.27, have been synthesized. Among

170 Chapter 6: Crystal Structures of Classical Superconductors
these, tc-(MDT-TTF)2AuI 2 is found to become superconducting at 4.5 K. The
orthorhombic structure contains donor molecule layers with dimers that are
rotated with respect to each other, similar to those found in the tc phases of the ET
salts. There are, however, two layers in the translation unit, related by a mirror
plane.
Crystallographic Data Sets
a. Criteria for Selection
Complete crystallographic data sets, including atom coordinates, are given for
111 compounds, representative for structure types found among classical super-
conductors. As a general rule for selection, at least one compound with a
superconducting transition temperature exceeding 4.2K should have been
reported for the structure type. Whenever one or more isotypic superconductors
are known, the set of atom coordinates is followed by a table listing cell
parameters and superconducting transition temperatures for selected isotypic
compounds. Compounds becoming superconducting at temperatures lower
than 4.2 K, including particular nonsuperconducting materials, have also been
considered in these tables. However, a lower limit around 1 or 2 K has sometimes
been fixed, without special indication.
Preference has been given, when possible, to recent literature references
and to references where the cell parameters and the critical temperature are
determined for the same sample. The historical aspect has thus not been
emphasized, which means that the original publications stating for the first
time the existence of a particular phase or its superconductivity may not be
mentioned. As a further consequence, the superconducting transition tempera-
tures listed here do not always correspond to the highest values reported in the
literature. Differences that were too large were, however, avoided. For systems
with a strong dependence on the chemical composition, an effort was made to
select a close-to-optimal composition, but also to respect the correspondence
between composition and cell parameters. Transition temperatures determined
for high pressure or on thin films were considered only occasionally.
The cell parameters listed in each table were taken from the first reference
indicated on the same line and the critical temperature, if not from the first, then
from the second reference. One or two literature references reporting structure
refinements, marked with an asterisk, are sometimes added. The term structure
refinement is here understood in a broad sense, depending on the class of
compound and the structure type. In some cases publications with additional data
are mentioned in remarks, without any claim on completeness. To avoid
differences due to small variations in experimental conditions used by different
!. Crystallographic Data Sets 171
authors, cell parameters for series of isotypic compounds were sometimes
preferably taken from the same reference.
b. Presentation and Notation
The general presentation of a data set is explained in Fig. 6.29 and the few
abbreviations used here are listed in Table 6.3. The Pearson code and the
Wyckoff sequence are defined in Section A, where the use of the
International
Tables for Crystallography
is also briefly explained. In a few particular cases a
slightly different presentation has been chosen. This is the case for the structures
Fig.
6.29.
Structure [Colloquial Strukturbericht
I p~rsonl spa~ Wyckoff]
type [ name notation I code ] group]
s~qu~nr I
MgAI204 type, spinel, H1 l,
cF56,
(227)
Fd3 m - ecb
Refined
Superconducting [Diffraction Temperature for Reliability
composition transition temperature [ method data collection factor
Li0.7sTi20 4,
Tc =
13.2 K, SX,
T=
223 K, R =0.0109
Cell Formula
parameters units in cell
a=8.4030A, Z=8
Site
label
Atom
Multiplicity and
Wyckoff letter
r \
WP x
Fractional
coordinates
Y
Occupation
factor
Occ.
Li 8(b) 3/s % 3/8
Ti
16(c) 0 0 0
O 32(e) 0.23685 0.23685 0.23685
0.75
Extract from the tables with explanation of items. For some tables reference is made to figures
in which the structure type is presented.
