Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

cells with a velocity greater than 400 m s
-1
. Despite its apparent crudeness, this technique is proving to be the most
effective way of transforming plants, especially important crop species such as soybean, corn, wheat, and rice. The gene-
gun technique affords an opportunity to develop genetically modified organisms (GMOs) with beneficial characteristics.
Such characteristics could include the ability to grow in poor soils, resistance to natural climatic variation, resistance to
pests, and nutritional fortification. These crops might be most useful in developing countries. The use of genetically
modified organisms is highly controversial at this point because of fears of unexpected side effects.
The first GMO to come to market was a tomato characterized by delayed ripening, rendering it ideal for shipment. Pectin
is a polysaccharide that gives tomatoes their firmness and is naturally destroyed by the enzyme polygalacturonase. As
pectin is destroyed, the tomatoes soften, making shipment difficult. DNA was introduced that disrupts the
polygalacturonase gene. Less of the enzyme was produced, and the tomatoes stayed fresh longer. However, the tomato's
poor taste hindered its commercial success.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
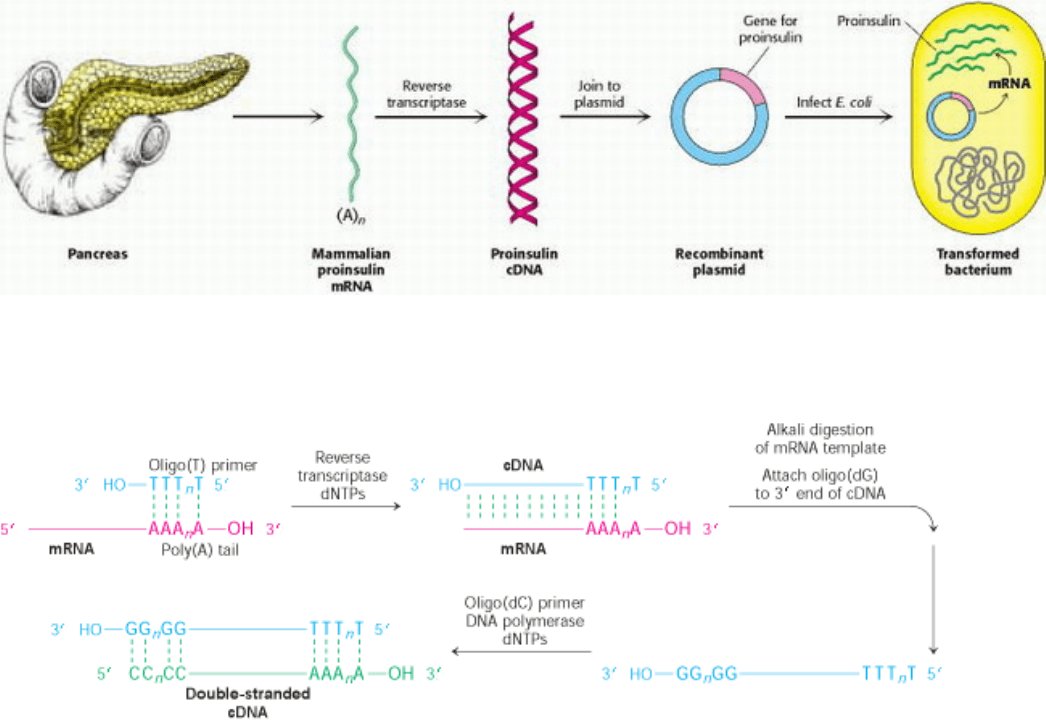
Figure 6.23. Synthesis of Proinsulin by Bacteria. Proinsulin, a precursor of insulin, can be synthesized by transformed
(genetically altered) clones of E. coli. The clones contain the mammalian proinsulin gene.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.24. Formation of a cDNA Duplex. A cDNA duplex is created from mRNA by using reverse transcriptase to
