Water and Wastewater Engineering
Подождите немного. Документ загружается.

13-18 WATER AND WASTEWATER ENGINEERING
Disinfection Practice
To be of practical use, disinfectants must possess the following properties:
1 . They must destroy the kinds and numbers of pathogens that may be introduced into
water within a practicable period of time over an expected range in water temperature.
2. They must meet possible fluctuations in com
position, concentration, and condition of the
water to be treated.
3. They must be neither toxic to humans and domestic animals nor unpalatable or otherwise
objectionable in the concentrations required for disinfection.
4. Their strength or concentration in the treated water must be determined easily, quickly,
and, preferably, automatically.
5. Their cost must be reasonable.
I deally, disinfectants should also possess the following characteristics:
1 . They should be safe and easy to store, transport, handle, and apply.
2. They should persi
st in a sufficient concentration to provide reasonable residual protection
against possible recontamination before use, or—because this is not a normally attain-
able property—the disappearance of residuals must be a warning that recontamination
may have taken place.
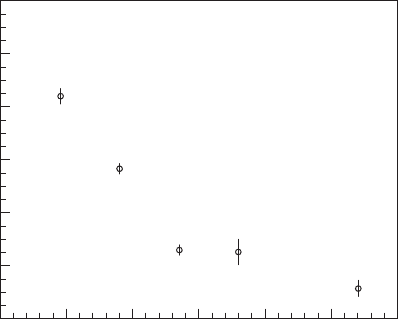
Effective germicidal dose, mW·s/cm
2
50
4
3
2
Log survival, log N/N
0
1
0
100 150 200 250 3000
6
5
FIGURE 13-7
Relationship between log survival of MS2 phage and effective germi-
cidal dose as determined in low pressure UV lamp collimated beam
system. (95 percent confidence interval shown.) ( Source: Linden and
Darby, 1997.)

DISINFECTION AND FLUORIDATION 13-19
Regulatory Context. Selection of an appropriate disinfec tion strategy for water treatment re-
quires a balance among three driving forces:
• Providing water free of pathogens. The regulatory focus for pathogen removal is on coli-
form bacteria, heterotrophic plate counts, Cryptosporidium oocysts, Giardia cysts, Legio-
nella, and viruses.
• Avoiding prod
uction of disinfection byproducts (DBPs). Trihalomethanes (THMs), haloace-
tic acids (HAAs), other halogenated organic compounds, ozone DBPs, oxidation byproducts,
and disinfectant residuals present a health risk. They must be limited in drinking water.
• Maintaining a disinfectant resi
dual in the distribution system. Residual disinfectant is pro-
vided to maintain the bacteriological quality, provide a rapid means for detection of system
contamination, and prevent regrowth of microorganisms.
The disinfectant strategy consists of two parts: primary di
sinfection and secondary disinfec-
tion. Primary disinfection refers to the first disinfectant used to achieve microbial inactivation.
Secondary disinfection refers to the second disinfectant used in a treatment system. Its objective
is to provide di
sinfection residual in the distribution system.
The selection of the primary disinfectant is bounded by four factors: (1) preceding treatment,
(2) total organic carbon (TOC) concentration, (3) bromide ion concentration, and (4) ability to
meet microbial inactivation requirements. If the upstream water treat
ment processes do not in-
clude filtration, the U.S. Environmental Protection Agency strongly discourages the use of ozone
or ozone/peroxide systems because of the potential production of ozone byproducts and biode-
gradable organic matter (BOM) that promotes regrowth in the distribution system (U.S. EPA,
1999). The presence of TOC concentrations above 2 m g/L favor
s selection of a primary disin-
fectant that will not produce DBPs. The reactions of ozone and ozone/peroxide with bromide ion
produces hypobromous acid and bromate ion. If the concentration of bromide ion exceeds 0.10
mg/L, ozone and ozone/peroxide may not be used (U.S. EPA, 1999).
