Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


proteins are shown in Table 13.1.
2. Ion channels exist in open and closed states. These channels undergo transitions from the closed state, incapable of
supporting ion transport, to the open state, through which ions can flow.
3. Transitions between the open and the closed states are regulated. Ion channels are divided into two classes: ligand-
gated channels and voltage-gated channels. Ligand-gated channels open and close in response to the binding of specific
chemicals, whereas voltage-gated channels open and close in response to the electrical potential across the membrane in
which they are found.
4. Open states of channels often spontaneously convert into inactivated states. Most ion channels do not remain in an
open state indefinitely but, instead, spontaneously transform into inactivated states that do not conduct ions. The
spontaneous transitions of ion channels from open to inactivated states act as built-in timers that determine the duration
of ion flow.
13.5.1. Patch-Clamp Conductance Measurements Reveal the Activities of Single
Channels
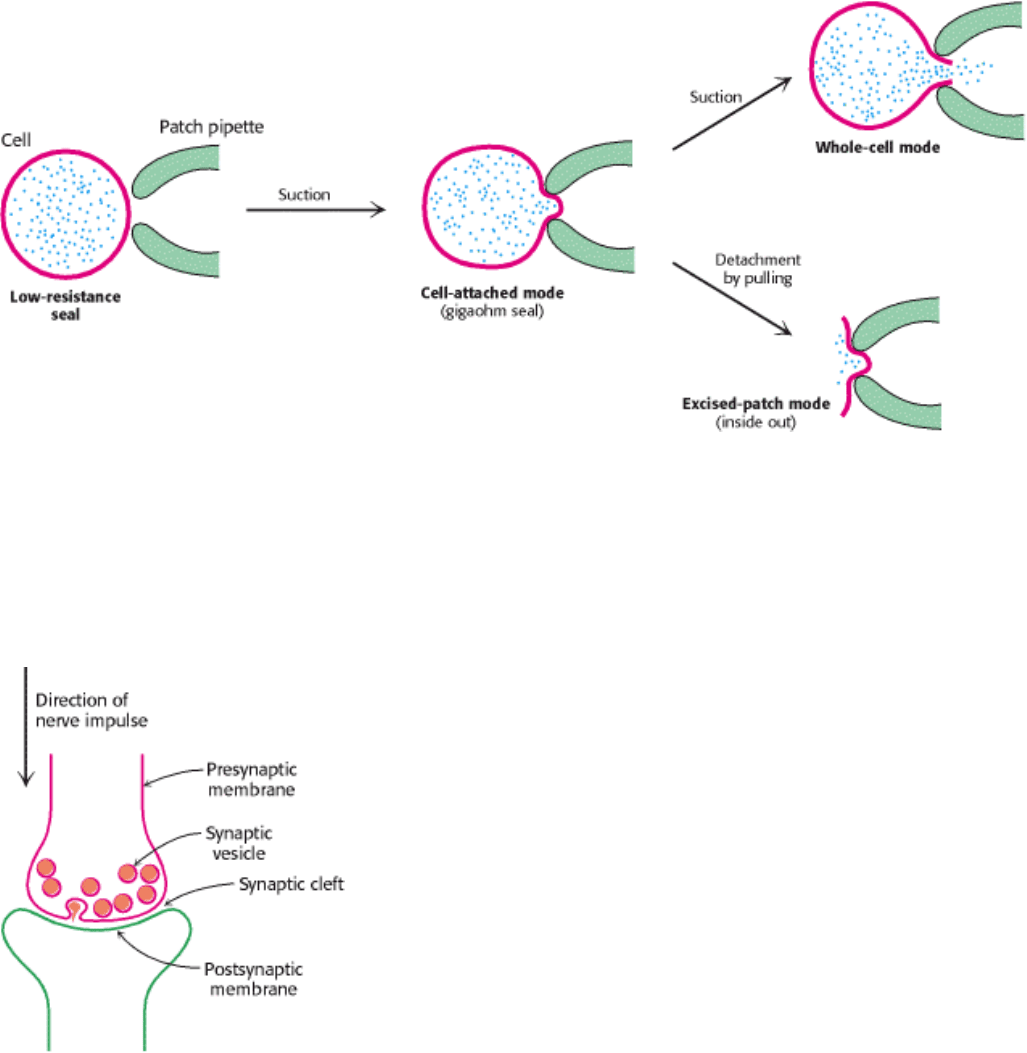
The study of ion channels has been revolutionized by the patch-clamp technique, which was introduced by Erwin Neher
and Bert Sakmann in 1976 (Figure 13.13). This powerful technique enables the measurement of the activity of a single
channel to be measured. A clean glass pipette with a tip diameter of about 1 µm is pressed against an intact cell to form a
seal. Slight suction leads to the formation of a very tight seal so that the resistance between the inside of the pipette and
the bathing solution is many gigaohms (1 gigaohm is equal to 10
9
ohms). Thus, a gigaohm seal (called a gigaseal)
ensures that an electric current flowing through the pipette is identical with the current flowing through the membrane
covered by the pipette. The gigaseal makes possible high-resolution current measurements while a known voltage is
applied across the membrane. In fact, patch clamping increased the precision of such measurements 100-fold. The flow
of ions through a single channel and transitions between the open and closed states of a channel can be monitored with
a time resolution of microseconds. Furthermore, the activity of a channel in its native membrane environment, even in an
intact cell, can be directly observed. Patch-clamp methods provided one of the first views of single biomolecules in
action. Subsequently, other methods for observing single molecules were invented, opening new vistas on biochemistry
at its most fundamental level.