synthesize a cDNA strand, first along the mRNA template and then, after digestion of the mRNA, along that same newly
synthesized cDNA strand.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
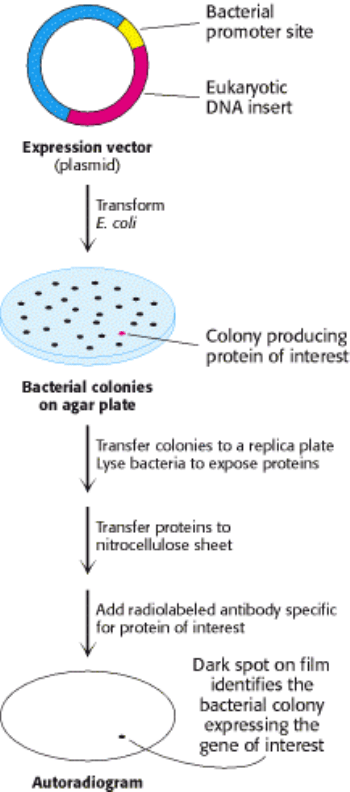
Figure 6.25. Screening of cDNA Clones. A method of screening for cDNA clones is to identify expressed products by
staining with specific antibody.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
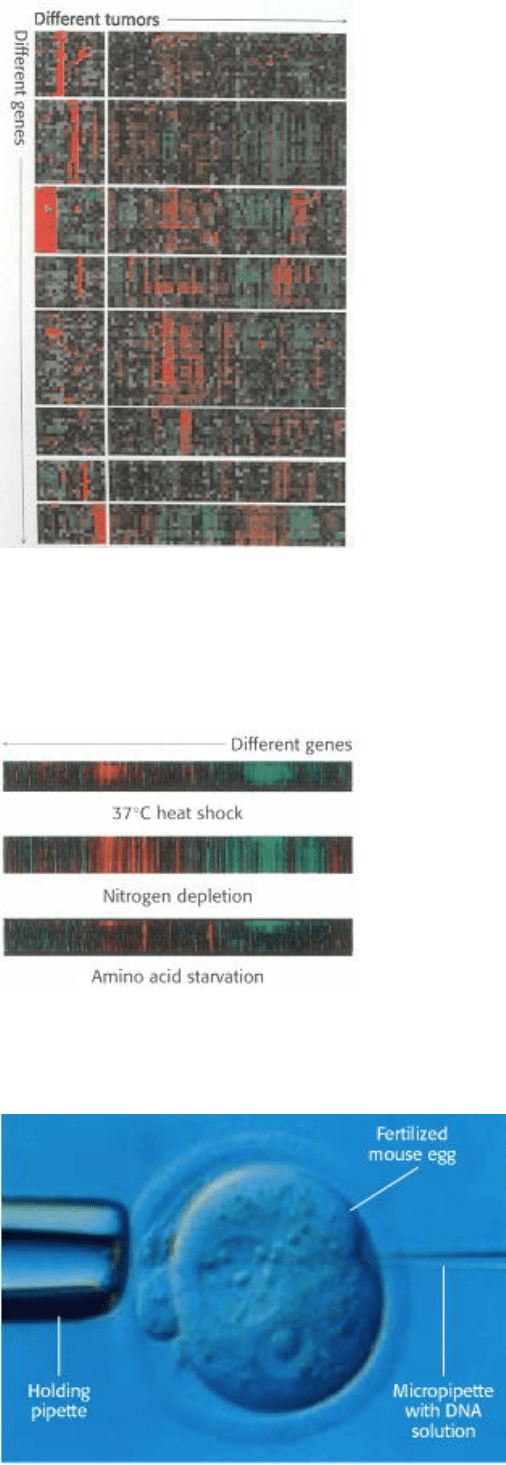
Figure 6.26. Gene Expression Analysis Using Microarrays. The expression levels of thousands of genes can be
simultaneously analyzed using DNA microarrays (gene chips). Here, analysis of 1733 genes in 84 breast tumor samples
reveals that the tumors can be divided into distinct classes based on their gene expression patterns. Red corresponds to
gene induction and green corresponds to gene repression. [Adapted from C. M. Perou et al., Nature 406(2000):747.]
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.27. Monitoring Changes in Yeast Gene Expression. This microarray analysis shows levels of gene
expression for yeast genes under different conditions. [Adapted from Iyer et al., Nature 409(2000):533.]
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
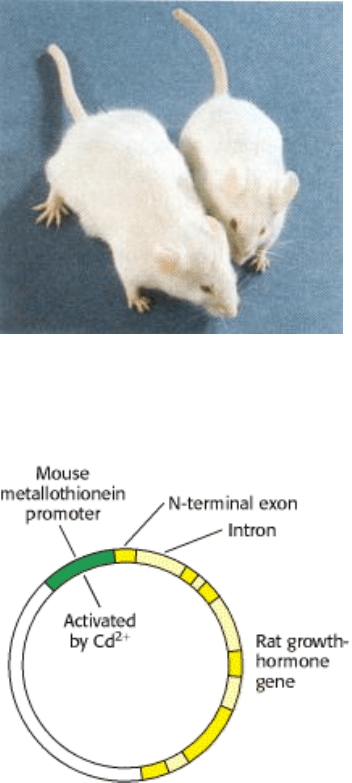
Figure 6.28. Microinjection of DNA. Cloned plasmid DNA is being microinjected into the male pronucleus of a
fertilized mouse egg.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.29. Transgenic Mice. Injection of the gene for growth hormone into a fertilized mouse egg gave rise to a giant
mouse (left), about twice the weight of his silbling (right). [Courtesy of Dr. Ralph Brinster.]