U.S. EPA uses the Ct concept to determ
ine the ability of the primary disinfectant to inac-
tivate three target organisms: Cryptosporidium oocysts , Giardia cysts, and viruses. The target
inactivation is based on inactivation expressed in a logarithmic form (“log-inactivation”):
LI ln
N
N
kCt
0
⎛
⎝
⎜
⎞
⎠
⎟
(13-27)
where LI log inactivation, dimensionless
N number of surviving microorganisms per unit volume
N
0
original number of organisms per unit volume
k rate constant for inactivation, min
1
C disinfectant concentration, mg/L
t contact time, min
LI can be converted to percent removal:
% removal
LI
100
100
10
⎛
⎝
⎜
⎞
⎠
⎟
(13-28)
13-20 WATER AND WASTEWATER ENGINEERING
EPA’s Long Term Enhanced Surface Water Treatment Rules (LT1ESWTR and LT2ESWTR)
target log-inactivation values are 3.0 for Giardia cysts and 4.0 for viruses. The log-inactiva-
tion required for Cryptosporidium oocysts is a function of the raw water Cryptosporidium oocyst
concentration as shown in Table 13-4 .
These credits assume a treated water turbidit
y 0.5 NTU for conventional and direct filtra-
tion and 1 NTU for slow-sand and diatomaceous earth filtration (Lin, 2001).
In some cases, where the degree of contamination is high, greater log removals may be
appropriate. EPA (1991) recommends the overall treatment requirement be adjusted based on the
degree of contamination as shown in Table 13-5
.
The selection of a secondary disinfectant depends on the selected primary disinfectant. Of
concern are the assimilable organic carbon (AOC) concentration, DBP formation potential
(DBPFP), and distribution system retention time. AOC is produced when a strong oxidant such as
ozone i
s used as a primary disinfectant with a high TOC concentration in the water. Without fur-
ther treatment, the finished water has a high potential to stimulate regrowth of microorganisms in
the distribution system. High AOC is defined as a concentration exceeding 0.10 mg/L after filtra-
tion. DBPFP is an indication that organic byproducts can be expec
ted to form in the distribution
system if chlorine is used. A high DBPFP is defined as a water meeting one of the following:
• THM seven-day formation exceeds the MCL of 0.08 mg/L.
• HAA5 seven-day formation exceeds the MCL of 0.06 mg/L.
TABLE 13-4
Additional Cryptosporidium log-inactivation requirements for filtered water
Raw water Cryptosporidium
oocysts concentration,
oocysts/L
Additional conventional filtration
treatment requirements including
softening
a
Additional direct filtration
requirements
a
0.075 No additional treatment No additional treatment
0.075 and 1.0 1 log treatment 1.5 log treatment
1.0 and 3.0 2 log treatment 2.5 log treatment
3.0 2.5 log treatment 3 log treatment
a
A dditional treatment requirements reflect a Cryptosporidium removal credit of 3 log for conventional, slow sand, or
diatomaceous earth filtration plants, and a 2.5 log credit for direct filtration plants.
Source: Code of Federal Regulations, 40 CFR 141.711, 2006.
TABLE 13-5
Recommended overall disinfection
a
as a function of raw water quality
Raw water concentration, microorganisms/100 L
Microorganism
removal/inactivation 1 1 and 10 10
Giardia cyst
3 log 4 log 5 log
Virus 4 log 5 log 6 log
a
Overall disinfection includes removal credits for treatment as well as inactivation by disinfectants.
DISINFECTION AND FLUORIDATION 13-21
Water that spends a long time in the distribution system allows for the reactions that form
THMs to proceed toward completion. A distribution system retention time is considered high if it
exceeds 48 hours (U.S. EPA, 1999).
Selection of Disinfectant. Although there are other disinfectants and combinations of disinfec-
tants, this discussion is limited to chlorine gas, liquid sodium hypochlorite, chloramines, chlorine
dioxide, ozone, and UV radiation.
A summary of disinfectant properties and consid erations in their selection are summarized
in Tables 13-6 and 13-7 .