172 Chapter 6:
Crystal Structures of Classical
Superconductors
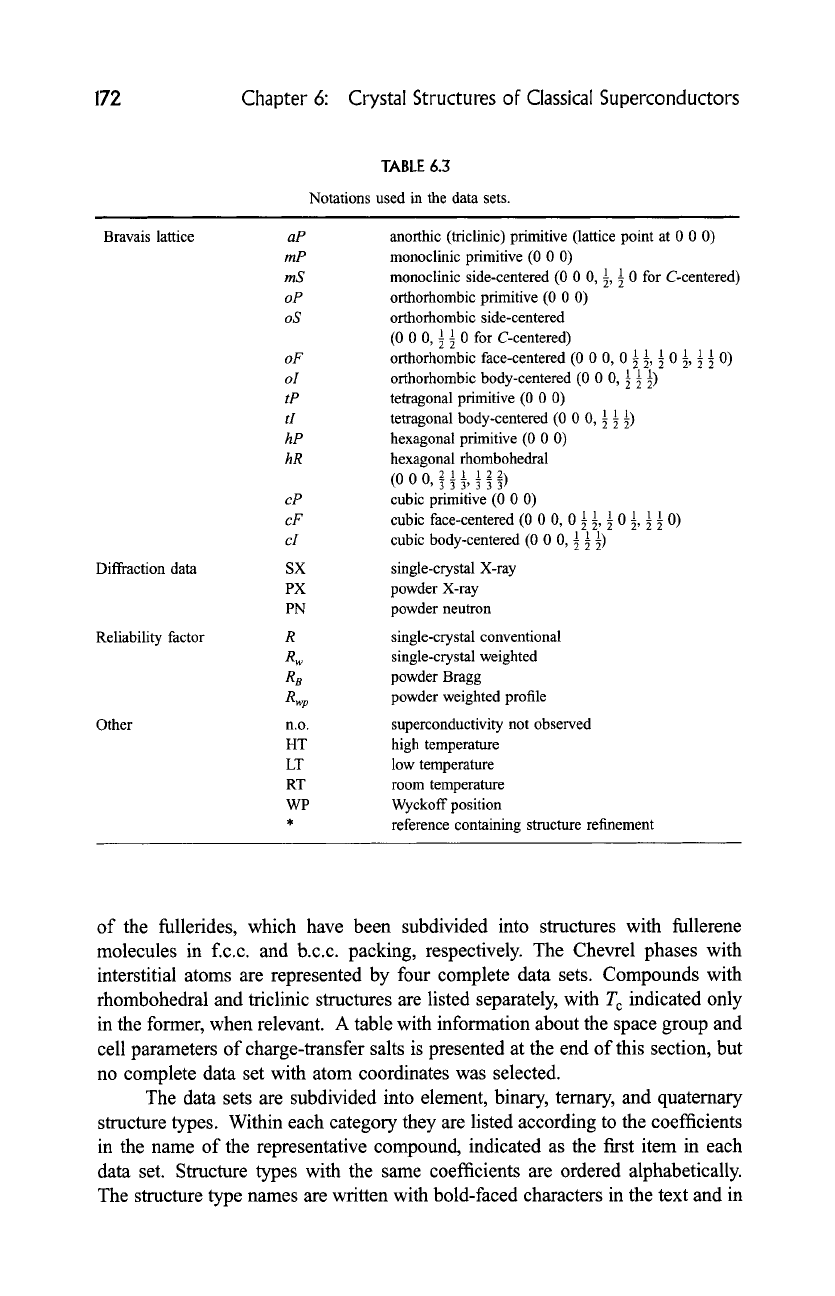
TABLE 6.3
Notations used in the data sets.
Bravais lattice
Diffraction data
Reliability factor
Other
aP
mP
mS
oP
oS
oF
oI
tP
tI
hP
hR
cP
cF
cI
SX
PX
PN
R
Rw
R8
Rwp
n.o.
HT
LT
RT
WP
anorthic (triclinic) primitive (lattice point at 0 0 0)
monoclinic primitive (0 0 0)
monoclinic side-centered (0 0 0, 1, 1 0 for C-centered)
orthorhombic primitive (0 0 0)
orthorhombic side-centered
(0 0 0, 1 89 0 for C-centered)
orthorhombic face-centered (0 0 0, 0 89 89 89 0 1, 1 89 0)
orthorhombic body-centered (0 0 0, 1 89 89
tetragonal primitive (0 0 0)
tetragonal body-centered (0 0 0, 89 89
hexagonal primitive (0 0 0)
hexagonal rhombohedral
cubic primitive (0 0 O)
cubic face-centered (0 0 O, 0 89 89 89 0 89 89 89 O)
cubic body-centered (0 0 O, 89 89 89
single-crystal X-ray
powder X-ray
powder neutron
single-crystal conventional
single-crystal weighted
powder Bragg
powder weighted profile
superconductivity not observed
high temperature
low temperature
room temperature
Wyckoff position
reference containing structure refinement
of the fullerides, which have been subdivided into structures with fullerene
molecules in f.c.c, and b.c.c, packing, respectively. The Chevrel phases with
interstitial atoms are represented by four complete data sets. Compounds with
rhombohedral and triclinic structures are listed separately, with T c indicated only
in the former, when relevant. A table with information about the space group and
cell parameters of charge-transfer salts is presented at the end of this section, but
no complete data set with atom coordinates was selected.