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.30. Rat Growth Hormone-Metallothionein Gene Construct. The gene for rat growth hormone (shown in
yellow) was inserted into a plasmid next to the metallothionein promoter, which is activated by the addition of heavy
metals, such as cadmium ion.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
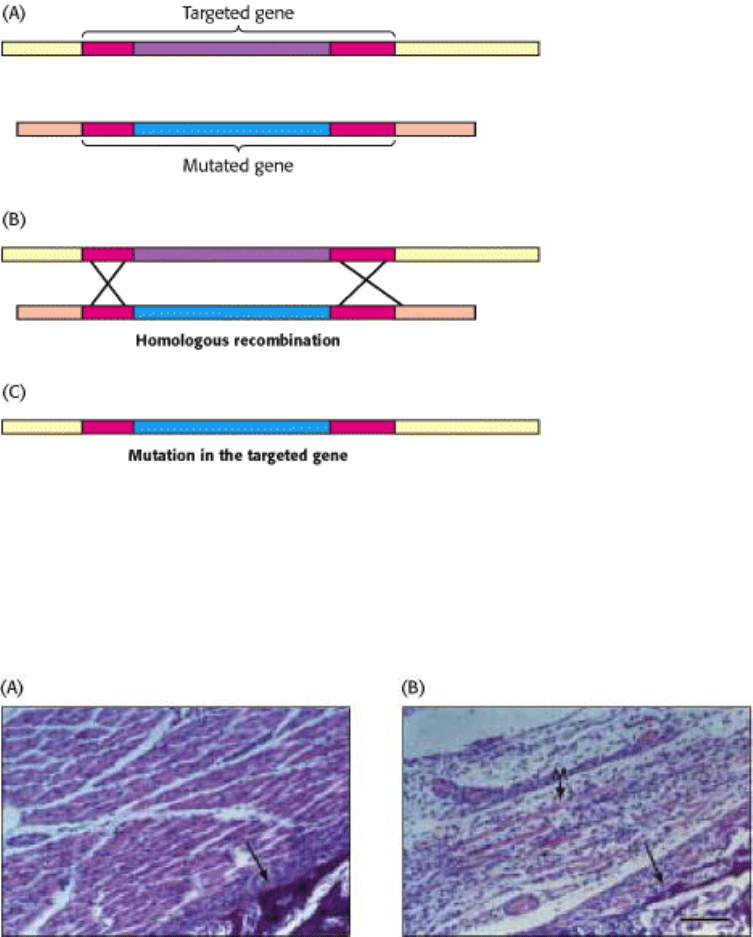
Figure 6.31. Gene Disruption by Homologous Recombination. (A) A mutated version of the gene to be disrupted is
constructed, maintaining some regions of homology with the normal gene (red). When the foreign mutated gene is
introduced into an embryonic stem cell, (B) recombination takes place at regions of homology and (C) the normal
(targeted) gene is replaced, or "knocked out," by the foreign gene The cell is inserted into embryos, and mice lacking the
gene (knockout mice) are produced.
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.32. Consequences of Gene Disruption. Sections of muscle from normal (A) and gene-disrupted (B) mice, as
viewed under the light microscope. Muscles do not develop properly in mice having both myogenin genes disrupted.
[From P. Hasty, A. Bradley, J. H. Morris, D. G. Edmondson, J. M. Venuti, E. N. Olson, and W. H. Klein, Nature 364
(1993):501.]