13.5.2. Ion-Channel Proteins Are Built of Similar Units
How do ion channels, vital to a wide array of biological functions, operate at a molecular level? We will examine three
channels important in the propagation of nerve impulses: the ligand-gated channel; the acetylcholine receptor channel,
which communicates the nerve impulse between certain neurons; and the voltage-gated Na
+
and K
+
channels, which
conduct the nerve impulse down the axon of a neuron.
Nerve impulses are communicated across most synapses by small, diffusible molecules called neurotransmitters, of
which one is acetylcholine, referred to as a cholinergic neurotransmitter because it is derived from choline (Section
12.3.1). The presynaptic membrane of a synapse is separated from the postsynaptic membrane by a gap of about 50 nm,
called the synaptic cleft. The end of the presynaptic axon is filled with synaptic vesicles, each containing about 10
4
acetylcholine molecules (Figure 13.14). The arrival of a nerve impulse leads to the synchronous export of the contents of
some 300 vesicles, which raises the acetylcholine concentration in the cleft from 10 nM to 500 µM in less than a
millisecond. The binding of acetylcholine to the postsynaptic membrane markedly changes its ionic permeabilities
(Figure 13.15). The conductance of both Na
+
and K
+
increases greatly within 0.1 ms, leading to a large inward current
of Na
+
and a smaller outward current of K
+
. The inward Na
+
current depolarizes the postsynaptic membrane and
triggers an action potential (Section 13.5.3). Acetylcholine opens a single kind of cation channel, which is almost equally
permeable to Na
+
and K
+
. This change in ion permeability is mediated by the acetylcholine receptor.

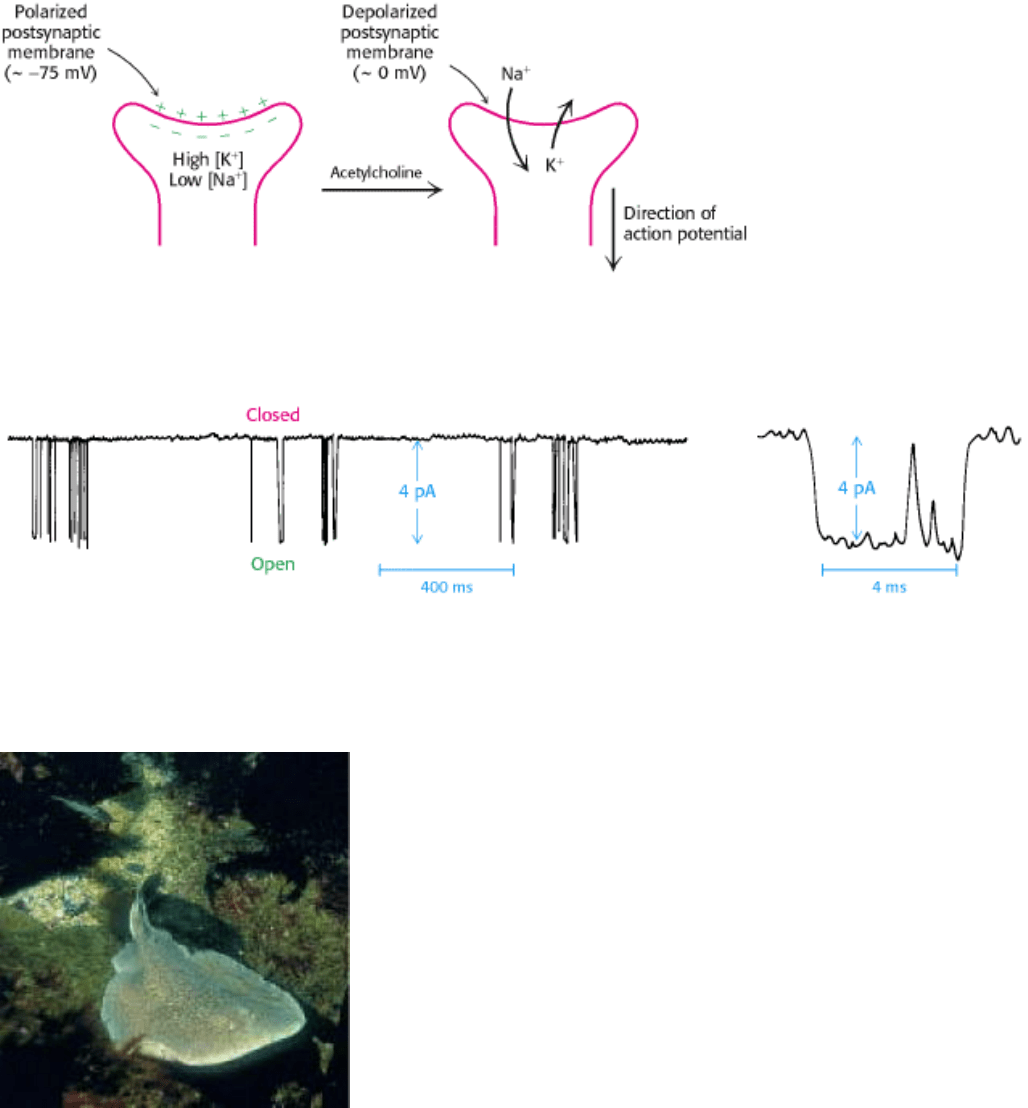
The acetylcholine receptor is the best-understood ligand-gated channel. The activity of a single such channel is
graphically displayed in patch-clamp recordings of postsynaptic membranes of skeletal muscle (Figure 13.16). The
addition of acetylcholine is followed by transient openings of the channel. The current, i, flowing through an open
channel is 4 pA (picoamperes) when the membrane potential, V, is -100 mV. An ampere is the flow of 6.24 × 10
18
charges per second. Hence, 2.5 × 10
7
ions per second flow through an open channel.