The data sets are subdivided into element, binary, ternary, and quaternary
structure types. Within each category they are listed according to the coefficients
in the name of the representative compound, indicated as the first item in each
data set. Structure types with the same coefficients are ordered alphabetically.
The structure type names are written with bold-faced characters in the text and in
J. Tabulated Data 173
Tables 6.1 and 6.2. References for type-defining compounds can be found in
TYPIX
(Parth6
et al.,
1993/1994; Cenzual
et al.,
1995), a compilation of
standardized data for inorganic compounds defining structure types, accompanied
by a brief crystal chemical characterization.
Tabulated Data
a. Element Structure Types
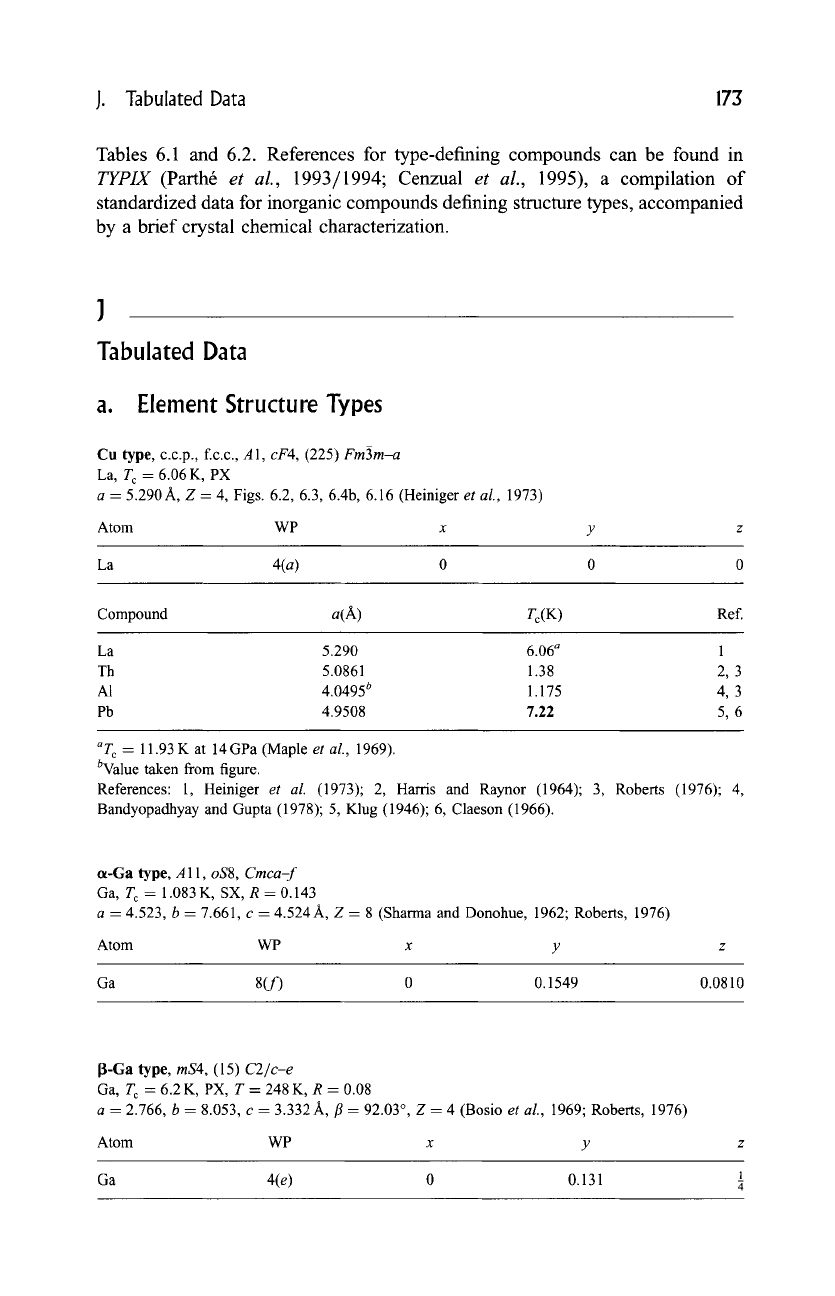
Cu type, c.c.p., f.c.c., A1,
cF4,
(225)
Fm~3m-a
La, T c = 6.06 K, PX
a - 5.290A, Z -- 4, Figs. 6.2, 6.3, 6.4b, 6.16 (Heiniger
et al.,
1973)
Atom WP x y z
La 4(a) 0 0 0
Compound a(A) Tc(K ) Ref.