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
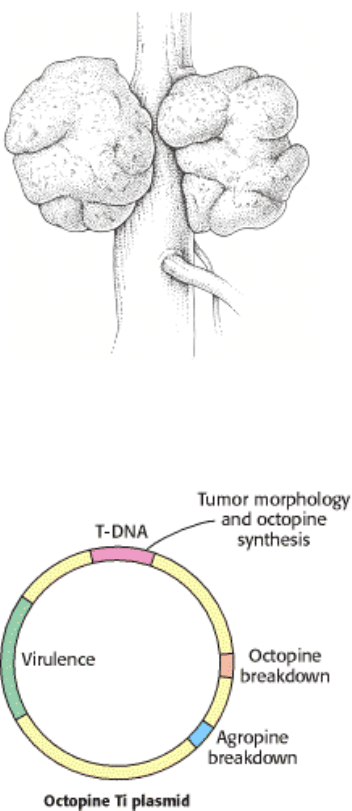
Figure 6.33. Tumors in Plants. Crown gall, a plant tumor, is caused by a bacterium (Agrobacterium tumefaciens) that
carries a tumor-inducing plasmid (Ti plasmid).
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
Figure 6.34. Ti Plasmids. Agrobacteria containing Ti plasmids can deliver foreign genes into some plant cells. [After
M. Chilton. A vector for introducing new genes into plants. Copyright ©1983 by Scientific American, Inc. All rights
reserved.]
I. The Molecular Design of Life 6. Exploring Genes 6.3. Manipulating the Genes of Eukaryotes
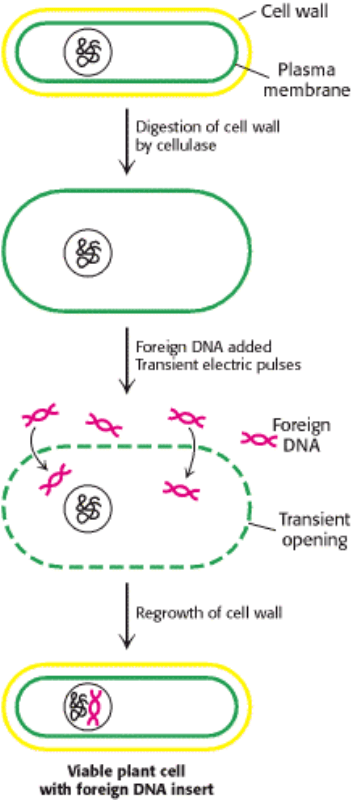
Figure 6.35. Electroporation. Foreign DNA can be introduced into plant cells by electroporation, the application of
intense electric fields to make their plasma membranes transiently permeable.
I. The Molecular Design of Life 6. Exploring Genes
6.4. Novel Proteins Can Be Engineered by Site-Specific Mutagenesis
Much has been learned about genes and proteins by analyzing mutated genes selected from the repertoire offered by
nature. In the classic genetic approach, mutations are generated randomly throughout the genome, and those exhibiting a
particular phenotype are selected. Analysis of these mutants then reveals which genes are altered, and DNA sequencing
identifies the precise nature of the changes. Recombinant DNA technology now makes it feasible to create specific
mutations in vitro.
6.4.1. Proteins with New Functions Can Be Created Through Directed Changes in DNA
We can construct new genes with designed properties by making three kinds of directed changes: deletions, insertions,
and substitutions.
Deletions.
A specific deletion can be produced by cleaving a plasmid at two sites with a restriction enzyme and ligating to form a
smaller circle. This simple approach usually removes a large block of DNA. A smaller deletion can be made by cutting a
plasmid at a single site. The ends of the linear DNA are then digested with an exonuclease that removes nucleotides from

both strands. The shortened piece of DNA is then ligated to form a circle that is missing a short length of DNA about the
restriction site.
Substitutions: Oligonucleotide-Directed Mutagenesis.
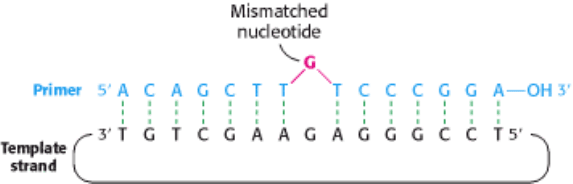
Mutant proteins with single amino acid substitutions can be readily produced by oligonucleotide-directed mutagenesis
(Figure 6.36). Suppose that we want to replace a particular serine residue with cysteine. This mutation can be made if (1)
we have a plasmid containing the gene or cDNA for the protein and (2) we know the base sequence around the site to be
altered. If the serine of interest is encoded by TCT, we need to change the C to a G to get cysteine, which is encoded by
TGT. This type of mutation is called a point mutation because only one base is altered. The key to this mutation is to
prepare an oligonucleotide primer that is complementary to this region of the gene except that it contains TGT instead of
TCT. The two strands of the plasmid are separated, and the primer is then annealed to the complementary strand. The
mismatch of 1 base pair of 15 is tolerable if the annealing is carried out at an appropriate temperature. After annealing to
the complementary strand, the primer is elongated by DNA polymerase, and the double-stranded circle is closed by
adding DNA ligase. Subsequent replication of this duplex yields two kinds of progeny plasmid, half with the original
TCT sequence and half with the mutant TGT sequence. Expression of the plasmid containing the new TGT sequence
will produce a protein with the desired substitution of serine for cysteine at a unique site. We will encounter many
examples of the use of oligonucleotide-directed mutagenesis to precisely alter regulatory regions of genes and to produce
proteins with tailor-made features.