The electric organ of Torpedo marmorata, an electric fish, is a choice source of acetylcholine receptors for study
because its electroplaxes (voltage-generating cells) are very rich in cholinergic postsynaptic membranes. The receptor is
very densely packed in these membranes (~20,000/µm
2
). An exotic biological material has been invaluable in the
isolation of acetylcholine receptors. Snake neurotoxins such as α -bungarotoxin (from the venom of a Formosan snake)
and cobratoxin block the transmission of impulses between nerve and muscle. These small (7-kd) basic proteins bind
specifically and very tightly to acetylcholine receptors and hence can be used as tags.
The acetylcholine receptor of the electric organ has been solubilized by adding a nonionic detergent to a
postsynaptic membrane preparation and purified by affinity chromatography on a column bearing covalently
attached cobratoxin. With the use of techniques presented in Chapter 4, the 268-kd receptor was identified as a pentamer
of four kinds of membrane-spanning subunits
α
2
, β, γ, and δ arranged in the form of a ring that creates a pore
through the membrane (Figure 13.17). The cloning and sequencing of the cDNAs for the four kinds of subunits (50 58
kd) showed that they have clearly similar sequences; the genes for the α, β, γ, and δ subunits arose by duplication and
divergence of a common ancestral gene. Each subunit has a large extracellular domain, followed at the carboxyl end by
four predominantly hydrophobic segments that span the bilayer membrane. Acetylcholine binds at the α
γ and α δ
interfaces. Electron microscopic studies of purified acetylcholine receptors demonstrated that the structure has
approximately fivefold symmetry, in harmony with the similarity of its five constituent subunits.
What is the basis of channel opening? A comparison of the structures of the closed and open forms of the channel would
be highly revealing, but such comparisons have been difficult to obtain. Cryoelectron micrographs indicate that the
binding of acetylcholine to the extracellular domain causes a structural alteration, which initiates rotations of the α-
helical rods lining the membrane-spanning pore. The amino acid sequences of these helices point to the presence of
alternating ridges of small polar or neutral residues (serine, threonine, glycine) and large nonpolar ones (isoleucine,
leucine, phenylalanine). In the closed state, the large residues may occlude the channel by forming a tight hydrophobic
ring (Figure 13.18). Indeed, each subunit has a bulky leucine residue at a critical position. The binding of acetylcholine
could allosterically rotate the membrane-spanning helices so that the pore would be lined by small polar residues rather