La 5.290 6.06 a 1
Th 5.0861 1.38 2, 3
A1 4.0495 b 1.175 4, 3
Pb 4.9508 7.22 5, 6
aT c
--
11.93 K at 14 GPa (Maple
et aL,
1969).
bValue taken from
figure.
References: 1, Heiniger
et aL
(1973); 2,
Harris and
Raynor (1964); 3, Roberts (1976); 4,
Bandyopadhyay and Gupta (1978); 5, Klug (1946); 6, Claeson (1966).
cx-Ga type, A 11,
oS8, Cmca-f
Ga, T c = 1.083 K, SX, R = 0.143
a = 4.523, b - 7.661, c = 4.524 A, Z = 8 (Sharma and Donohue, 1962; Roberts, 1976)
Atom WP x y
Ga 8(f) 0 0.1549
0.0810
~-Ga type,
mS4,
(15)
C2/c-e
Ga, T c = 6.2 K, PX, T = 248 K, R = 0.08
a = 2.766, b = 8.053, c = 3.332 A, fl = 92.03 ~ Z = 4 (Bosio
et al.,
1969; Roberts, 1976)
Atom WP x y
Ga 4(e) 0 0.131
174
Chapter 6: Crystal Structures of Classical Superconductors
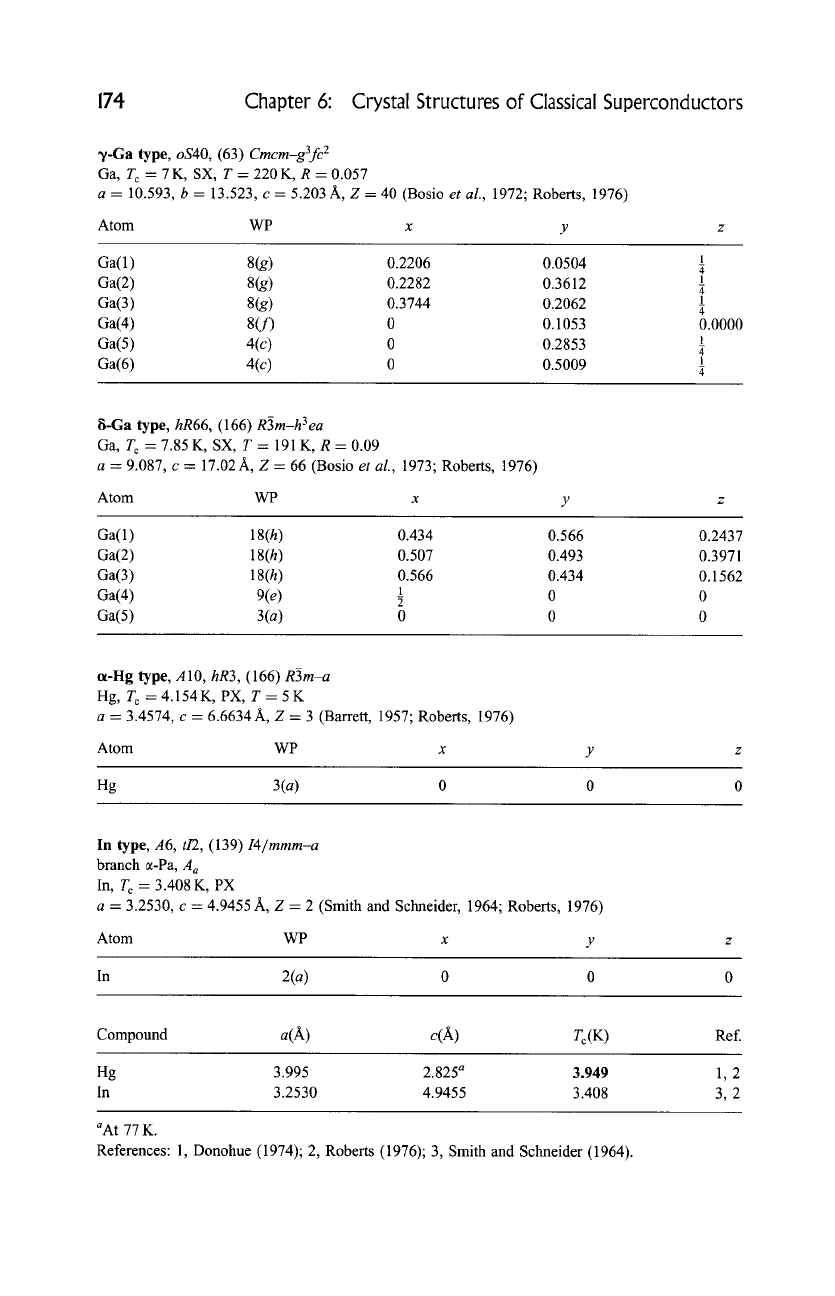
~/-Ga type, oS40, (63)
Cmcm-g3fc 2
Ga, T c=7K,SX, T=220K, R=0.057
a = 10.593, b = 13.523, c = 5.203 A, Z = 40 (Bosio
et al.,
1972; Roberts, 1976)
Atom WP x
Ga(1) 8(g) 0.2206 0.0504
Ga(2) 8(g) 0.2282 0.3612
Ga(3) 8(g) 0.3744 0.2062
Ga(4) 8(f) 0 0.1053