Insertions: Cassette Mutagenesis.
In another valuable approach, cassette mutagenesis, plasmid DNA is cut with a pair of restriction enzymes to remove a
short segment (Figure 6.37). A synthetic double-stranded oligonucleotide (the cassette) with cohesive ends that are
complementary to the ends of the cut plasmid is then added and ligated. Each plasmid now contains the desired mutation.
It is convenient to introduce into the plasmid unique restriction sites spaced about 40 nucleotides apart so that mutations
can be readily made anywhere in the sequence.
Designer Genes.
Novel proteins can also be created by splicing together gene segments that encode domains that are not associated in
nature. For example, a gene for an antibody can be joined to a gene for a toxin to produce a chimeric protein that kills
cells that are recognized by the antibody. These immunotoxins are being evaluated as anticancer agents. Entirely new
genes can be synthesized de novo by the solid-phase method. Furthermore, noninfectious coat proteins of viruses can be
produced in large amounts by recombinant DNA methods. They can serve as synthetic vaccines that are safer than
conventional vaccines prepared by inactivating pathogenic viruses. A subunit of the hepatitis B virus produced in yeast is
proving to be an effective vaccine against this debilitating viral disease.
6.4.2. Recombinant DNA Technology Has Opened New Vistas
Recombinant DNA technology has revolutionized the analysis of the molecular basis of life. Complex chromosomes are
being rapidly mapped and dissected into units that can be manipulated and deciphered. The amplification of genes by
cloning has provided abundant quantities of DNA for sequencing. Genes are now open books that can be read. New
insights are emerging, as exemplified by the discovery of introns in eukaryotic genes. Central questions of biology, such
as the molecular basis of development, are now being fruitfully explored. DNA and RNA sequences provide a wealth of
information about evolution. Biochemists now move back and forth between gene and protein and feel at home in both
areas of inquiry.
Analyses of genes and cDNA can reveal the existence of previously unknown proteins, which can be isolated and
purified (Figure 6.38A). Conversely, purification of a protein can be the starting point for the isolation and cloning of its
gene or cDNA (Figure 6.38B). Very small amounts of protein or nucleic acid suffice because of the sensitivity of
recently developed microchemical techniques and the amplification afforded by gene cloning and the polymerase chain
reaction. The powerful techniques of protein chemistry, nucleic acid chemistry, immunology, and molecular genetics are
highly synergistic.
New kinds of proteins can be created by altering genes in specific ways. Site-specific mutagenesis opens the door to
understanding how proteins fold, recognize other molecules, catalyze reactions, and process information. Large amounts
of protein can be obtained by expressing cloned genes or cDNAs in bacteria or eukaryotic cells. Hormones, such as
insulin, and antiviral agents, such as interferon, are being produced by bacteria. Tissue plasminogen activator, which is
administered to a patient after a heart attack, is made in large quantities in mammalian cells. A new pharmacology, using
proteins produced by recombinant DNA technology as drugs, is beginning to significantly alter the practice of medicine.
Recombinant DNA technology is also providing highly specific diagnostic reagents, such as DNA probes for the
detection of genetic diseases, infections, and cancers. Human gene therapy has been successfully initiated. White blood
cells deficient in adenosine deaminase, an essential enzyme, are taken from patients and returned after being transformed
in vitro to correct the genetic error. Agriculture, too, is benefiting from genetic engineering. Transgenic crops with
increased resistance to insects, herbicides, and drought have been produced.
I. The Molecular Design of Life 6. Exploring Genes 6.4. Novel Proteins Can Be Engineered by Site-Specific Mutagenesis
Figure 6.36. Oligonucleotide-Directed Mutagenesis. A primer containing a mismatched nucleotide is used to produce
a desired change in the DNA sequence.