than by large hydrophobic ones. The wider, more polar pore would then be open to the passage of Na
+
and K
+
ions.
13.5.3. Action Potentials Are Mediated by Transient Changes in Na
+
and K
+
Permeability
We turn now from ligand-gated channels to voltage-gated channels, which are responsible for the propagation of nerve
impulses. A nerve impulse is an electrical signal produced by the flow of ions across the plasma membrane of a neuron
and is the fundamental means of communication in the nervous system. The interior of a neuron, like that of most other
cells, has a high concentration of K
+
and a low concentration of Na
+
. These ionic gradients are generated by an ATP-
driven pump (Section 13.2.1). In the resting state, the membrane potential is -60 mV. A nerve impulse, or action
potential, is generated when the membrane potential is depolarized beyond a critical threshold value (i.e., from -60 to -40
mV). The membrane potential becomes positive within about a millisecond and attains a value of about +30 mV before
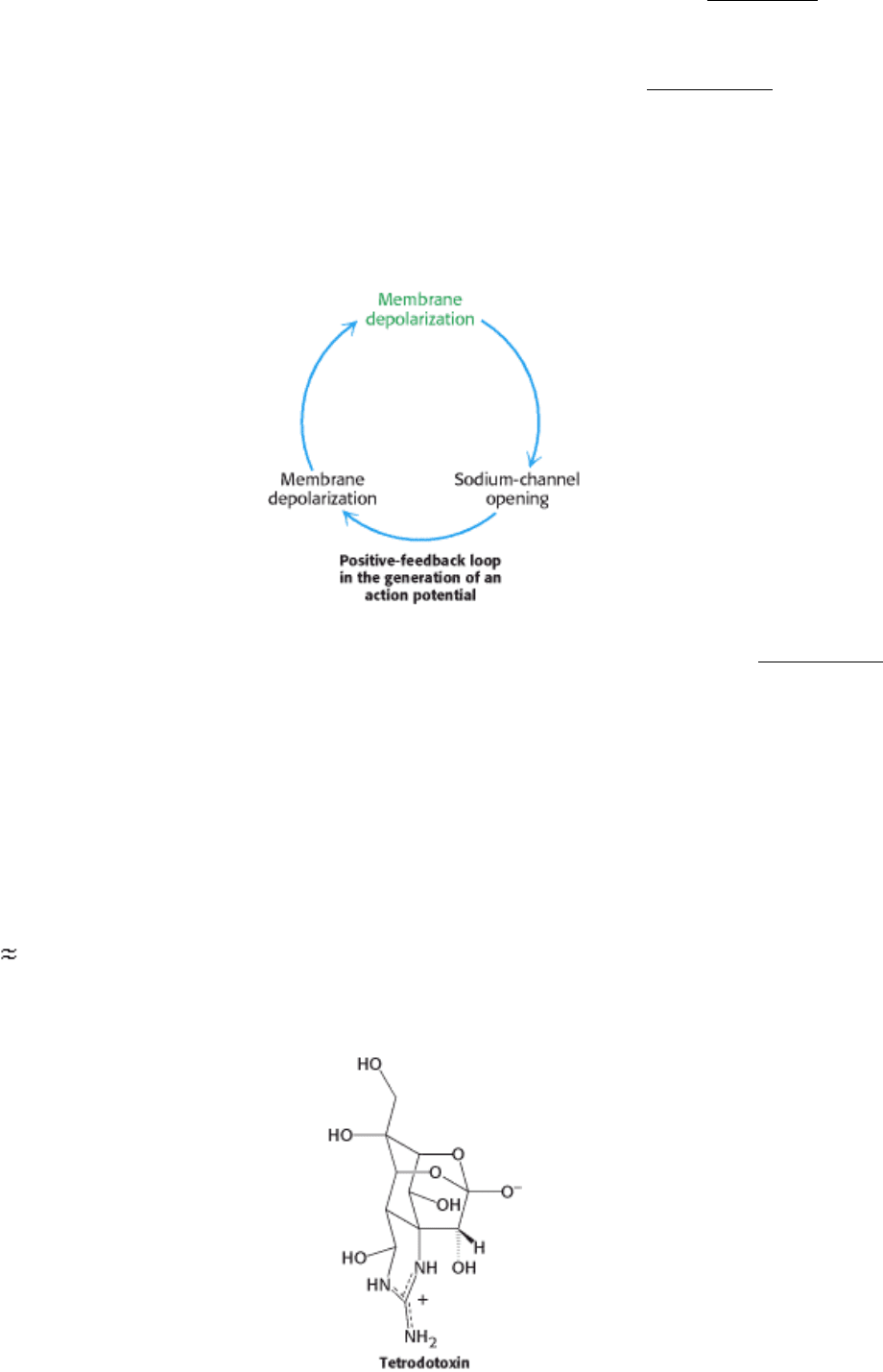
turning negative again. This amplified depolarization is propagated along the nerve terminal (Figure 13.19)
Ingenious experiments carried out by Alan Hodgkin and Andrew Huxley revealed that action potentials arise from large,
transient changes in the permeability of the axon membrane to Na
+
and K
+
ions (see Figure 13.19A). Two kinds of
voltage-sensitive channels, one selectively permeable to Na
+
and the other to K
+
, were defined. The conductance of the
membrane to Na
+
changes first. Depolarization of the membrane beyond the threshold level leads to an opening of Na
+
channels. Sodium ions begin to flow into the cell because of the large electrochemical gradient across the plasma
membrane. The entry of Na
+
further depolarizes the membrane, and so more gates for Na
+
are opened. This positive
feedback between depolarization and Na
+
entry leads to a very rapid and large change in membrane potential, from about
-60 mV to +30 mV in a millisecond.
Sodium channels spontaneously close and potassium channels begin to open at about this time (see Figure 13.19B).
Consequently, potassium ions flow outward, and so the membrane potential returns to a negative value. The resting level
of -60 mV is restored in a few milliseconds as the K
+
conductance decreases to the value characteristic of the
unstimulated state. Only a very small proportion of the sodium and potassium ions in a nerve cell, of the order of one in a
million, flows across the plasma membrane during the action potential. Clearly, the action potential is a very efficient
means of signaling over large distances.