Ga(5) 4(c) 0 0.2853
Ga(6) 4(c) 0 0.5009
1
4
1
4
1
4
0.0000
1
4
1
4
8-Ga type,
hR66,
(166)
R3m-h 3 ea
Ga, T c = 7.85K, SX, T = 191K, R = 0.09
a = 9.087, c = 17.02A, Z = 66 (Bosio
et al.,
1973; Roberts, 1976)
Atom WP x
Ga(1 ) 18(h) 0.434 0.566 0.2437
Ga(2) 18(h) 0.507 0.493 0.3971
Ga(3) 18(h) 0.566 0.434 0.1562
0 0
Ga(4) 9(e)
Ga(5) 3(a) 0 0 0
a-I-Ig type, A 10,
hR3,
(166)
R3m-a
Hg, T c = 4.154 K, PX, T = 5 K
a -- 3.4574, c = 6.6634 A, Z = 3 (Barrett, 1957; Roberts, 1976)
Atom WP x y
Hg 3(a) 0 0 0
In type, A6, t/2, (139)
I4/mmm-a
branch ~-Pa,
A a
In, T c = 3.408 K, PX
a = 3.2530, c = 4.9455 A, Z = 2 (Smith and Schneider, 1964; Roberts, 1976)
Atom WP x y
In 2(a) 0 0 0
Compound a(A) c(A) T c (K)
Ref.
Hg 3.995 2.825 a 3.949
In 3.2530 4.9455 3.408
1,2
3,2
aAt 77 K.
References: 1, Donohue (1974); 2, Roberts (1976); 3, Smith and Schneider (1964).
J. Tabulated Data 175
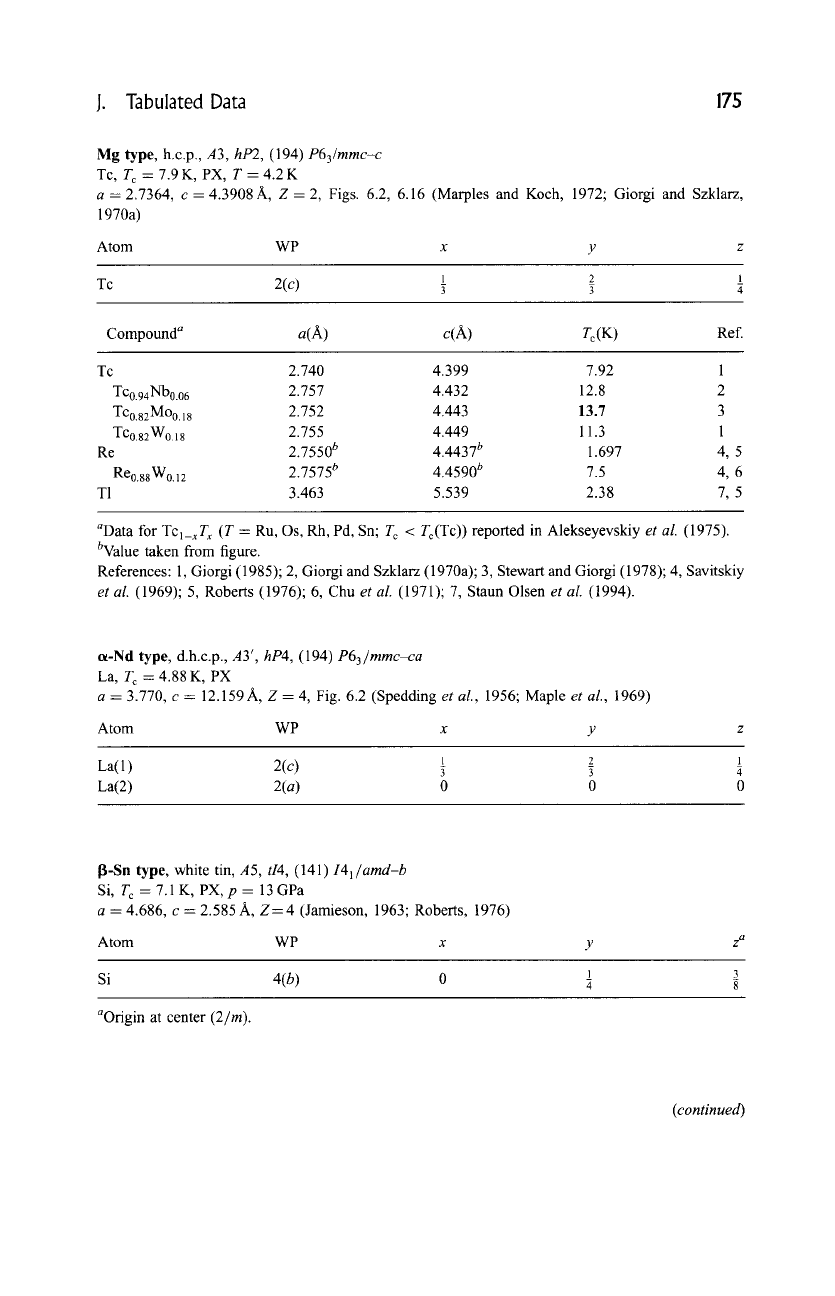
Mg
type, h.c.p., A3, hP2, (194)
P63/mmc-c
Tc, T c=7.9K,PX, T=4.2K
a--2.7364, c- 4.3908A, Z = 2, Figs. 6.2, 6.16 (Marples and Koch, 1972; Giorgi and Szklarz,
