Труды Всемирного конгресса Международного общества солнечной энергии - 2007. Том 2
Подождите немного. Документ загружается.

Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
782
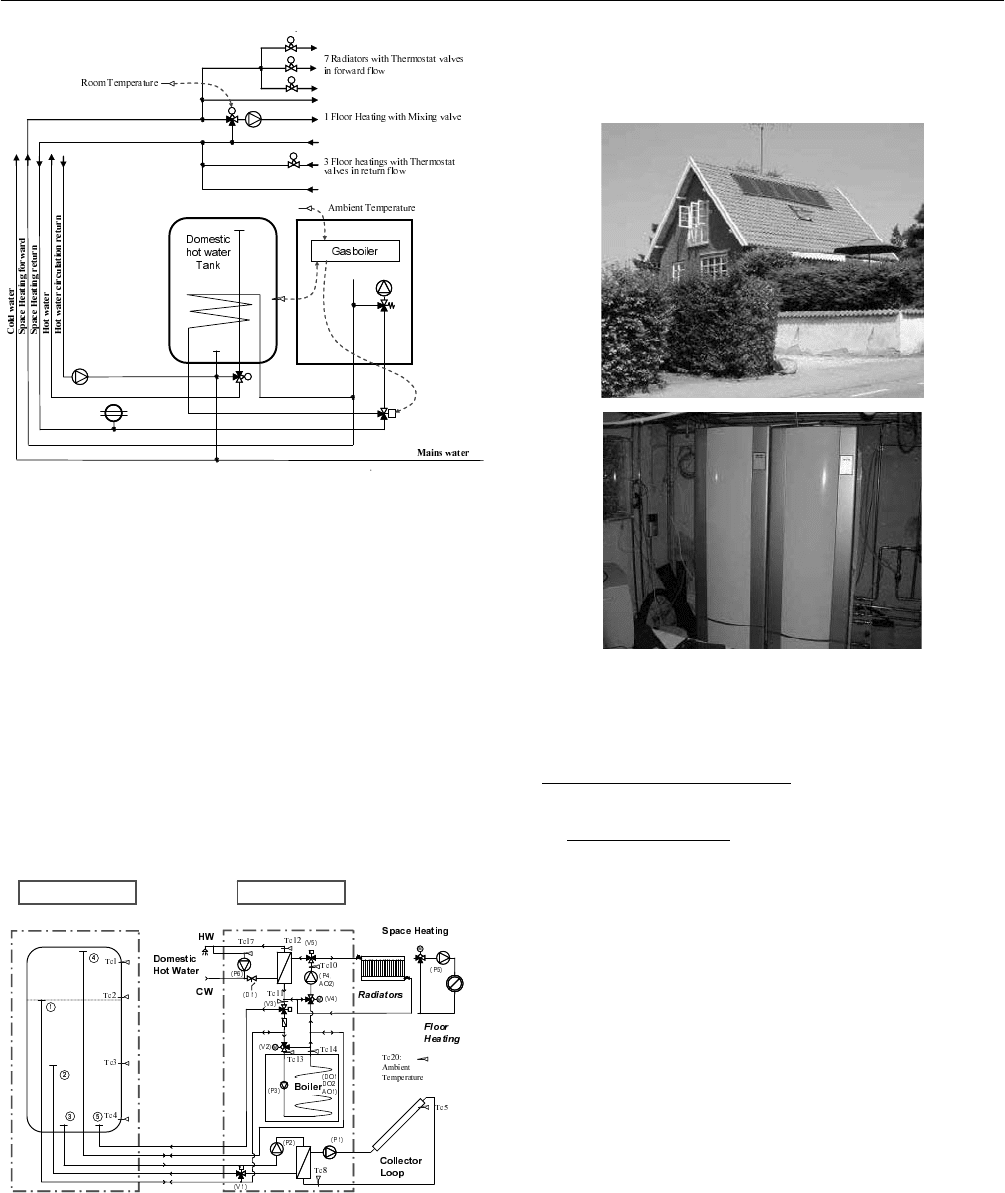
Fig. 2: Schematic sketch of the energy system before
installation of the solar heating system.
Both the technical unit and the heat storage unit are built
into 60 x 60 cm units by METRO THERM A/S. Due to this
prefabrication, the installation of the system is easy and the
risk of installation mistakes is reduced. The design of the
units provides good operation conditions for the
condensing natural gas boiler and for the solar collectors
and high energy savings for the system.
Figure 3 shows a schematical sketch of the two units as
well as the solar collector loop and space heating system.
Solar Store Unit
Technical Unit
Fig. 3: Schematic sketch of the solar/natural gas heating
system.
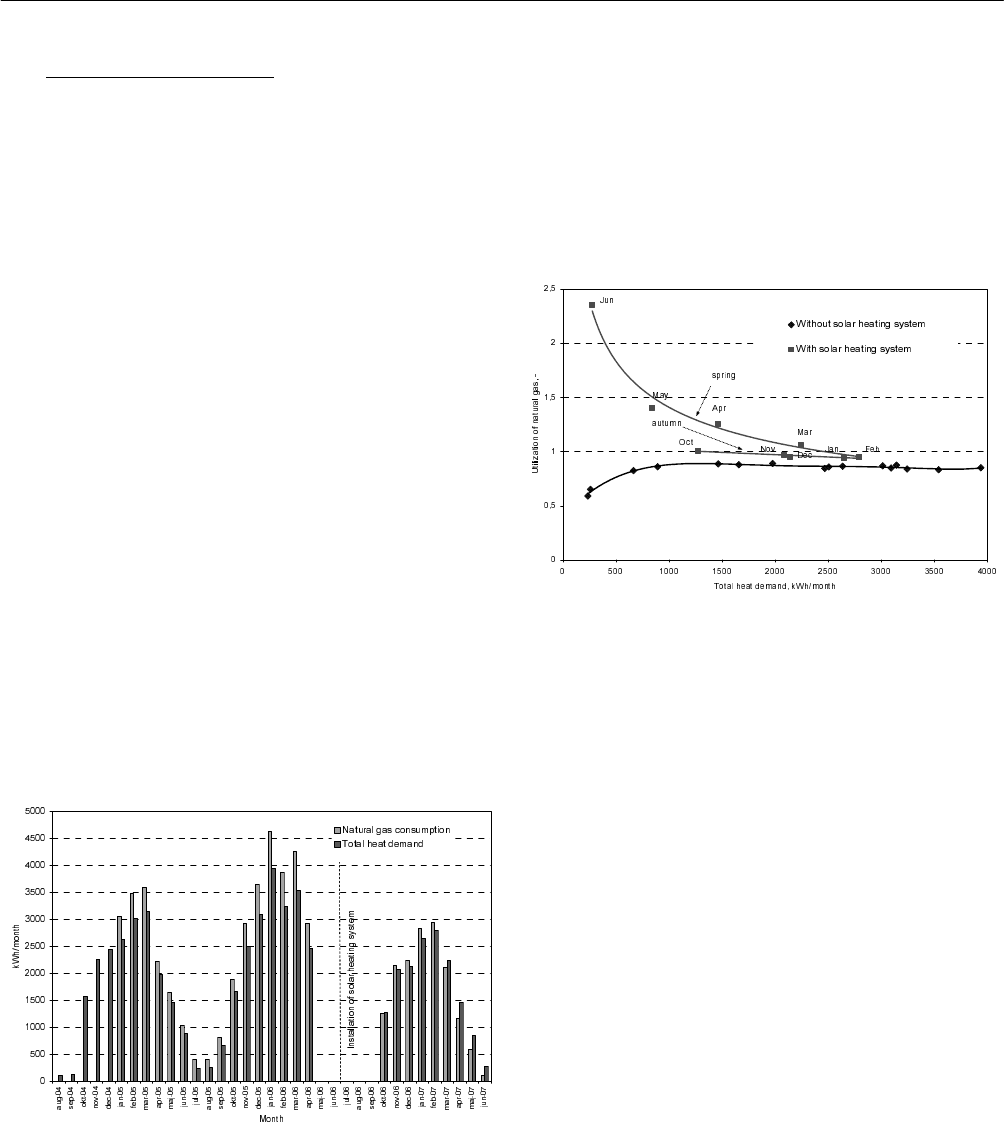
Figure 4 shows the house after installation of the solar
heating system.