13.5.4. The Sodium Channel Is an Example of a Voltage-Gated Channel
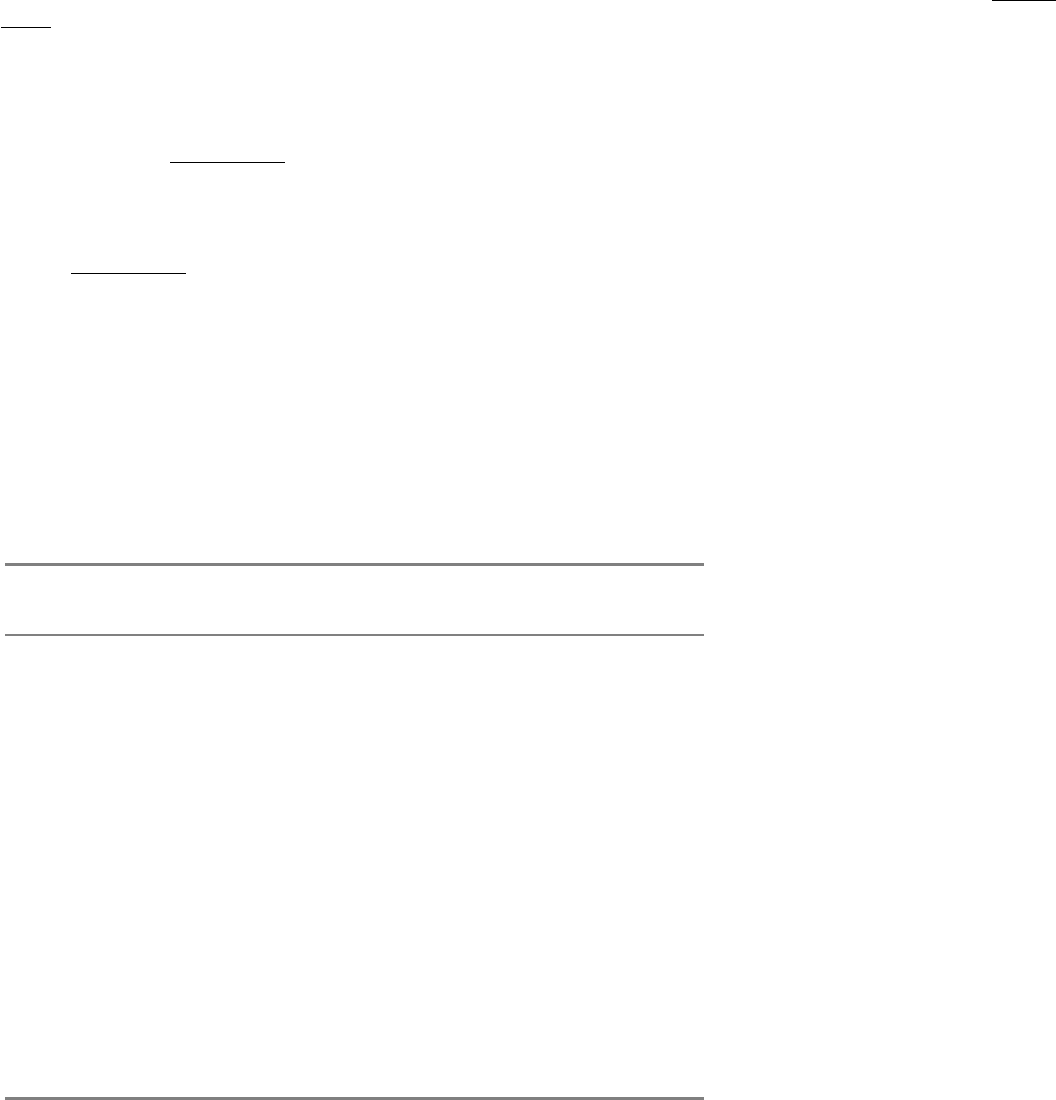
Like the acetylcholine receptor channel, the sodium channel also was purified on the basis of its ability to bind a specific
neurotoxin. Tetrodotoxin, an organic compound isolated from the puffer fish, binds to sodium channels with great
avidity (K
i
1 nM). The lethal dose of this poison for an adult human being is about 10 ng. The sodium channel was
first purified from the electric organ of electric eel, which is a rich source of the protein forming this channel. The
isolated protein is a single chain of 260 kd.

The availability of purified protein enabled Shosaku Numa and coworkers to clone and sequence the cDNA for the
sodium channel from the electroplax cells of the eel electric organ and then from the rat. Subsequently, a large
number of sodium channel cDNAs have been cloned from other sources, and sequence comparisons have been made.
The eel and rat cDNA sequences are approximately 61% identical, which indicates that the amino acid sequence of the
sodium channel has been conserved over a long evolutionary period. Most interesting, the channel contains four internal
repeats, or homology units, having similar amino acid sequences, suggesting that gene duplication and divergence have
produced the gene for this channel. Hydrophobicity profiles indicate that each homology unit contains five hydrophobic
segments (S1, S2, S3, S5, and S6). Each repeat also contains a highly positively charged S4 segment; arginine or lysine
residues are present at nearly every third residue. Numa proposed that segments S1 through S6 are membrane-spanning
α helices (Figure 13.20). The positively charged residues in S4 segments act as the voltage sensors of the channel. The
purification of calcium channels and the subsequent cloning and sequencing of their cDNAs revealed that these proteins
are homologous to the sodium channels and have quite similar architectures; each protein comprises four imperfectly
repeated units, each of which has regions corresponding to segments S1 through S6.
We can thus note similarities between ligand-gated and voltage-gated channels. Like the acetylcholine receptor, the
sodium channel is constructed of similar units. The acetylcholine receptor has five units, whereas the sodium channel has
four units that have been fused into a single polypeptide chain. The acetylcholine receptor is composed of similar but
noncovalently attached subunits.
