Yamaguchi H. Engineering Fluid Mechanics (Fluid Mechanics and Its Applications)
Подождите немного. Документ загружается.


50 2 Conservation Equations in Continuum Mechanics
»
»
»
¼
º
«
«
«
¬
ª
0
0
0
2
1
12
13
23
AA
AA
AA
a
T
(
2.3.7)
where
a
T is the skew-symmetric part of the tensor T derived from the fol-
lowing relationship
as
TT
TTTTTTT
2
1
2
1
(
2.3.8)
where
s
T is the symmetric part of the tensor T .
Equation (2.3.6) implies the fact that A is the vector of the tensor T ,
indicating that A can be obtained from the tensor T and conversely
a
T
can be found from the pseudovector A as follows
T:İ A
(
2.3.9)
and
A İT
2
1
a
(
2.3.10)
Employing Eqs. (2.3.3) and (2.3.5) to the integral equation of Eq. (2.3.2),
we have the resultant integral equation
³³
¸
¹
·
¨
©
§
u
VV
dV
dV
Dt
D
Ag
u
x Tේ
UU
(2.3.11)
The volume integral vanishes identically since the volume is arbi-
trary, so that
Ag
u
x
¸
¹
·
¨
©
§
u T
UU
Dt
D
(2.3.12)
The left hand side vanishes identically by Cauchy’s equation of motion
(see given by Eq. (2.2.6)), the conservation law of linear momentum, con-
sequently, we reach the conclusion that A =0. This implies from Eq.
(2.3.10), that the skew-symmetric part of the stress tensor T vanishes, so
that the stress is written
s
TT or
jiij
TT
(2.3.13)
2.3 Angular Momentum Conservation 51
Considering the angular momentum of the linear momentum equation
(Cauchy’s equation of motion), for a non-polar fluid it can be concluded
that the stress tensor is symmetric. If, in other words, the stress tensor of a
continuum medium in motion is symmetric, the angular momentum of a
linear momentum equation is always conserved so that the motion of fluid
can only be determined by the linear momentum equation.
In treating a polar fluid, however, an angular momentum due to a long-
range force may be exerted on a fluid particle, and likewise for the body
force per unit mass from distant surroundings. As displayed schematically
in Fig. 2.4, for example, the extra angular momentum we introduce to a
polar fluid may be a body couple f
U
in addition to the body torque
gx
U
u
, i.e. f per unit mass. In similar fashion, a surface couple
n
C
per
unit surface may also be introduced to the surface of a fluid particle, as a
surface traction couple, due to a short-range force, and likewise for the sur-
face torque
n
tx u . The total angular momentum L of a fluid particle is
considered in a certain way that L may consist of the sum of the moment
of linear momentum ux
U
u per unit mass and an internal angular momen-
tum (or intrinsic angular momentum s ) per unit mass, which accounts for
the local spin field of a material element. Thus, in consideration of the bal-
ance of total angular momentum, we have
³³
³
uu
u
S
nn
V
V
dSdV
dV
tD
D
Dt
D
Ctxfgx
sux
L
UU
UU
(
2.3.14)
In Eq. (2.3.14),
n
t can be given by Cauchy’s stress formula, as seen earlier
in Eq. (1.6.8), i.e.
T
ˆ
nt
n
. Analogously,
n
C can also be found by a
similar expression given below, since
n
C arises from diffusive transport
of internal angular momentum where
c is called the couple stress tensor.
c
ˆ
nC
n
(2.3.15)
Introducing Eqs. (1.6.8) and (2.3.15) to Eq. (2.3.14) yields, after tensor
calculus likewise deriving Eq. (2.3.5), we have
^`
³
³
uu
u
V
V
dV
dV
Dt
D
Tc xgxf
sux
UU
U
(
2.3.16)
52 2 Conservation Equations in Continuum Mechanics
Applying the Reynolds’ transport theorem and the continuity equation to
the left hand side of Eq. (2.3.16), and vanishing the volume integration due
to an arbitrary volume, we have
Axgxfsux uu u Tc
UUU
tD
D
(
2.3.17)
Equation (2.3.17) is an equation of the total angular momentum of general
form for polar fluids. In order to exploit the conservation of angular mo-
mentum, Eq. (2.3.17) can be further reduced to simpler form with the fol-
lowing procedure. That is, first taking the vector product of
x to
Cauchy’s equation of motion, we can obtain
Tu u ේxgx
u
x
UU
Dt
D
(
2.3.18)
and then using the relationship
DtDDtD uxux u
, Eq. (2.3.17) can
be reduced to the following expression after subtracting Eq. (2.3.18) from
(2.3.17) as follows
Af
s
cේ
UU
Dt
D
(
2.3.19)
This is a resultant equation for the internal angular momentum for a polar
fluid.