Fig. 4: Demonstration house with collectors on the roof and
the solar tank and technical unit in the basement.
3. ENERGY MEASUREMENTS
3.1 Monitoring Systems
The space heating demand, the hot water consumption, the
heat loss of the circulation pipe, the natural gas
consumption and the electricity consumption of the natural
gas boiler and the heating system were measured in periods
before and after installation of the solar heating system. Further,
the heat transferred from the natural gas boiler to the hot water
tank was measured before installation of the solar heating
system. Furthermore, the heat produced by the solar collectors,
the heat produced by the boiler as well as the electricity
consumption of the solar heating system were measured in the
period after installation of the solar heating system.
Based on the measurements it is possible to estimate the
energy savings of the solar heating system.
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
783
3.2 Measured Energy Quantities
The measurements started in August 2004 and will be
continued until December 2007. Not all the energy
quantities mentioned in section 3.1 were measured during
the whole measuring period. A constant energy quantity of
10.67 kWh per m
3
natural gas is assumed.
The total heat demand of the house, that is: Space heating
demand + domestic hot water consumption + heat loss from
circulation pipe, and the natural gas consumption month by
month appear from figure 5. It is clear that both the heat
demand and the natural gas consumption is much lower after
the installation of the solar heating system than before. The
reasons for the reduced natural gas consumption are the
decreased heat demand due to warm weather in the autumn
of 2006 and in the start of 2007 and the new energy system
with solar collectors and an improved natural gas boiler.
Further, it is noticed that the difference between the natural
gas consumption and the total heat demand in the winter
months is lower after installation of the solar heating system
than before. In the spring, summer and autumn the natural
gas consumption is lower than the total heating demand after
installation of the solar heating system. Before installation of
the solar heating system the natural gas consumption is for
all months higher than the total heat demand.
Fig. 5: Total heat demand and natural gas consumption for
the house during the measuring period.
Figure 6 shows the measured utilization of the natural gas
as a function of the total heat demand month by month. The
utilization of the natural gas is defined as: (space heating
demand + hot water consumption + heat loss from
circulation pipe)/natural gas consumption. The utilization
of natural gas is strongly increased by the solar heating
system, especially for low heat demands. Further, the
utilization of natural gas after installation of the solar
heating system is much higher in the sunny spring than in
the less sunny autumn.
Fig. 6: Utilization of the natural gas as a function of the
total heat demand.
Based on the total monthly measured heat demand and the
curves of figure 6 it is possible to estimate the natural gas
consumption for the house for all months without and with
the solar heating system, if only the heat demands are
measured. Consequently, the energy savings for the house
achieved by the solar heating system can be determined for
all months, where the total heat demand is measured.
However, so far the utilization curve for the solar heating
system is not covering July, August and September due to a
lack of measurements in these months. Therefore energy
savings for the summer months are not estimated with a
good accuracy.
The yearly natural gas savings for the solar heating system
is for the period May’05-April’06 estimated to 4100 kWh.
This corresponds to a reduction of the natural gas
consumption of 14.4%. Additional electricity savings of 60
kWh are estimated. The total estimated yearly energy
savings are 4200 kWh, corresponding to about 620 kWh
per m² collector. For other periods with durations of one
year the energy savings will be different. For increasing
heat demand the energy savings will increase.
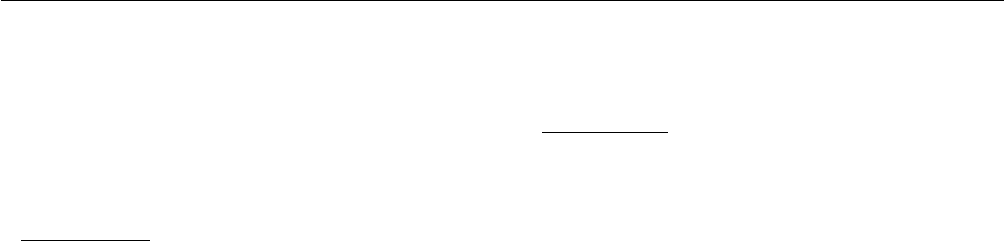
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
784
The measurements will be continued. Therefore the
accuracy of the estimates of the energy savings will be
improved, and the variations of energy savings from one
year to another caused by weather variations and variations
of user habits can be determined.
4. CONCLUSION
Measurements of the heat demand and energy consumption
in a demonstration house before and after installation of a
new developed solar heating/natural gas heating system
based on a condensing natural gas boiler and 6.75 m² solar
collectors have been carried out.