TABLE 13-6
Summary of disinfectant properties (based on typical disinfectant application)
Condition Chlorine Ozone Chlorine dioxide Permanganate Chloramine Ozone/peroxide Ultraviolet
Produce THM with TOC y s nny s n
Produce oxidized organicssy ssn y s
Produce halogenated organics y s nny s n
Produce inorganic byproducts n s y nn s n
Produce BOM s y s nn y n
MRDL applies y n y n y nn
Lime softening impa
cts y nn n y n y
Turbidity impacts n s nnns y
Meet giardia - 2.0 log
yy y nn n y
Meet giardia - 2.0 log
n yy nn n y
Meet crypto - 2.0 log
n yy nn n y
Meet crypto - 2.0 log
n y
y
a
nn n y
Meet virus - 2.0 log yy y nn n y
Meet virus - 2.0 log yy y nn n y
Secondary disinfectant y n s n y nn
Operator skill (1 low; 5 high) 1 55 12 53
Applicable to large utilities yy y y y y n
Applicable to small utilities yy y y y y y
y yes, n no, s sometimes
a
Ct values to achieve 2.0 log inactivation are very high at common water temperatures.
TABLE 13-7
Consideration for selecting disinfectant
Consideration Cl
2
NaOCl O
3
ClO
2
Chloramine UV
Residual persistence Low Low None Moderate Very low None
pH dependenceYes Yes SomeSomeYes None
Safety concerns Very high Moderate High Very high Moderate Moderate
Complex equipment Yes No Very Yes Yes Yes
(continued)
13-22 WATER AND WASTEWATER ENGINEERING
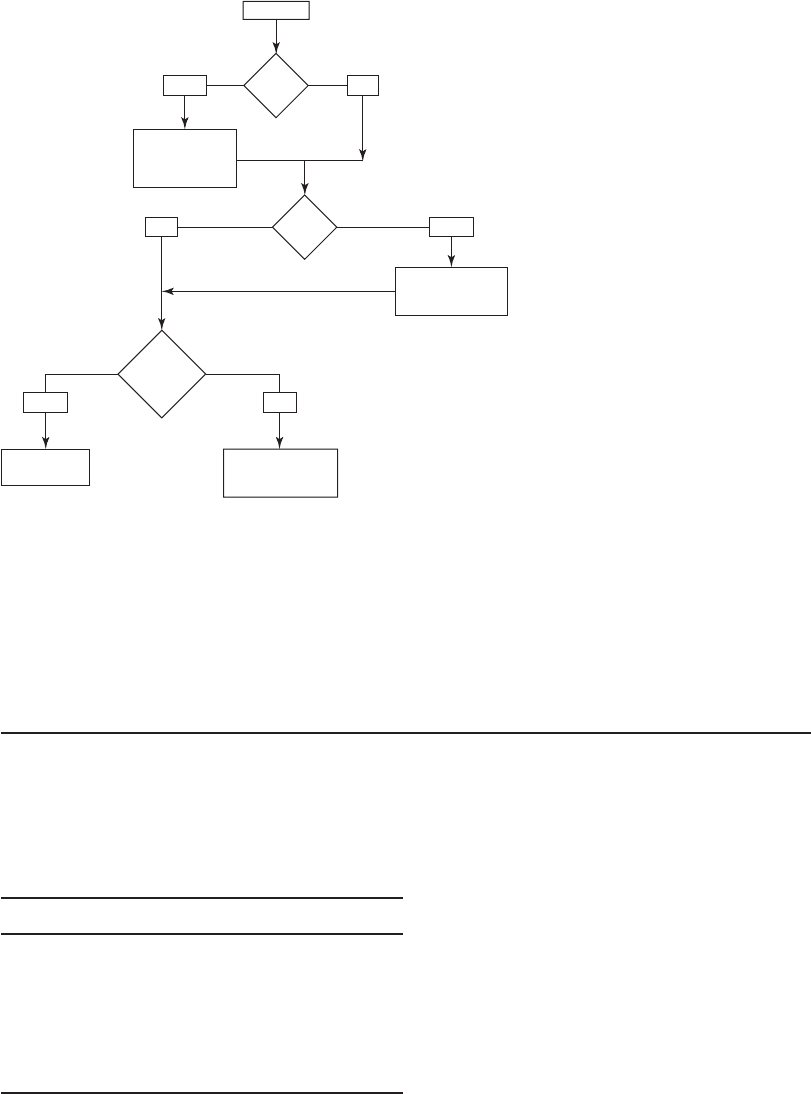
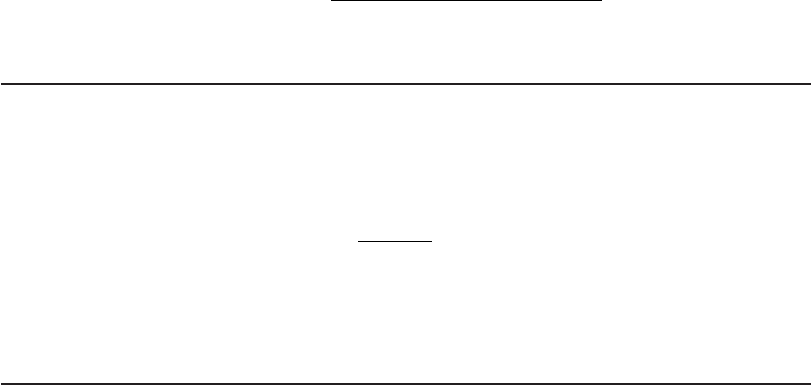