In the case of a polar fluid, the skew-symmetric part of the stress ten-
sor
a
T from Eq. (2.3.10) would be generated by an effect of the body cou-
ple
f
U
and the diffusion of the surface couple c
to the net change of
the internal angular momentum
DtDs
U
. Equation (2.3.19) is a non-
conservation form. The conservation form of the equation for the internal
angular momentum can be reduced to the following form, after the mass
conservation is taken in account, so as to yield the following, where
us
U
is called the spin flux.
Afus
s
w
w
c
UU
U
)(
t
(
2.3.20)
2.4 Energy Conservation
The energy conservation of a continuum medium can be considered from the
first law of thermodynamics, when the law is applied to the thermodynamic
u
u

2.4 Energy Conservation 53
system of a particle. The first law of thermodynamics in a dynamic system
implies the conservation of thermal energy and work, which means that
WQukd
G
G
)(
(2.4.1)
where W
G
and Q
G
are the work done by the system, and the heat sup-
plied to the system respectively.
k
and
u
in Eq. (2.4.1) are the kinetic en-
ergy and the internal energy of the system respectively. When the system is
at equilibrium, Eq. (2.4.1) can be written with a unit of power by
WQ
t
W
t
Q
dt
ukd
)(
G
G
G
G
(2.4.2)
where W
and
Q
are the work output by the system and the heat input to
the system respectively.
In consideration of the first law of thermodynamics as applied to a sys-
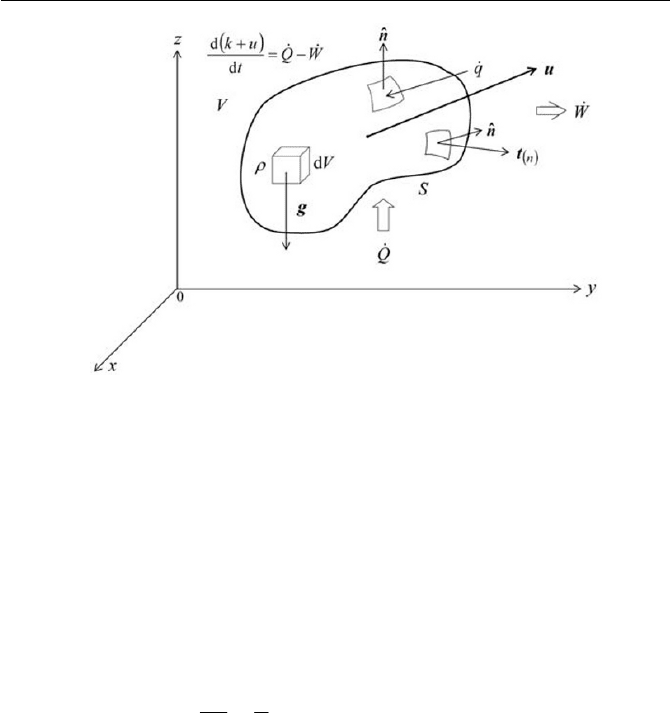
tem of a certain fluid particle as depicted in Fig. 2.5, we may be able to ob-
tain the work output
W
in the first place, taking a dot product to the forces
of the fluid particle as follows
³³
VS
n
dVdSW ugut
U
(
2.4.3)
The first term of the right hand side is the work output by a surface force
and the second term is the work output by a body force. While applying a
dot product of
u
to Cauchy’s equation of motion from Eq. (2.2.6), we can
have the following expression
uguu
ugu
u
¸
¸
¹
·
¨
¨
©
§
U
UU
ේ)(ේ
)ේ(
2
2
:TT
T
Dt
D
(
2.4.4)
Equation (2.4.4) can also be written by a volume integral form as
dVdV
dVdV
Dt
D
VV
VV
³³
³³
uu
uuu
gT
:T
U
U
)(
ේ)(
2
1
(
2.4.5)
54 2 Conservation Equations in Continuum Mechanics
Fig. 2.5 The first law of thermodynamics to a fluid particle
Denoting Cauchy’s stress formula from Eq. (1.6.8), i.e.
T
ˆ
nt
n
and
applying Gauss’ divergence theorem to the surface integral in Eq. (2.4.3),
we can write the work output W
as
dVdVW
VV
ugu
³³
U
T
(2.4.6)
Thus, equating the right hand side of Eq. (2.4.5) with Eq. (2.4.6), we can
newly express the work output
W
as
dVdV
Dt
D
W
VV
³³
uuu ේ
2
1
:T
U
(
2.4.7)
Equation (2.4.7) indicates that the work output of a system of a fluid parti-
cle can be divided into two parts; the change of kinetic energy and the rate
at which the internal stresses do work.