13.5.5. Potassium Channels Are Homologous to the Sodium Channel
The purification of potassium channels proved to be much more difficult because of their low abundance and the
lack of known high-affinity ligands comparable to tetrodotoxin. The breakthrough came in studies of mutant fruit
flies that shake violently when anesthetized with ether. The mapping and cloning of the gene, termed shaker, responsible
for this defect revealed the amino acid sequence encoded by a potassiumchannel gene. The availability of this gene
sequence has led to the cloning of potassium-channel cDNAs from many other organisms. Shaker cDNA encodes a 70-
kd protein that has regions that correspond to one of the homology units of the sodium channel containing the membrane-
spanning segments S1 through S6. Thus, a potassium-channel subunit is homologous to one of the repeated homology
units of the sodium and calcium channels. Consistent with this hypothesis, four potassium-channel subunits come
together to form a functional channel. Subsequently, other potassium channels were discovered, including some from
bacteria, which contain only the two membrane-spanning regions corresponding to segments S5 and S6. This and other
information pointed to the region between S5 and S6 as a key component of the ion-channel pore in the potassium
channel and in the sodium and calcium channels as well. The sequence relationships between these ion channels are
summarized in Figure 13.21.
13.5.6. The Structure of a Potassium Channel Reveals the Basis of Rapid Ion Flow with
Specificity
Structural Insights, The Potassium Channel, examines the structural basis of
the potassium channel's ion specificity and high conductivity in further detail.
Scientists were slowly discovering the likely structures of ion channels through a combination of patch-clamp methods,
site-directed mutagenesis, and other methods. However, progress was limited by the lack of a high- resolution three-
dimensional structure. The need was met by the determination of the structure of a bacterial potassium channel by x-ray
crystallography in 1998. The resulting structural framework is a source of insight into many aspects of ion-channel
function, including specificity and rapidity of ion flow.
As expected, the potassium channel is a tetramer of identical subunits, each of which includes two membrane-spanning
α helices. The four subunits come together to form a pore in the shape of a cone that runs through the center of the
structure (Figure 13.22). Beginning from the inside of the cell, the pore starts with a diameter of approximately 10 Å and
then constricts to a smaller cavity with a diameter of 8 Å. Both the opening to the outside and the central cavity of the

pore are filled with water, and a K
+
ion can fit in the pore without losing its shell of bound water molecules.
Approximately two-thirds of the way through the membrane, the pore becomes more constricted (3-Å diameter). At that
point, any K
+
ions must give up their water molecules and interact directly with groups from the protein. The channel
structure effectively reduces the thickness of the membrane from 34 Å to 12 Å by allowing the solvated ions to penetrate
into the membrane before the ions must directly interact with the channel (Figure 13.23).
For potassium ions to relinquish their water molecules, other polar interactions must replace those with water. The
restricted part of the pore is built from residues between the two transmembrane helices (which correspond to segments
S5 and S6 in the sodium channel). In particular, a five-amino-acid stretch within this region functions as the selectivity
filter that determines the preference for K
+
over other ions (Figure 13.24). The stretch has the sequence Thr-Val-Gly-Tyr-
Gly, which is nearly completely conserved in all K
+
channels and had already been identified as a signature sequence
useful for identifying potential K
+
channels. This region lies in a relatively extended conformation and is oriented such
that the peptide carbonyl groups are directed into the channel, facilitating interaction with the potassium ions.
Potassium channels are 100-fold as permeable to K
+
as to Na
+
. How is this high degree of selectivity achieved? The
narrow diameter (3 Å) of the selectivity filter of the potassium channel enables the filter to reject ions having a radius
larger than 1.5 Å. However, a bare Na
+
is small enough (Table 13.2) to pass through the pore. Indeed, the ionic radius of
Na
+
is substantially smaller than that of K
+
. How then is Na
+
rejected?
We need to consider the free-energy cost of dehydrating the Na
+
and K
+
ions, given that they cannot pass through this
part of the channel bearing a retinue of water molecules. The key point is that the free-energy costs of dehydrating these
ions are considerable [Na
+
, 72 kcal mol
-1
(301 kJ mol
-1
), and K
+
, 55 kcal mol
-1
(203 kJ mol
-1
)]. The channel pays the
cost of dehydrating K
+
by providing compensating interactions with the carbonyl oxygen atoms lining the selectivity
filter. However, these oxygen atoms are positioned such that they do not interact very favorably with Na
+
, because it is
too small (Figure 13.25). The higher cost of dehydrating Na
+
would be unrecovered, and so Na
+
would be rejected. The
ionic radii of oxygen, potassium, and sodium are 1.4, 1.33, and 0.95 Å, respectively. Hence a ring of oxygen atoms
positioned so that the K
+
O distance is 2.73 Å (1.4 + 1.33 Å) would be optimal for interaction with K
+
compared with
the shorter Na
+
O bonds (0.95 + 1.4 = 2.35 Å) optimal for interaction with Na
+
. Thus, the potassium channel avoids
closely embracing Na
+
ions, which must stay hydrated and hence are impermeant.
13.5.7. The Structure of the Potassium Channel Explains Its Rapid Rates of Transport
In addition to selectivity, ion channels display rapid rates of ion transport. A structural analysis provides an appealing
explanation for this proficiency. The results of such studies revealed the presence of two potassium-binding sites in the
constricted regions of the potassium channel that are crucial for rapid ion flow. Consider the process of ion conductance.