Figures 13-8 and 13-9 provide a means for narrowing the choices of primary and secondary
disinfectant, respectively. The selection flow diagrams do not address the role of microbial growth
in settling tanks and filters or the presence of manganese, iron, and sulfides in the raw water.
Where biological growth is a proble
m in settling tanks and filters, some alternative means of
reducing DBPs include reducing NOM, the use of chlorine dioxide as a pretreatment followed by
chlorine and chloramine, and ozonation followed by DBP removal with anthracite biofilters or
granular activated carbon (GAC).
Consideration Cl
2
NaOCl O
3
ClO
2
Chloramine UV
Equipment reliability Good Very good Good Good Good Moderate
Process control Well developed Well developed Developing Developing Well developed Developing
O&M requirements Low Low High High Low Low
TABLE 13-7 (continued)
Consideration for selecting disinfectant
Sources: Haas, 1999; Hesby, 2005; MWH, 2005.
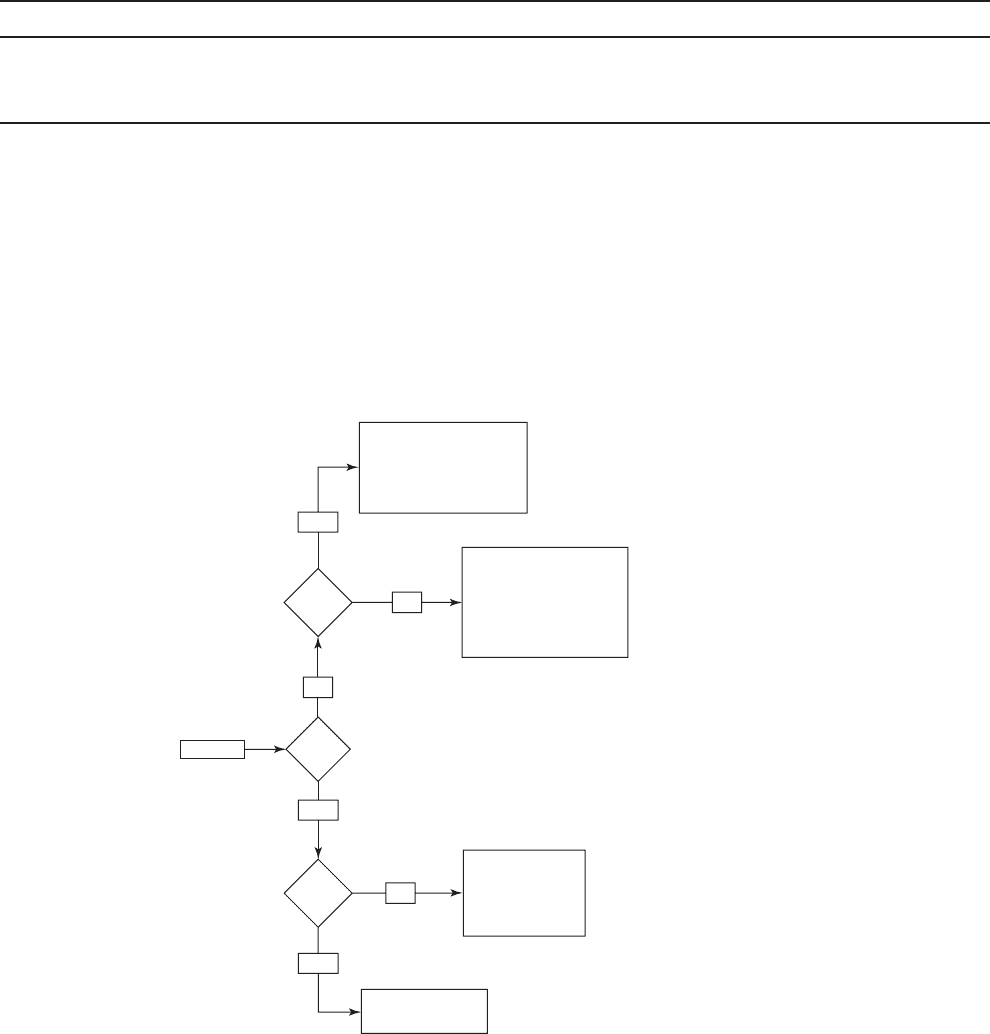
High
TOC?
Start
Yes
Chlorine dioxide
Chlorine
UV
Need bench or pilot study
Chlorine dioxide
Chlorine
Ozone
UV
Interactive disinfectants
Chlorine dioxide
UV
High DBPFP
Ozone / BAC
Chlorine dioxide
UV
Yes
Yes
No
High
bromide
?
High
bromide
?
No
No
FIGURE 13-8
Flow diagram to narrow selection of a new primary
disinfectant for systems that filter.