Based on the measurements the energy savings by the solar
heating system were estimated to 4200 kWh per year,
corresponding to 620 kWh/m² collector per year. The
energy savings will vary from year to year. In years with a
high heat demand the energy savings will be high. In years
with a low heat demand the energy savings will be lower
5. REFERENCES
(1) T. Larsson, “Enkätundersökning om energibesparing
och drift med solfångare”, Intern rapport 00:00, Örebro
University, 2000.
(2) A. Thür, L.J. Shah & S. Furbo, “Energy savings for
solar heating systems”, Solar Energy Vol. 80, Issue 11,
pp. 1463-1474, 2006.
(3) S. Furbo, L.J. Shah, C. Holm Christiansen & K. Vinkler
Frederiksen, “Kedeleffektiviteter for oliefyr og natur-
gaskedler i enfamiliehuse”, report R-072. Department
of Civil Engineering, Technical University of Denmark,
2004.
(4) A. Thür, “Compact Solar Combisystem. High
Efficiency by Minimizing Temperatures”, report R-160,
Department of Civil Engineering, Technical University
of Denmark, 2007.
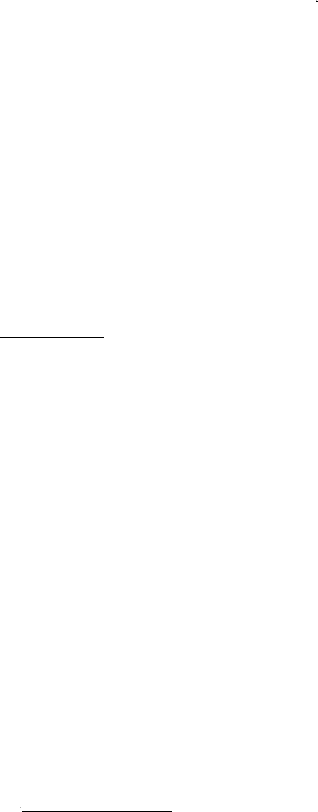
EXPERIMENTAL STUDIES OF SOLAR HEAT PIPE USED TO OPERATE ABSORPTION
CHILLER IN CONDITIONS OF VIETNAM
LE CHI HIEP
Ho Chi Minh City University of Technology
268 Ly Thuong Kiet st., Dist.10, Ho Chi Minh City, Vietnam
lechihiep@gmail.com
HOANG AN QUOC
Ho Chi Minh City University of Technical Education
01 Vo Van Ngan st., Thu Duc Dist, Ho Chi Minh City, Vietnam
hanquoc@hcmute.edu.vn
HOANG DUONG HUNG
Danang University of Technology
54 Nguyen Luong Bang St, Lien Chieu Dist, Danang City, Vietnam
hdhung@ud.edu.vn
ABSTRACT
Several models of solar heat pipe have been fabricated and
tested. The experiments show that the flat plate model
could be used to operate absorption chiller in the climate of
southern part of Vietnam. Two main advantages of the
selected solar heat pipe are low cost and easy fabrication at
local conditions. It is expected that the selected solar heat
pipe could attract attention of the community to develop the
application of solar energy in Vietnam.
Based on the current demand, the paper presents the
experimental studies of the first generation of low cost
solar heat pipe. The paper also discusses the ability of
application of solar air conditioning in Vietnam and
recommends the suitable diagram mixing solar energy with
other heat source to operate stably the system.
1.
INTRODUCTION
Currently, in Vietnam, solar energy is exploited mainly to
supply hot water, besides it is also used to supply people in
remote areas with electricity in small scale. But, because of
hot climate, solar energy is now also evaluated as one of
possible and available energy resources that can be used to
satisfy the air conditioning demand to fulfil the energy
conservation policy of the country.
One of the appropriate systems that can produce the needed
cooling capacity for air conditioning purpose is absorption
chiller which has been manufactured largely in
commercialization scale. Thus, the rising problem now is
the solar device that can be used to supply absorption
chiller with hot water at high enough temperature but at
low enough cost, and its fabrication must not need high
technology and high investment. Obviously, it is not easy to
fulfil at the same time these contradictions.
In order to find out the most appropriate option in
conditions of Vietnam, the experiments have been done on
two models: (i) tubular solar heat pipe and (ii) flat plate
solar heat pipe. Because of difficulties in keeping the
vacuum, tubular solar heat pipe is divided in two options:
evacuated and non-evacuated, whereas flat plate solar heat
pipe is non-evacuated one.
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
786
2.
DESCRIPTION OF EXPERIMENTAL MODELS
2.1
Tubular Solar Heat Pipe
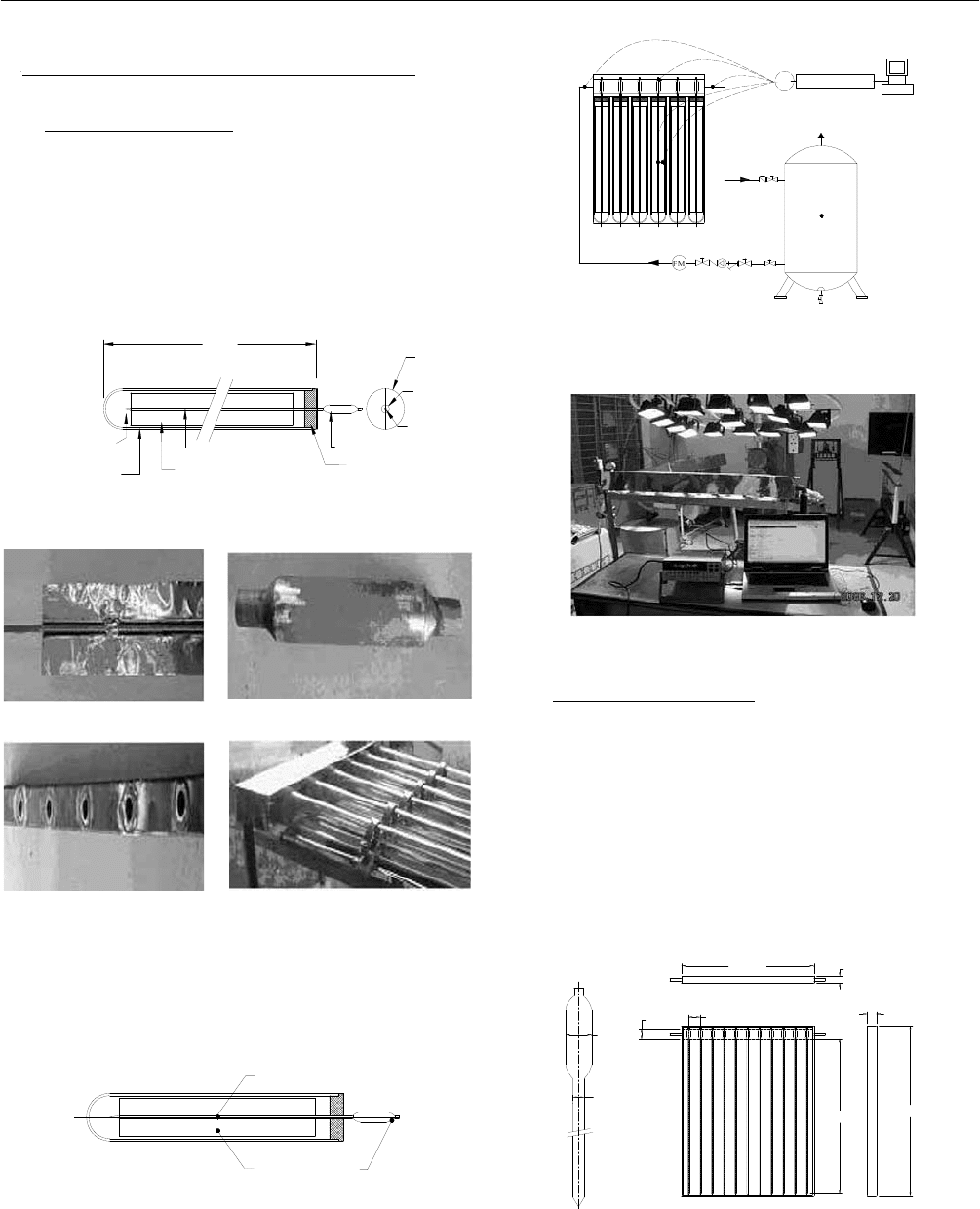
The experimental model consists of 6 heat pipes, each is
put in a 100mm diameter and 2000mm length glass tube.