One K
+
ion proceeds into the channel and through the relatively unrestricted part of the channel. It then gives up most or
all of its coordinated water molecules and binds to the first site in the selectivity filter region, a favorable binding site. It
can then jump to the second site, which appears to have comparable binding energy. However, the binding energy of the
second site presents a free-energy barrier, or trap, preventing the ion from completing its journey; there is no energetic
reason to leave the second ion-binding site. However, if a second ion moves through the channel into the first site, the
electrostatic repulsion between the two ions will destabilize the initially bound ion and help push it into solution (Figure
13.26). This mechanism provides a solution to the apparent paradox of high ion selectivity (requiring tight binding sites)
and rapid flow.
The structure determined for K
+
channels is a good start for considering the amino acid sequence similarities, as
well as the structural and functional relations, for Na
+
and Ca
2+
channels because of their homology to K
+
channels. Sequence comparisons and the results of mutagenesis experiments have also implicated the region between
segments S5 and S6 in ion selectivity in the Ca
2+
channels. In Ca
2+
channels, one glutamate residue of this region in
each of the four units plays a major role in determining ion selectivity. The Na
+
channel's selection of Na
+
over K
+
depends on ionic radius; the diameter of the pore is sufficiently restricted that small ions such as Na
+
and Li
+
can pass
through the channel, but larger ions such as K
+
are significantly hindered (Figure 13.27).
Residues in the positions corresponding to the glutamate residues in Ca
2+
channels are major components of the
selectivity filter of the Na
+
channel. These residues are aspartate, glutamate, lysine, and alanine in units 1, 2, 3, and 4,
respectively (the DEKA locus). Thus, the potential fourfold symmetry of the channel is clearly broken in this region,
providing one explanation of why Na
+
channels comprise single large polypeptide chains rather than a noncovalent
assembly of four identical subunits.
13.5.8. A Channel Can Be Inactivated by Occlusion of the Pore: The Ball-and-Chain
Model
The potassium channel and the sodium channel undergo inactivation within milliseconds of channel opening (Figure
13.28). A first clue to the mechanism of inactivation came from exposing the cytoplasmic side of either channel to
trypsin; cleavage by trypsin produced a trimmed channel that stayed persistently open after depolarization. A second clue
was the finding that alternatively spliced variants of the potassium channel have markedly different inactivation kinetics;
these variants differed from one another only near the amino terminus, which is on the cytoplasmic side of the channel.
A mutant Shaker channel lacking 42 amino acids near the amino terminus opened in response to depolarization but did
not inactivate (see Figure 13.28). Most revealing, inactivation was restored by adding a synthetic peptide corresponding
to the first 20 residues of the native channel.
These experiments strongly support the ball-and-chain model for channel inactivation that had been proposed years
earlier (Figure 13.29). According to this model, the first 20 residues of the potassium channel form a cytoplasmic unit
(the ball) that is attached to a flexible segment of the polypeptide (the chain). When the channel is closed, the ball rotates
freely in the aqueous solution. When the channel opens, the ball quickly finds a complementary site in the pore and
occludes it. Hence, the channel opens for only a brief interval before it undergoes inactivation by occlusion. Shortening
the chain speeds inactivation because the ball finds its target more quickly. Conversely, lengthening the chain slows
inactivation. Thus, the duration of the open state can be controlled by the length and flexibility of the tether.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Table 13.1. Relative permeabilities for selected ion channels
Na
+
channel K
+
channel
Acetylcholine receptor Chloride channel
Li
+
0.93 < 0.01 0.87 < 0.01
Na
+
1.00 < 0.01 1.00 < 0.01
K
+
0.09 1.00 1.11 < 0.01
Rb
+
< 0.01 0.91
Cs
+
< 0.01 < 0.08 1.42
NH
4
+
0.16 0.13 1.79
H
3
NOH
+
0.94 < 0.03 1.92
H
2
NNH
3
+
0.59 < 0.03
H
3
CNH
3
+
< 0.01 < 0.02
Cl
-
< 0.01 < 0.01 < 0.01 1.00
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.13. Patch-Clamp Modes. The patch-clamp technique for monitoring channel activity is highly versatile. A
high-resistance seal (gigaseal) is formed between the pipette and a small patch of plasma membrane. This configuration
is called cell attached. The breaking of the membrane patch by increased suction produces a low-resistance pathway
between the pipette and interior of the cell. The activity of the channels in the entire plasma membrane can be monitored
in this whole-cell mode. To prepare a membrane in the excised-patch mode, the pipette is pulled away from the cell. A
piece of plasma membrane with its cytosolic side now facing the medium is monitored by the patch pipette.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.14. Schematic Representation of a Synapse.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.15. Membrane Depolarization. Acetylcholine depolarizes the postsynaptic membrane by increasing the
conductance of Na
+
and K
+
.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.16. Patch-Clamp Recordings of the Acetylcholine Receptor Channel. Patch-clamp recordings illustrate
changes in the conductance of an acetylcholine receptor channel in the presence of acetylcholine. The channel undergoes
frequent transitions between open and closed states. [Courtesy of Dr. D. Colquhoun and Dr. B. Sakmann.]