( Source: Adapted from U.S. EPA, 1999.)
DISINFECTION AND FLUORIDATION 13-23
Iron, manganese, and sulfides ex ert an oxidant demand and, in the case of iron and man-
ganese, will form precipitates as a result of oxidation. The precipitates, in addition to being an
aesthetic problem, will interfere with the disinfection process.
The use of the flow charts and boundary conditions in sele
cting primary and secondary dis-
infectants is illustrated in Example 13-5 .
Example 13-5. Select the primary and secondary disinfectants for the town of Stillwater which uses
the Noir River for its water supply. The design flow rate is 18,500 m
3
/ d. The water is treated by con-
ventional coagulation, sedimentation, and filtration. The time for water to reach the most distant cus-
tomer at the minimum demand flow rate is 31 hours. The Noir River water analysis is shown below.
Noir River water analysis
Constituent Concentration
TOC 5 mg/L
Bromide Not detected
Turbidity 10–500 NTU
Giardia cysts
1/100 L
Virus 1/100 L
Cryptosporidium oocysts
1.1–2.0/L
High
AOC?
High
DBPFP?
Extended
distribution
time?
Start
Yes
No
Yes
No
Yes
Chlorine
Chloramine
∗
Chlorine
Chlorine dioxide
Chloramine
∗
Treatment to
reduce DBPFP
No
Biological
treatment
BAF or GAC
*Chloramine is a potential problem because NH
3
promotes regrowth and because of chloramine
reactions that release Pb and Cu from pipes.