The working fluid inside the heat pipe is pure water. The
drawing and the construction of the tested tubular solar heat
pipe are shown in Figures 1 and 2, respectively.
2000
?00
?2
?
Rubber
Selective Absorber
Evaporator
Condenser
Evacuated Tube
Glass Tube
Fig. 1
Fig. 2
The arrangement of measuring devices and the total tested
system are presented in Figures 3, 4 and 5.
TC3
TC4
TC5
Fig. 3
TC1
TC2
T
TC3
TC4 TC5
Circulating pump
Hot Water
Storagered Tank
Solar collector
TC
Data acquisition
Fig. 4
Fig. 5
2.2 Flat Plate Solar Heat Pipe
The flat plate solar collector fabricated at 1050mm width
and 1650mm length consists of 11 heat pipes. The diameter
and the length of evaporator section (including adiabatic
section) are 13mm and 1500mm, the diameter and the
length of condenser section are 25mm and 100mm,
respectively. The tested flat plate solar heat pipe are shown
in Figues 6 and 7.
1020
7 0
1 650
7 0
2 5
13
9 1
1 500
100
Fig. 6
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
787
Fig. 7
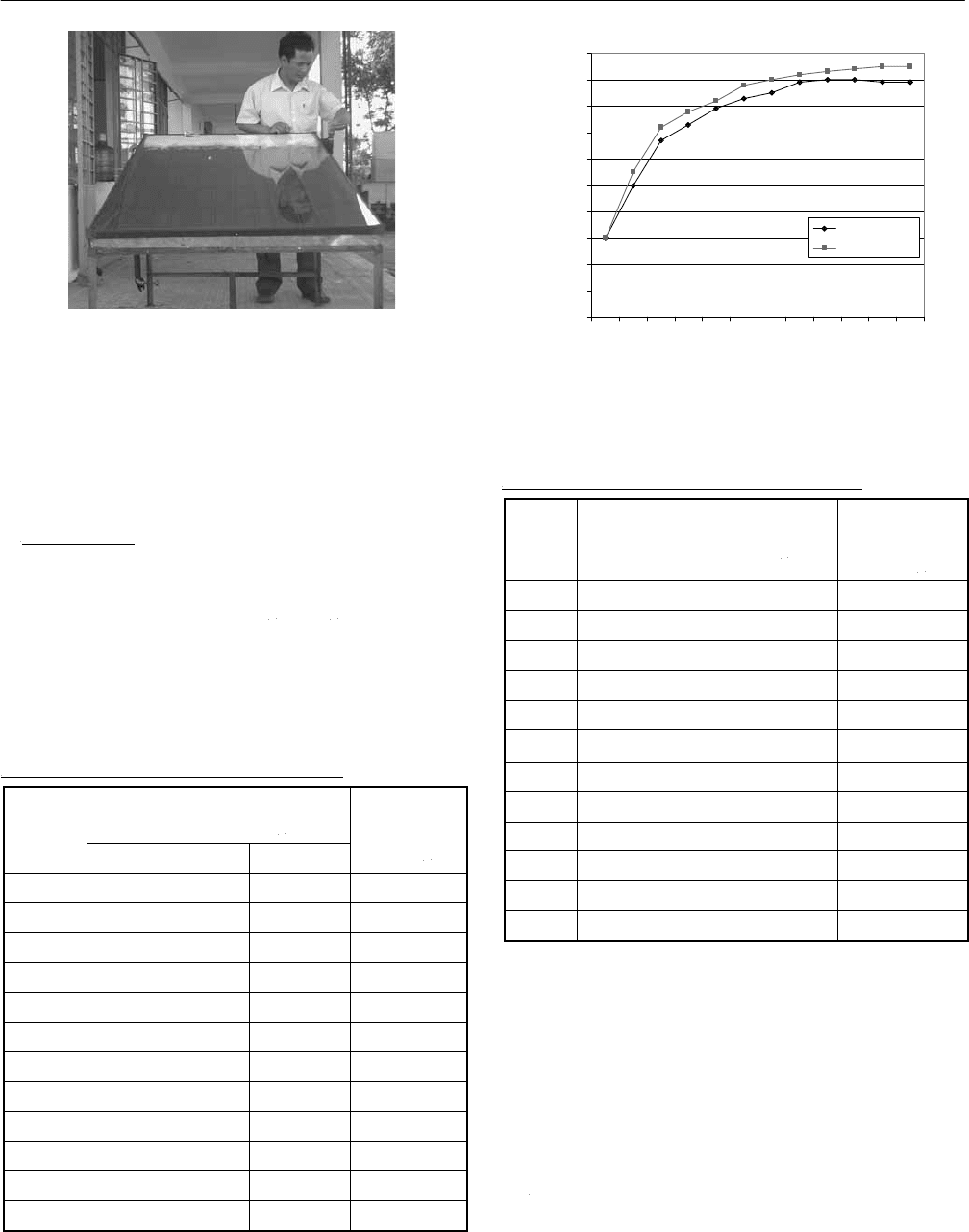
In all experiments, temperatures are measured by using
thermoelectric couples with 5mm length sensor. The
recorded data are sent immediately to Multi Data
Acquisiton System (20 inputs) which is connected directly
to a computer.