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
The torpedo (Torpedo marmorata, also known as the electric ray) has an electric organ, rich in acetylcholine receptors,
that can deliver a shock of as much as 200 V for approximately 1 s. [Yves Gladu/Jacana/ Photo Researchers.]

I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.17. Schematic Representation of the Closed Form of the Acetylcholine Receptor Channel. In the closed
state, the narrowest part of the pore is occluded by side chains coming from five helices. [Courtesy of Dr. Nigel Unwin.]
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.18. Opening of the Acetylcholine Channel Pore. Large hydrophobic side chains (L) occlude the pore of the
closed form of the acetylcholine receptor channel. Channel opening is probably mediated by the tilting of helices that
line the pore. Large residues move away from the pore and small ones (S) take their place. [After N. Unwin. Neuron 3
(1989):665.]
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.19. Membrane Potential. Depolarization of an axon membrane results in an action potential. Time course of
(A) the change in membrane potential and (B) the change in Na
+
and K
+
conductances.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
A puffer fish is regarded as a culinary delicacy in Japan. [Fred Bavendam/ Peter Arnold.]
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
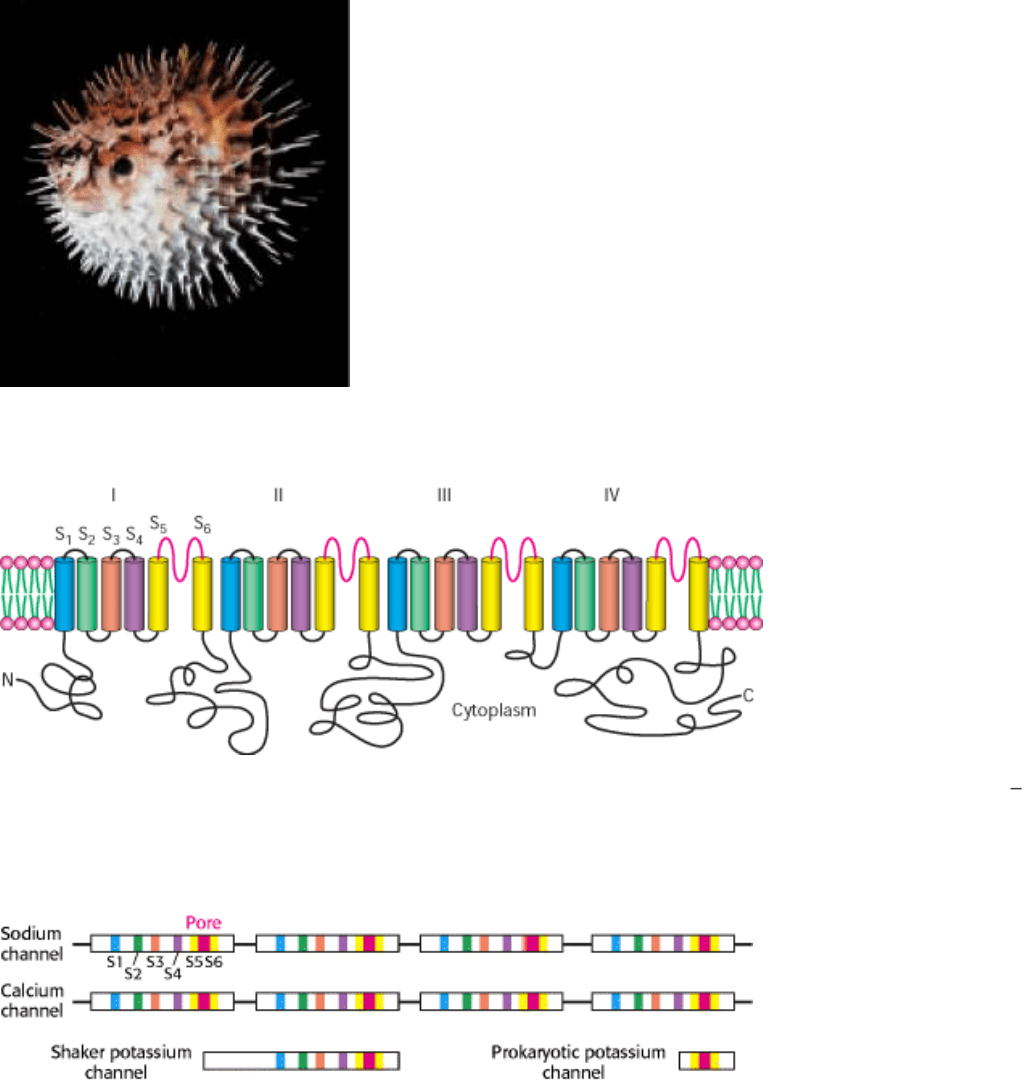
Figure 13.20. The Sodium Channel. The Na
+
channel is a single polypeptide chain with four repeating units (I IV).
Each repeat probably folds into six transmembrane helices. The loops (shown in red) between helices 5 and 6 of each
domain form the pore of the channel.
I. The Molecular Design of Life 13. Membrane Channels and Pumps 13.5. Specific Channels Can Rapidly Transport Ions Across Membranes
Figure 13.21. Sequence Relationships of Ion Channels. Like colors indicate structurally similar regions of the sodium,
calcium, and potassium channels. These channels exhibit approximate fourfold symmetry.