FIGURE 13-9
Flow diagram to narrow selection of a
new secondary disinfectant.
( Source: Adapted from U.S. EPA,
1999.)

13-24 WATER AND WASTEWATER ENGINEERING
Solution:
a . The concentration of TOC is greater than 2 mg/L so it is considered high. Bromide was
not detected. From Figure 13-8 , the primary disinfectant alternatives are ozone, chlorine
dioxide, and UV.
b. A comparison of ozone, chlorine dioxide, and UV in Table 13-6 reveals the following
highlights:
• Neither ozone nor ClO
2
are clearly superior with respect to production of byproducts
(THM, oxidized organic matter, halogenated organic matter, inorganic byproducts ,
and BOM). UV is clearly superior.
• Ozone and UV do not have MRDLs (Maximum Residual Disinfectant Level) while
ClO
2
does.
• All three can achieve 2.0 log Giardia inactivation and 2.0 virus inactivation.
From Table 13-5 , the required log removal for Giardia cysts and virus when the
concentration is 1/100 L is 3 and 4 logs, respectively. The conventional filtration
credit for Giardia cyst removal is
2.5 and 2 for viruses. Therefore, all can meet the
additional disinfection inactivation required.
• The concentration of ClO
2
or UV dose to meet a 2.0 log inactivation for Cryptospo-
ridium oocysts that is required for the concentration found in the Noir River is very
high (See Table 13-4 and Ct tables* ). At the required dose, the potential for exceed-
ing both the ClO
2
MRDL of 0.8 mg/L and chlorite DBP limit of 1.0 mg/L is high.
c. From Table 13-7 , with the exception of safety concerns, ozone and ClO
2
are very com-
parable. UV has moderate safety concerns and moderate equipment reliability concerns.
d. Based on this analysis, ozone is selected as the primary disinfectant with the understand-
ing that there will be no residual and that AOC will be a problem.
e. From Fig
ure 13-9 , the flow path leads to a requirem ent for biological treatment and a
high DBPFP.
f. The distribution system time is 31 hours. This is less than the 48-hour criterion. This
leads to ClO
2
or chloramines as the secondary disinfectant choices. Because chloramine
is rated as (yes) and ClO
2
i s rated s (sometimes) as a secondary disinfectant in Table 13-6 ,
chloramine is selected.
Comments:
1 . Over time, with improvements in reliability and O&M, the UV alternative will become
more attractive than the ozone option selected here.
2. Other strategies may be more appropriate. For example:
• Removal of NOM m
ay permit consideration of alternative disinfectants.
• An improved watershed management program to lower the Cryptosporidium dis-
charges from agricultural runoff may lower the overall log removal/inactivation re-
quirement and thus open consideration of other disinfectants.
*Ct tables may be found at www.mhprofessional.com/wwe or in the Code of Federal Regulations.
DISINFECTION AND FLUORIDATION 13-25
Weight Percent Chlorine. Chlorine in one of its forms is the most common disinfectant used
in the United States. It is available commercially in pressurized vessels that contain both liqui-
fied and gaseous fractions. Sodium hypochlorite (NaOCl), also known as bleach, is a liquid form.
Calcium hypoc
hlorite (Ca(OCl)
2
· 4H
2
O), also known as HTH
®
, is sold as a granule, powder,
and tablet.
The relative amount of chlorine present in these compounds may be expressed as weight
percent chlorine. The weight percent chlorine is a measure of the amount of chlorine being pur-
chased or being supplied. It is used to calculate the feed rate to produce the d
esired dose of chlo-
rine for compounds other than chlorine gas as well as the storage volume required and equivalent
operating cost for chemicals. It is defined as
Weight chlorine
GMW of chlorine in compou
%
nnd
GMW of compound
()100%
(13-29)
Example 13-6. Estimate the weight percent chlorine in HTH
®
.
Solution. Using the molecular weights of Cl
2
and HTH
®
, the weight percent chlorine is
Weight chlorine%%
2 35 45
214 90
100 329
()
.