3.
DISCUSSION
Experiments have been done on the two models. The mean
ambient temperature is around 31
o
C - 33
o
C and the wind
speed is 1m/s - 3m/s. In order to evaluate the efficiency of
each option, Tables 1 and 2 show the values which are
considered as the mean of recorded data, Figures 8 and 9
present the variation of water temperature on time.
TABLE 1: TUBULAR SOLAR HEAT PIPE
Water temperature at the
middle of the tank (
o
C )
Time
Non-evacuated Evacuated
Total solar
radiation
(W/m
2
)
8:00 30 30 370
8:30 50 55 500
9:00 67 72 650
9:30 73 78 720
10:00 79 82 750
10:30 83 88 800
11:00 85 90 850
11:30 89 92 850
12:00 90 93 890
12:30 90 94 890
13:00 89 95 850
13:30 89 95 850
0
10
20
30
40
50
60
70
80
90
100
8
:
0
0
8
:
3
0
9
:0
0
9
:3
0
1
0
:
0
0
1
0
:
3
0
1
1
:0
0
1
1
:
3
0
1
2
:
0
0
1
2
:
3
0
1
3
:0
0
1
3
:
3
0
Time
Temperature (C
)
Non Evacuated
Evacuated
Fig. 8: The variation of water temperature on time / tubular
solar heat pipe.
TABLE 2: FLAT PLATE SOLAR HEAT PIPE
Time
Water temperature at
the middle of the tank (
o
C )
Total solar
radiation
(W/m
2
)
8:00 30 350
8:30 49 450
9:00 67 490
9:30 76 655
10:00 79 780
10:30 85 790
11:00 88 810
11:30 90 850
12:00 92 890
12:30 93 870
13:00 93 820
13:30 94 810
Tables 1, 2 and Figures 8 and 9 lead us to the primary
remark that the efficiency of evacuated tubular solar heat
pipe is higher than that of non-evacuated tubular solar heat
pipe. But, in comparison with flat plate solar heat pipe, the
efficiency of evacuated tubular solar heat pipe is only a
little higher than that of flat plate solar heat pipe. The
experiments show that, in general, evacuated tubular solar
heat pipe can supply hot water at temperature higher than
75
o
C from around 9:40AM, whereas non-evacuated tubular
solar heat pipe can supply hot water at high enough
temperature only after 10:30AM. Table 2 also shows that
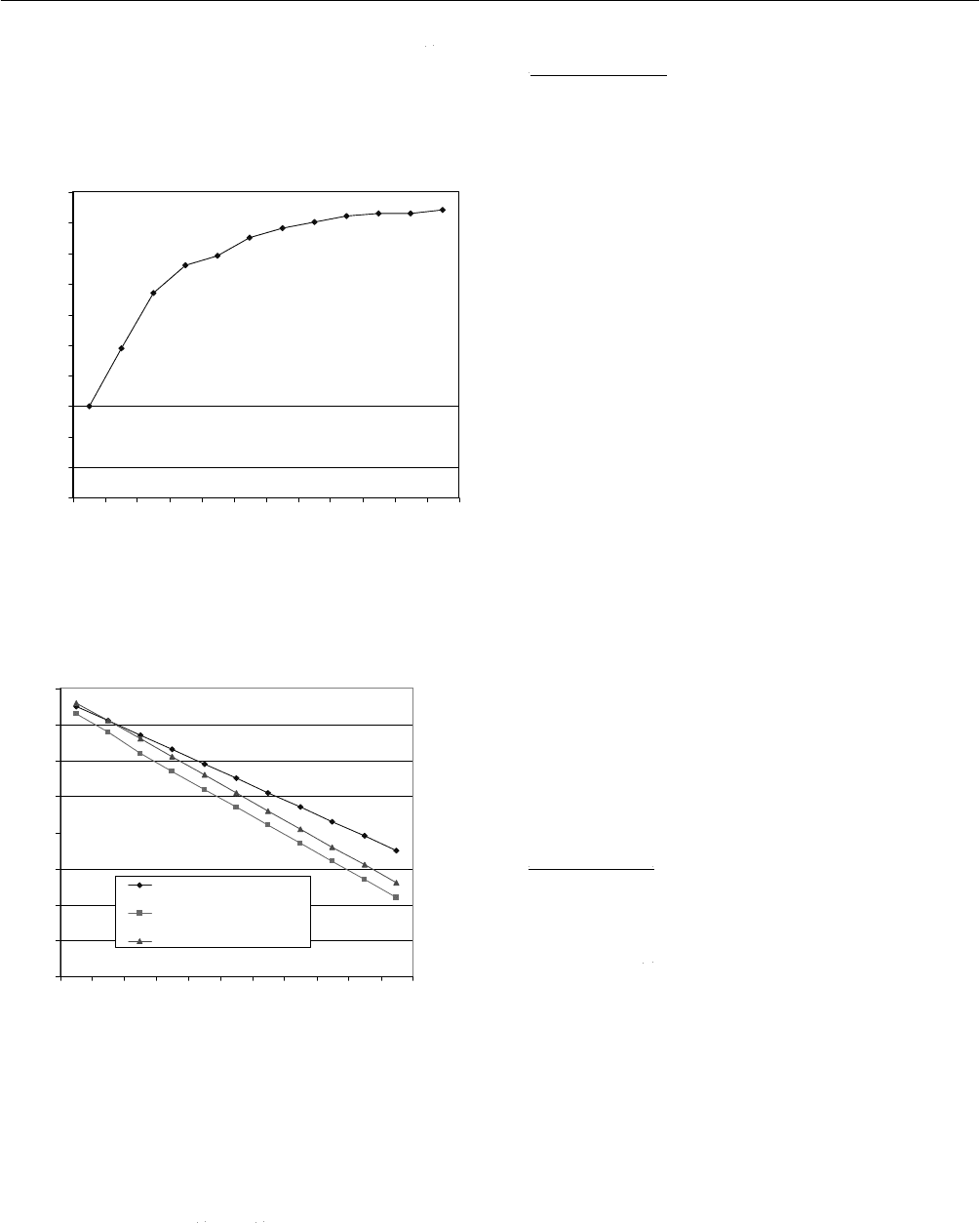
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
788
flat plate solar heat pipe can supply hot water at 79
o
C at
around 10:00AM. To make everything clearer, the
efficiency comparison of tested options is presented in
Figure 10.