Let us examine further the scenario where
T is not symmetric. Denot-
ing again
sa
TTT
in Eq. (2.3.8), and Ȧe uේ in Eq. (1.1.18), the
work output due to the internal stress can be written as
Ȧ:Te:T
Ȧe:TT:T
as
as
ේ u
(
2.4.8)
2.4 Energy Conservation 55
second term of Eq. (2.4.8) from Eq. (1.1.29) for the spin tensor
Ȧee
¸
¸
¹
·
¨
¨
©
§
w
w
w
w
İ
ˆˆ
Ȧ
2
1
2
1
i
j
j
i
ji
x
u
x
u
(
2.4.9)
Furthermore,
a
T as given by Eq. (2.3.10), utilizing these identities, we can
reduce the term to
AȦ
ȦA
¸
¹
·
¨
©
§
¸
¹
·
¨
©
§
2
1
2
1
4
1
2
1
2
1
pkkp
pkijpijk
a
A
A
ZG
ZHH
İİȦ:T ᧶
(
2.4.10)
As a result, Eq. (2.4.10) indicates that the skew-symmetric part of the
stress tensor does produce an output work, owing to the vorticity. However,
as easily demonstrated when the stress is symmetric, the work output due
to the internal stress is simply shown by the deformation as
e:TT
s
uේ:
(
2.4.11)
The heat input of the system of a fluid particle is conceived to consist of
heat transferred to the system through the surface and heat generated in the
system, so that
Q
can be written as
³³
VS
bdVdQ
U
Sq
(
2.4.12)
Here, q is the heat flux vector; the negative sign is assigned toward the
surface, i.e. opposite to the surface direction
n
ˆ
. Moreover, b is the
amount of heat generated per unit mass in the system. Equation (2.4.12)
can be converted into a volume integral by applying Gauss’ divergence
theorem as follows
³
V
dVbQ
U
q
(
2.4.13)
The total change of the system energy expressed with
k
and
u
in Eq.
(2.4.2) can be written as
Thus, we have decomposed u into the symmetric and skew-symmetric
parts so that other products vanish identically. Let us further consider the
56 2 Conservation Equations in Continuum Mechanics
dVu
Dt
D
dt
ukd
V
2
1
¸
¹
·
¨
©
§
UU
uu
(
2.4.14)
As we can see,
u
is the internal energy per unit mass. The energy pos-
sessed by the system may include spin energy if the continuum has an in-
ternal structure and field energy derived from an externally imposed filed,
depending upon the circumstance and property of the continuum in motion.
Generally speaking, within the continuum mechanics we write the energy
equation of a fluid, by substituting Eqs. (2.4.7) and (2.4.13) together with
Eq. (2.4.14) into Eq. (2.4.2), we can obtain the equation of the energy con-
servation as
dVbdVu
tD
D
VV
ේේ
UU
qu᧩:T
(
2.4.15)
Note that the power W
is chosen as to the work input to the system in Eq.
(2.4.15), where the sign of plus is assigned. After vanishing the volume in-
tegral from both sides of equation (2.4.15) for an arbitrary volume and
with Reynolds’ transport theorem to the right hand side of Eq. (2.4.15), we
can obtain the resultant equation to yield
bu
t
ȡ
UU
w
w
uq ේ:ේේ T᧩
(2.4.16)
This is the conservation equation of energy, which is called the Neumann
energy equation in the conservation form. The equation can also be re-
duced to the non-conservation form, as practiced previously by consider-
ing the equation of mass continuity, which yield the form
b
tD
uD
UU
uq ේ:ේ T᧩
(
2.4.17)
The Neumann energy equation given by Eq. (2.4.16) or (2.4.17) is an ex-
pression derived from the first law of thermodynamics. The equations con-
tain thermodynamic properties, such as
u and
U
, so that the equations can
be further expanded thermodynamically in order to define the state of con-
tinuum undergoing thermal process.
2.5 Thermodynamic Relations
The state of a thermodynamic system can be determined by its thermody-
namic properties, which are connected by its relationship to the general term

2.5 Thermodynamic Relations 57
0 ),,( Tvpf
(
2.5.1)
As such,
p
is the thermodynamic pressure, or simply the pressure,
v
is
the specific volume
U
1 v
and
T
is the absolute temperature. Equation
(2.5.1) is called the equation of state, where its functional form depends
upon the state of the thermodynamic properties of the substance contained
in the system. Any one of the three variables in Eq. (2.5.1) can be ex-
pressed as a function of the other two by solving Eq. (2.5.1). This means
that the thermodynamic state is completely determined by two remaining
thermodynamic properties. An important concept to note here is the state
of equilibrium, which we can determine through the thermodynamic state
from Eq. (2.5.1). The state of equilibrium is that property which does not
vary over time when the external conditions remain unchanged.