().
.
99 33or %
Comment. If HTH
®
i s selected to supply the required chlorine dose, the mass of HTH
®
to be
provided must be (1/0.3299) or about three times the mass of chlorine required assuming the
HTH
®
is 100% pure.
Safety Precautions and Chemical Handling. Gaseous chlorine is most often employed by
larger utilities. It is normally stored in its shipping container. The recommended standards for
chlorine are provided here in detail because of the extreme hazard of the gas and the wide use of
chlorine gas for disinfec
tion (GLUMRB, 2003):
• Chlorine gas feed and storage shall be enclosed and separated from other operating areas.
The chlorine room shall be:
• provided with a shatter resistant inspection window installed in an interior wall,
• constructed in such a manner that all openings between the chlorine room and the
remainder of the plant are sealed, and
• provided with doors eq
uipped with panic hardware, assuring ready means of exit and
opening outward only to the building exterior.
• The room shall be constructed to provide the following:
• e a ch room shall have a ventilating fan with a capacity that provides one complete air
change per minute,
13-26 WATER AND WASTEWATER ENGINEERING
• the ventilating fan shall take suction near the floor as far as practical from the door and
air inlet,
• air inlets should be through louvers near the ceiling,
• separate switches for the fan and lights shall be located outside the chlorine room and at
the inspection window,
• vents from the feeders and storage shall discharge to the outs
ide atmosphere through
chlorine gas collection and neutralization systems,
• floor drains are discouraged. Where provided, the floor drains shall discharge to the out-
sid e of the building and s hall not be connected to other internal or external drainage
systems.
• Chlorinator rooms should be heated to 15
C and be protected from excessive heat.
• Pressurized chlorine feed lines shall not carry chlorine gas beyond the chlorinator room.
• A continuous chlorine sensor and alarm is recommended.
A scrubber system that is activated in the event of a chlorine leak is recommend
ed. Section
80.303 of Article 80 of the Uniform Fire Code provides design guidance. The scrubber system
uses sodium hydroxide to neutralize the chlorine gas (AWWA, 2006).
Because of safety and security concerns, many utilities have s
witched to hypochlorite. The
decision to switch is a complex management decision because the cost of NaOCl is significantly
higher than the cost of gaseous chlorine.
Sodiu m hypochlorite (als o called liquid bleach) may be stored in the original shipping
containers
or in compatible containers. Fiber-glass reinforced plastic (FRP) tanks, spe-
cific polyethylene fabricated for NaOCL storage, and carbon steel tanks lined with ru bber
or polyvinyl chloride (PVC) are recommended (Hesby, 2005; MWH, 2005). Most small to
medium-sized plants feed hypochlorite with po
sitive displacement diaphragm metering
pumps or peristaltic metering pumps. The peristaltic pumps are preferred because they oper-
ate without vapor lock. Schedule 80 PVC piping provides good service if it is not exposed
to sunlight. When sunlight exposure is unavoidable, chlorinated pol
yvinyl (CPVC) is recom-
mended (MWH, 2005). Ball valves should not be used because they lock down, trap NaOCL
off-gases, and explode.
A m ajor design issue with the storage of NaOCl is its stability. Commercial bleach is gen-
erally shipped as 12 to 15 percent available Cl
2
at a pH 12. If the pH is held above 11, the
rate of decay is very low (Gordon et al., 1997). Concentration and temperature are important
considerations in storage. For exam ple, if a 15 weight percent solution is diluted to 7.5 percent,
its half-life will increase from 50 to about 140 days if it is stored at 25 C. If it is stored at 7.5
weight percent and 15 C, its half-life will be on the order of 500 days (Gordon et al., 1997;
MWH, 2005). At the other end of the spectrum, the crystallization temperatures are: 22C
for a 15 trade percent (weight per unit volume so that 1 percent corresponds to a weight of
10 g of available chlorine per liter) s olution; 17C for a 12.5 percent solution; 12C for a
10 percent solution. The im
plications for design of a NaOCl storage facility are that it should
be protected from sunlight, kept at an ambient temperature less than 20 C but greater than
12C, and the NaOCl should be diluted on receipt from 15 to 7.5 weight percent. Because
DISINFECTION AND FLUORIDATION 13-27
it is delivered at a pH of about 12, dilution induced low-pH decay is normally not a problem
(MWH, 2005). However, the dilution water should be soft because the high pH of NaOCl will
precipitate hardness from a hard water.