0
10
20
30
40
50
60
70
80
90
100
8
:
00
8
:30
9
:
0
0
9
:
30
10:00
1
0
:30
1
1
:0
0
11:3
0
12
:0
0
1
2
:30
1
3
:0
0
13:3
0
Time
Temperature (C
)
Fig. 9: The variation of water temperature on time/flat plate
solar heat pipe.
0
10
20
30
40
50
60
70
80
0 0,01 0,02 0,03 0,04 0,05 0,06 0,07 0,08 0,09 0,1
(T-Ta)/I
Collector efficency (%
Heat pipe Evacuated tubular
collector
Heat pipe non-evacuated
tubular collector
Flate Heat
p
i
p
e collector
Fig. 10: The efficiency comparison.
Based on the primary tested data, the experiments on flat
plate solar heat pipe have been done. In this case, the
collector was connected with a 40litre tank, the results
show that - from 9AM to 2PM - the mean increase of water
temperature is around 30
o
C - 85
o
C/unit, the mean collector
efficiency is around 42.5%.
4.
CONCLUSIONS
Due to difficulties in keeping the vacuum, the flat plate
solar heat pipe should be selected to supply absorption
chiller with hot water. Main advantages of flat plate solar
heat pipe are low cost and easy fabrication, that means flat
plate solar heat pipe can be fabricated at any in-country
small workshop. This option suits the current situation in
Vietnam where there has not been yet any industry
referring to solar collector.
The experiments show that it is possible to supply small
absorption chiller with hot water produced by flat plate
solar heat pipe. In order to operate stably and to reduce the
solar collector surface, solar absorption chiller system
should include additional heat source.
It is determined that solar energy itself can operate the
system from 10AM to 2PM, before 10AM and after 2PM the
system can be operated by mixing solar energy and
additional heat source. Besides cooling, hot water supply for
household purposes is also considered as another function of
the system as well as of the selected solar heat pipe.
The total cost of the tested unit (W h L = 1050mm h
1650mm) is around 230USD. It is expected that the total
cost of the flat plate solar heat pipe could be reduced
significantly whenever solar collector is manufactured
largely in commercialization scale.
5.
REFERENCES
(1) Jorge Facao and Armando C. Oliveira, “Simulation of
The Thermal Behaviour of A Hybrid Heat Pipe Solar
Collector”, 1
st
International Conference on Sustainable
Energy Technologies, 12-14 June 2002, Porto, Portugal
(2) G.Oliveti, N.Arcuri, “Solar radiation utilisability
method in heat pipe panels”, Solar Energy 1996,
Vol.57, No.5, pp.345-360
(3) Hee-Youl Kwak, Chang-Yong Choi, “Long term
thermal performance of evacuated tubular solar
collector system for industrial process heat in Korea”,
Korea Institute of Energy research, Korea
(4) Li, Z.F. and K. Sumathy, “Experimental Studies on a
Solar Powered Air Conditioning System with
Partitioned Hot Water Storage Tank,” Solar Energy, Vol.
71, No. 5, 2001, 285-297

EVALUATION OF THE THERMAL PERFORMANCE OF A SOLAR WATER HEATING
THERMOSYPHON VERSUS A TWO-PHASE CLOSED THERMOSYPHON USING
DIFFERENT WORKING FLUIDS
A. Ordaz-Flores
Posgrado en Ingeniería (Energía),
Universidad Nacional Autónoma de México
Privada Xochicalco s/n
Temixco, Morelos, 62580, México
O. García-Valladares, V. H. Gómez
Centro de Investigación en Energía,
Universidad Nacional Autónoma de México
Privada Xochicalco s/n
Temixco, Morelos, 62580, México
ABSTRACT
A water heating closed two-phase thermosyphon solar
system was designed and built. The system consists of a
flat plate solar collector coupled to a thermotank by a
continuous copper tubing in which the working fluid
circulates. The working fluid evaporates in the collector
and condensates in the thermotank transferring its latent
heat to the water through a coil heat exchanger. The tested
fluids are acetone and R134a. The thermal performance of
the proposed systems is compared with a conventional solar
water thermosyphon under the same operating conditions.
Advantages of a two-phase system include the elimination
of freezing, fouling, scaling and corrosion. Geometry and
construction materials are the same except for the closed
circuit presented in the two-phase system. Data were
collected from temperature and pressure sensors throughout
the two systems. Early results suggest that R134a may
provide a better performance than acetone for this kind of
systems.
1. INTRODUCTION
The increasing interest of preserving the non renewable
resources has led to focus on sustainable growing, based
mainly on using renewable energy. Renewable energy
sources are the Sun, biomass, hydrogen, wind, etc. The Sun
leads to thermosolar and photovoltaic technologies, mainly.
The current work has special interest in the thermosolar
technology, particularly in the Domestic Water Heating
Systems (DWHS).
Solar domestic water heating systems permit to diminish
the consumption of liquid gas and electricity, helping to
reduce the quantity of pollutants expelled to the atmosphere.
In 2004, Kalogirou [1] studied the environmental impact of
energy utilization and the potential benefits to swap
conventional for solar assisted systems. He estimated that,
for the case of solar water heating, one of the two most
widely used renewable energy, the savings would reach up
to 80%. Hence, the importance of solar water heating.
The most common currently available solar equipments are
the thermosyphons in which the water is heated in a flat
plate solar collector and storaged in a thermotank.
Problems like freezing, corrosion, scaling and fouling
presented in this kind of systems are eliminated in
two-phase systems [2].
In 1979, Soin et al. [2] described an experimental set up to
evaluate the performance of a solar collector with a phase
change working fluid. They used acetone and petroleum
ether as working fluids, because of their high boiling and
condensation heat transfer coefficient. They demonstrated
that the collector efficiency increases linearly with liquid
level.
A refrigerant, trichlorofluoromethane, was used by
Schreyer in 1981 [3] to evaluate the energy recovery in a
Proceedings of ISES Solar World Congress 2007: Solar Energy and Human Settlement
790
solar collector coupled to a heat exchanger, and the latter to
a storage tank. The primary loop was passive and the
secondary needed a recirculation pump. His system
recovered up to 83% energy at low collector temperature
difference.