1970a)
Atom WP x y z
1 2 1
Tc
'3[ "~
Compound a a(A) c(A) T c (K) Ref.
Tc 2.740 4.399 7.92 1
Tc0.94Nb0.06 2.757 4.432 12.8 2
TC0.gzMoo.18 2.752 4.443 13.7 3
TCo.82W0.18 2.755 4.449 11.3 1
Re 2.7550 b 4.4437 b 1.697 4, 5
Reo.88Wo.12 2.7575 b 4.4590 b 7.5 4, 6
T1 3.463 5.539 2.38 7, 5
aData for
TCl_xTx
(T = Ru, Os, Rh, Pd, Sn; T c < Tc(Tc)) reported in Alekseyevskiy
et al.
(1975).
bValue taken from figure.
References: 1, Giorgi (1985); 2, Giorgi and Szklarz (1970a); 3, Stewart and Giorgi (1978); 4, Savitskiy
et al.
(1969); 5, Roberts (1976); 6, Chu
et al.
(1971); 7, Staun Olsen
et al.
(1994).
o~-Nd type, d.h.c.p., A3',
hP4,
(194)
P63/mmc-ca
La, T c = 4.88 K, PX
a -- 3.770, c = 12.159 A, Z -- 4, Fig. 6.2 (Spedding
et al.,
1956; Maple
et al.,
1969)
Atom WP x y
1 2 1
La(1) 2(c) 5 5 a
La(2) 2(a) 0 0 0
I~-Sn type, white tin, A5,
tI4, (141) 141/amd-b
Si, T c = 7.1 K, PX, p = 13 GPa
a = 4.686, c = 2.585 A, Z= 4 (Jamieson, 1963; Roberts, 1976)
Atom WP x
1 3
Si
4(b) 0 ~ g
aOrigin at center (2/m).
(continued)