In some situations, when a continuum is in motion with chemical
reaction, a relaxation process or in a large temperature gradient, that is a
process that results in the inability of the system to reach the state of
equilibrium in the time available, some processes have to be considered by
the states of non-equilibrium. However, the majority of processes in
engineering fluid mechanics are in the state of equilibrium, and the system
undergoes the reversible process where the process is connected only
between those initial and final states which are states of equilibrium.
As introduced in Eq. (2.4.1), the first law of thermodynamics in a dy-
namic system of a continuum, the internal energy
u can be regarded as in-
dependent of the kinematics of the motion of flow in the limit of the equi-
librium thermodynamics (thermostatics) as follows
WQdu
G
G
(2.5.2)
The first law of thermodynamics, demonstrated by Eq. (2.5.2), gives the
conservation of energy in quantity, but does not have any information on
the quality of the energy. The work done by the system
W
G
and the heat
supplied to the system
Q
G
are not thermodynamic properties, which can-
not be determined by being given two equilibrium states between a trans-
formation process. However,
W
G
may be determined by a known reversi-
ble process of work transfer, considering
p
and
v
at two given
equilibrium points of states as follows
pdvW
G
(2.5.3)
It is the
Q
G
that can not be determined by any other known thermody-
namic properties, but only by the thermodynamic property
s, the entropy.
The second law of thermodynamics gives a corollary that there exists a

58 2 Conservation Equations in Continuum Mechanics
thermodynamic property of a system such that a change in its value from
state 1 to 2 is equal to
s
ss
T
Q
G
³
12
2
1
(
2.5.4)
For any reversible process,
Q
G
can be written by the change (the differen-
tiation) of the entropy as
TdsQ
G
(2.5.5)
Thus, Eq. (2.5.2) can be written with Eqs. (2.5.3) and (2.5.5) as follows
pdvTdsdu
(2.5.6)
or
¸
¸
¹
·
¨
¨
©
§
U
1
pdTdsdu
(2.5.7)
Obtaining a new thermodynamic property s, we have the following
thermodynamic relationship between the thermodynamic properties of
sTvp ,,, :
vs
s
p
v
T
¸
¹
·
¨
©
§
¸
¹
·
¨
©
§
(
2.5.8)
p
s
s
v
p
T
¸
¹
·
¨
©
§
¸
¸
¹
·
¨
¨
©
§
(2.5.9)
Tv
v
s
T
p
¸
¹
·
¨
©
§
¸
¹
·
¨
©
§
(2.5.10)
T
p
p
s
T
v
¸
¸
¹
·
¨
¨
©
§
w
w
¸
¹
·
¨
©
§
(2.5.11)
Equations (2.5.8) to (2.5.11) are called the Maxwell equations, which
form the basis for obtaining further important thermodynamic relationships
which may be utilized for evaluation of thermal properties of continuum
substance.
'
2.5 Thermodynamic Relations 59
Among others, an important thermal property is the specific heat,
which is a quantity that gives the heat supplied to the system when the
temperature difference is given, so that
cdTQ
G
(2.5.12)
where c is the specific heat. Substituting Eq. (2.5.12) to the first law of
thermodynamics Eq. (2.5.2) and denoting
pdvW
G
from Eq. (2.5.3), we
have the following relationships;
pdvducdT
(2.5.13)
or
vdpdhcdT
(
2.5.14)
Such that h is defined as
pvuh
¸
¸
¹
·
¨
¨
©
§
U
1
pu
(
2.5.15)
In Eq. (2.5.15) h is the enthalpy per unit mass, which is a specific energy
function. From Eqs. (2.5.13) and (2.5.14), therefore, we can obtain two
kinds of specific heat:
v
v
T
u
c
¸
¹
·
¨
©
§
w
w
(2.5.16)
and
p
p
T
h
c
¸
¹
·
¨
©
§
w
w
(
2.5.17)
v
c
denotes the specific heat evaluated at constant volume (constant den-
sity) and
p
c
denotes the specific heat evaluated at constant pressure.
Considering the thermodynamic relations, we are now in position to
expand the Neumann energy equation of Eq. (2.4.17) by decomposing the
total stress tensor
T into IJ and
p
as described in Eq. (1.6.13). Denoting
that
p
in Eq. (1.6.13) is regarded as the thermodynamic pressure in the
state of equilibrium, so that Eq. (2.4.17) becomes
bp
Dt
Du
UU
uIJuq ᧶
(
2.5.18)