Evaluation of R134a, among others, as replacing working
fluids of ozone depletion promoting chlorofluorocarbons,
was made by Calm and Didion [4]. They concluded that
there is no perfect fluid to prevent every environmental
impact. R134a has a high latent heat of vaporization, does
not contributes to ozone depletion but, yet low, does have
impact on global warming.
Ong and Haider-E-Alahi [5] studied the performance of a
heat pipe filled up with R134a, and found that the heat flux
transferred increased with high refrigerant flow rates, high
fill ratios and greater temperature difference between bath
and condenser.
More recently, Hussein [6,7] studied a two-phase closed
thermosyphon with the heat exchanger (condenser) in the
solar collector, however, he did not mention the working
fluid used. He carried out both experimental and numerical
tests and set some dimensionless variables to determine
adequate storage dimensions for the tank to improve the
solar energy gain.
Finally, a thermosyphon heat-pipe solar collector was
studied by Esen and Esen [8] in 2005 to evaluate its
thermal performance using three different working fluids,
R134a, R407C and R410A. They found that the latter
offered the highest solar energy collection.
In this work, R134a was chosen due to its availability, low
cost and small impact to environment. On the other hand,
acetone was chosen as another working fluid because it
avoids the high pressures reached with the former.
2. EXPERIMENT
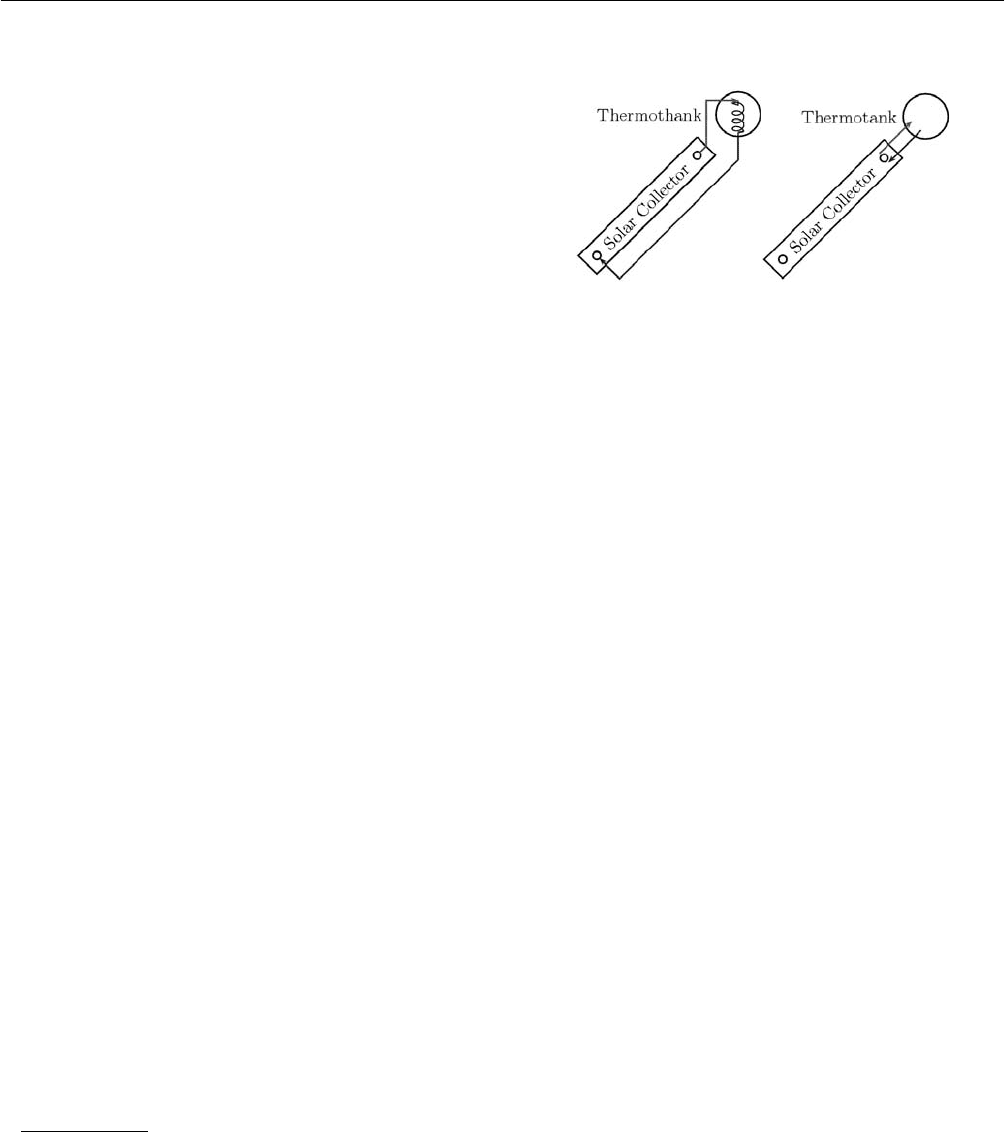
A water heating two-phase closed thermosyphon and a
conventional natural thermosyphon are compared
simultaneously. The two-phase system consists of a flat
plate solar collector coupled to a thermotank by a copper
tubing circuit in which the working fluid circulates. A
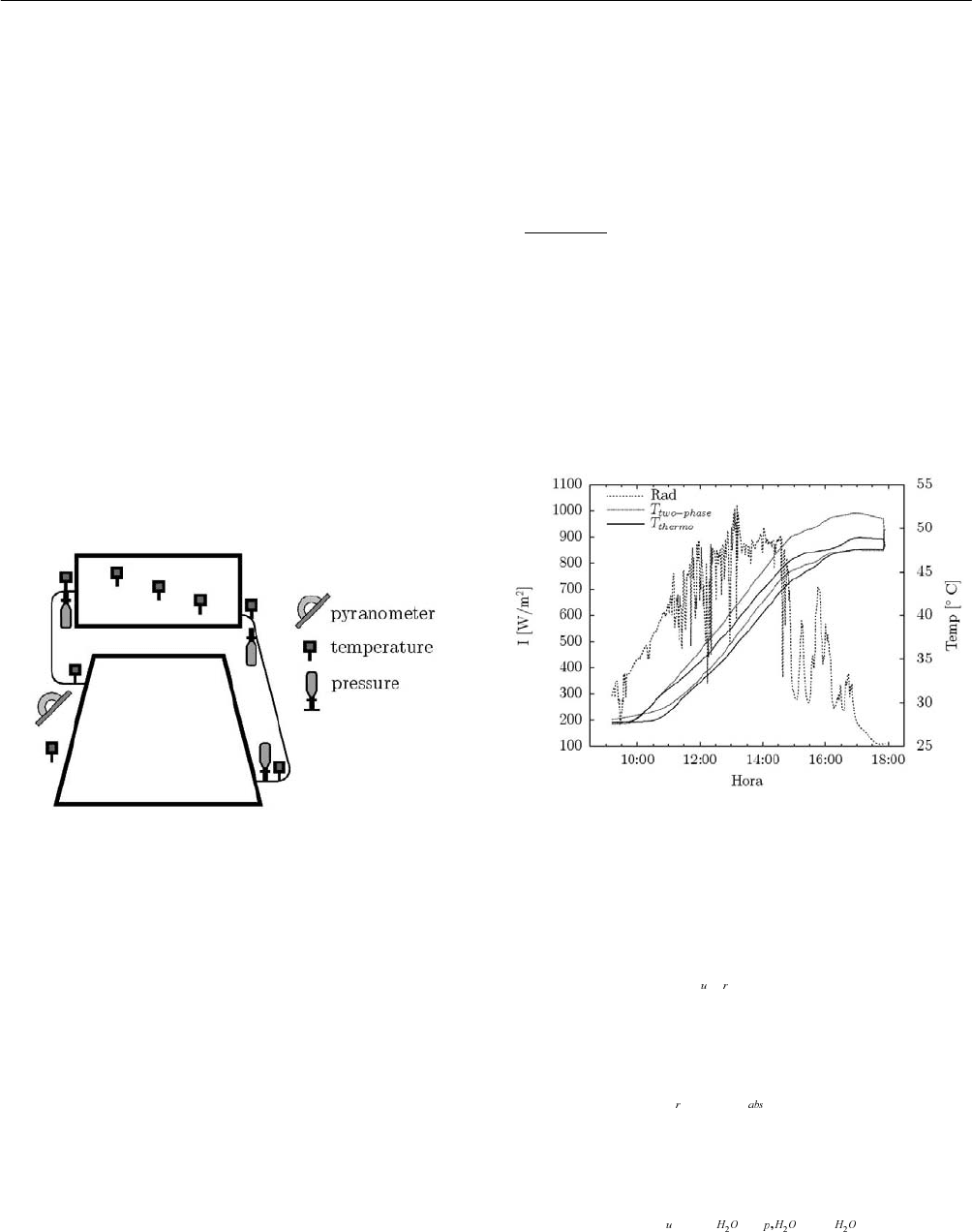
scheme of the systems is shown in Figure 1.
Fig. 1: Scheme of the two-phase closed thermosyphon and
the conventional thermosyphon.
Focusing on the fluid refrigerant behaviour, the solar
collector is the evaporator of the system and the copper coil
immersed in the thermotank is the condenser. The incoming
solar radiation makes the temperature of the refrigerant in
the collector to grow higher to reach the saturation liquid
state. From this point, the working fluid starts to evaporate
to reach the saturated vapour state and even the superheated
vapour zone. As the refrigerant has a higher temperature
than the water, the former donates its phase change latent
heat to the latter and leaves the thermotank as slightly
subcooled liquid to come back to the solar collector to
repeat the cycle.
Both systems have the same geometry, except for the coil
presented in the two-phase system. Each collector has an
absorption area of 1.62 m
2
and the volume capacity of each
thermotank is 160 l.
Refrigerant R134a and acetone were used as working fluids
in the two-phase system. Refrigerant R134a is one of the
replacing working fluids of chlorofluorocarbons since it
does not contribute to ozone depletion. At atmospheric
pressure, R134a evaporates at −26.1°C [9] and acetone at
56.05°C [10]. At their respective boiling points, their