A proprietary system for on-site generation of hypochlorite was introduced in the United
States in the 1980s. It uses electrolyti
c decomposition of NaCl to produce a NaOCl feed solution.
Because the hypochlorite solution is generated on demand, it has the safety advantages of hypo-
chlorite without the problem of degrad ation while the NaOCl solution is in storage. However,
power consumption is significant.
Ozone at concentrations greater than 23 percent is explo
sive. At ambient temperature and
pressure, it rapidly decays. Unlike chlorine, it cannot be stored under pressure but must be gen-
erated on-site. The corona discharge method of generation is commonly used for drinking wa-
ter disinfection. In this method, oxygen is passed through an elec
tric field that is generated by
applying a high voltage across electrodes separated by a dielectric material. As oxygen passes
through the electric field, it is broken down to oxygen singlets (O •). These react with oxygen
to form O
3
. The source of oxygen is either ambient air or commercially supplied liquid oxygen
(LOX). Air-fed ozone systems were used widely prior to the mid -1990s. The LOX-fed systems
have become the system of choice since about 1995 (Rakness, 2005). Regardless of the sou rce,
the feed gas must be prepared. The system design goals for the feed gas are: temperature 30 C ;
100 percent removal of particulate matter 0.3 micrometers ( m) in diameter, 95 percent re-
moval of particulate matter 0.1 m; hydrocarbons 4 to 5 ppm, dew point in the range
65C to 100
C (Dimitriou, 1990). Typical components include: air compressor, after-cooler,
refrigerative drying, vapor/liquid separator, prefilters, desiccant dryer, particulate after-filter, and
controls. Even with gas transfer efficiencies of 90 to 99 percent, the off-gas from the ozone reac-
tor may have ozone concentrations on the order of 500–1600 ppm (v/v). This exceeds the o
ccupa-
tional exposure limit of 0.10 ppm (v/v). The ozone in the off-gas can be destroyed thermally with
or without a catalyst. Without a catalyst the required temperature is 300C–350 C at a 5 second
residence time. The use of a catalyst lowers the required temperature to between 30C and
70 C
(AWWARF, 1991).
Chlorine dioxide cannot be stored because it is not safe. The pure gas may explode as a result
of high temperatures, exposure to light, changes in pressure, or exposure to organic contaminants
(Hesby , 2005). Therefore, it is generated on-site. Most generation techniques use a
chlorine/
sodium chlorite mixture. GLUMRB (2003) recommends that sodium chlorite be stored by itself
in a separate room and preferably in a separate building detached from the water treatment facil-
ity. The storage structure must be of noncombustible materials. Positive displa
cement feeders are
used.
Chloramine is formed on-site by reacting ammonia with chlorine as shown in Equations 13-5
through 13-7 . Monochloramine is the desired compound for chloramine disinfection. Dichlo-
ramine is a disinfectant, but it also produces
undesirable tastes and odors. In addition to being
poorly soluble, nitrogen chloride is a foul smelling gas. Monochloramine is formed until the mass
ratio of Cl
2
/NH
3
e xceeds 4 (Hesby, 2005). The recommended mass ratio is in the range between
4.5:1 and 5:1 because this minimizes the concentration of unreacted ammonia (AWWA, 2006).
The rate of the reaction is s trongly influenc ed by pH with the highest rate at pH 6 or lower.
However, at this pH the amount of dichloramine formed is significant. At pH 8 and above the
amount of dichloramine is not significant. A pH between 7 and 8 appears to be the best com-
promise.