I. The Molecular Design of Life 6. Exploring Genes 6.4. Novel Proteins Can Be Engineered by Site-Specific Mutagenesis
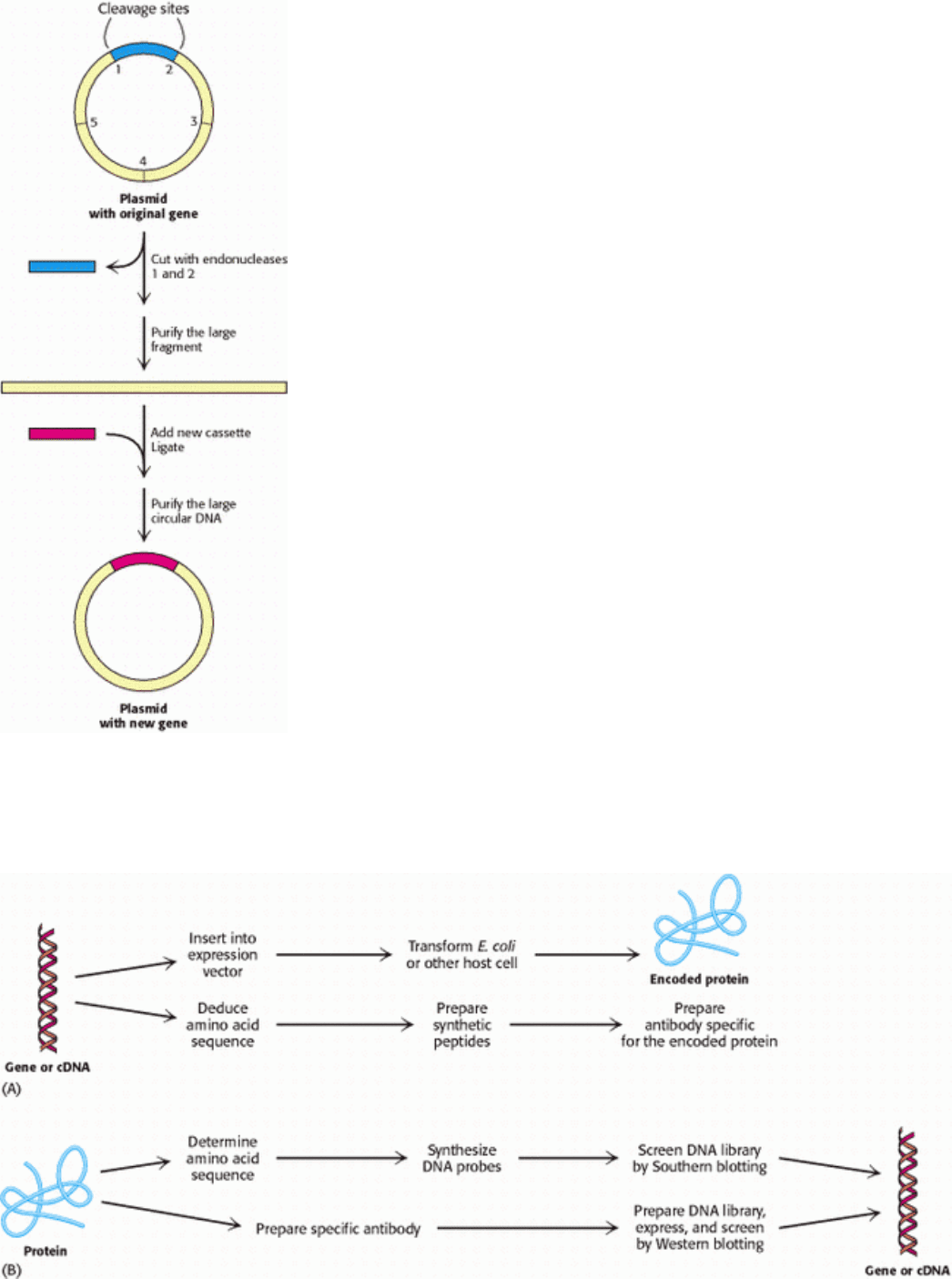
Figure 6.37. Cassette Mutagenesis. DNA is cleaved at a pair of unique restriction sites by two different restriction
endonuclease. A synthetic oligonucleotide with ends that are complementary to these sites (the cassette) is then ligated to
the cleaved DNA. The method is highly versatile because the inserted DNA can have any desired sequence.
I. The Molecular Design of Life 6. Exploring Genes 6.4. Novel Proteins Can Be Engineered by Site-Specific Mutagenesis
Figure 6.38. The Techniques of Protein Chemistry and Nucleic Acid Chemistry Are Mutually Reinforcing. (A)