enthalpies of vaporization are 216.98 kJ/kg for R134a and
501.03 kJ/kg for acetone. The combination of boiling point
temperature (the lowest, the best) and heat of vaporization
(the highest, the best) will show which of the fluids is more
suitable for these operating conditions. In early tests, the
two-phase system was loaded up to 78% when operating
with R134a and up to 63% when operating with acetone.
To carry out the experimental characterisation, it was
supposed that the thermotank is filled up with water during
3 SOLAR COLLECTOR TECHNOLOGIES AND SYSTEMS
791
the morning, with neither load nor any human intervention
until the next day in the morning when the whole water is
removed from the thermotank and filled up back again for
the next day of test.
The stratifications profile in each thermotank is monitored
by three sensors placed at different altitudes; this
arrangement permits to determine both the final
temperature reached in the water and the efficiency. The
temperatures and pressures of the working fluid in the
circuit are important to establish the state of the fluid
throughout the equipment, as well as the maximum
pressures of operation in the days of tests. An ambient
temperature sensor was used to monitor this parameter,
which is particularly important at night to estimate the
thermal losses during the night period. Finally, a spectral
pyranometer was used to monitor the incoming solar
radiation on the collector plane. A schema of the set of
sensors for the two-phase system is shown in Figure 2.
Fig. 2: Scheme of the set of sensors for the two-phase
system.
The test was carried out in the Solar Platform of the Centro
de Investigación en Energía of the Universidad Nacional
Autónoma de México, located in Temixco, Morelos State,
México, at 18º50.36’ N latitude and at 99º14.07’ W
longitude, with an altitude of 1219 m osl. The yearly
average ambient temperature is 23.09 ºC with a yearly
average solar irradiance on the horizontal plane of 20.28
MJ/m
2
. The tests began from the late days of May on until
the late days of June of 2007.
A data acquisitor was used to monitor and register the data
from the set of sensors. The typical one-day-long tests
began at 9:00 h and ended at 8:30 h of the next morning.
After 9:00 h, there was no human intervention during the
performance of the equipment. Daily, at 18:00 h, the data
was collected to establish the energy obtained from the Sun
during the radiation period and calculate the efficiency,
useful heat and the increase of water temperature in the
thermotank.
3. RESULTS
Figure 3 shows the radiation and the stratification profile of
the water temperatures of the experimental systems for one
day of test. The morning and the early afternoon were
sunny until around 3:00 pm with an average solar radiation
on the collector plane of 546.44 W/m
2
. The cloudy period
seems to diminish the gain of heat in the conventional
system more than in the phase change system.
Fig. 3: Radiation and stratification profile for one day of
test.
The efficiency and useful heat of the system are calculated
from the equations:
/ 100qqη ()=×
(1)
where
qIA tΔ()( )( )= (2)
and
qm c TΔ()( )( )= (3)
