Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

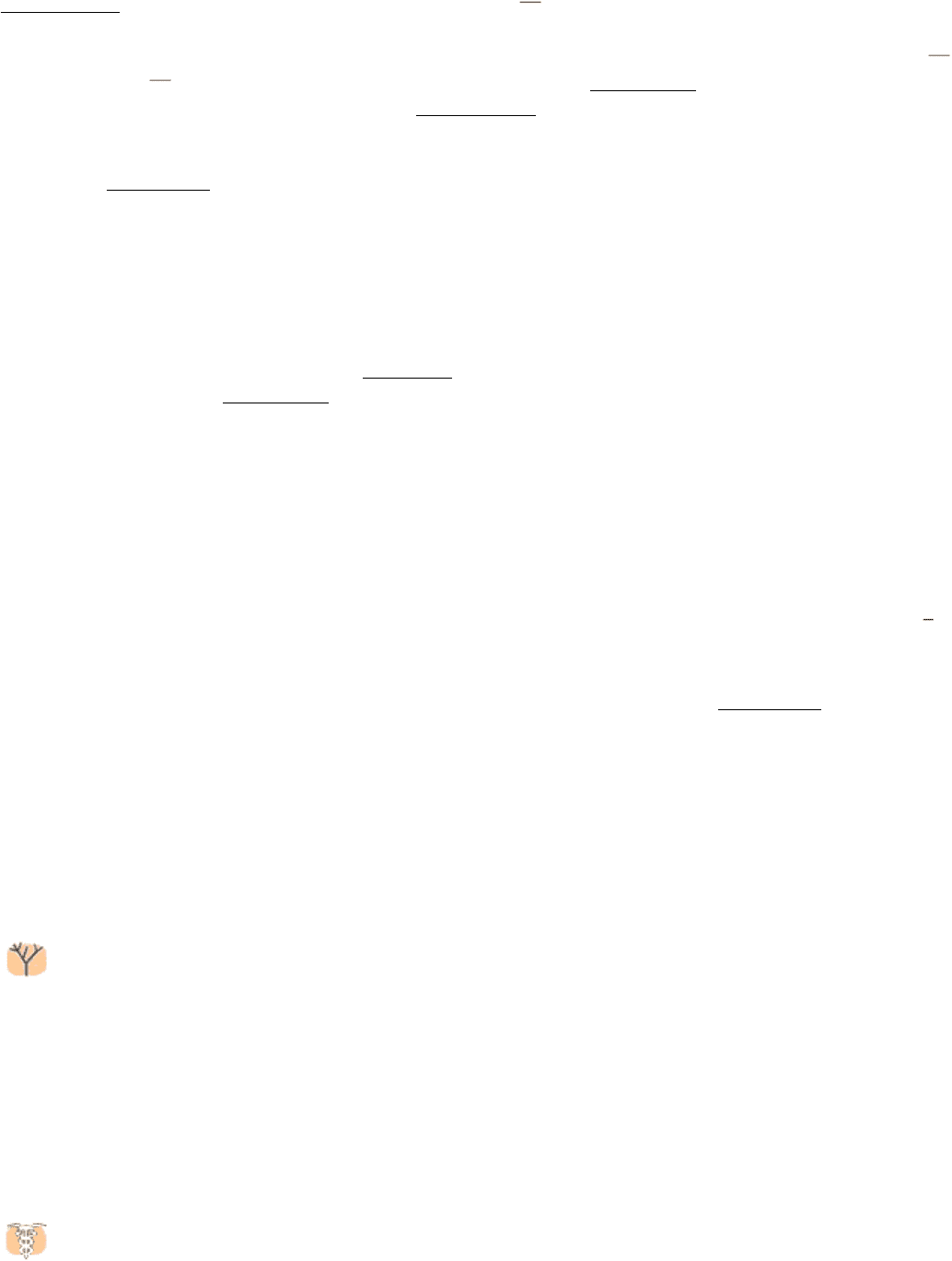
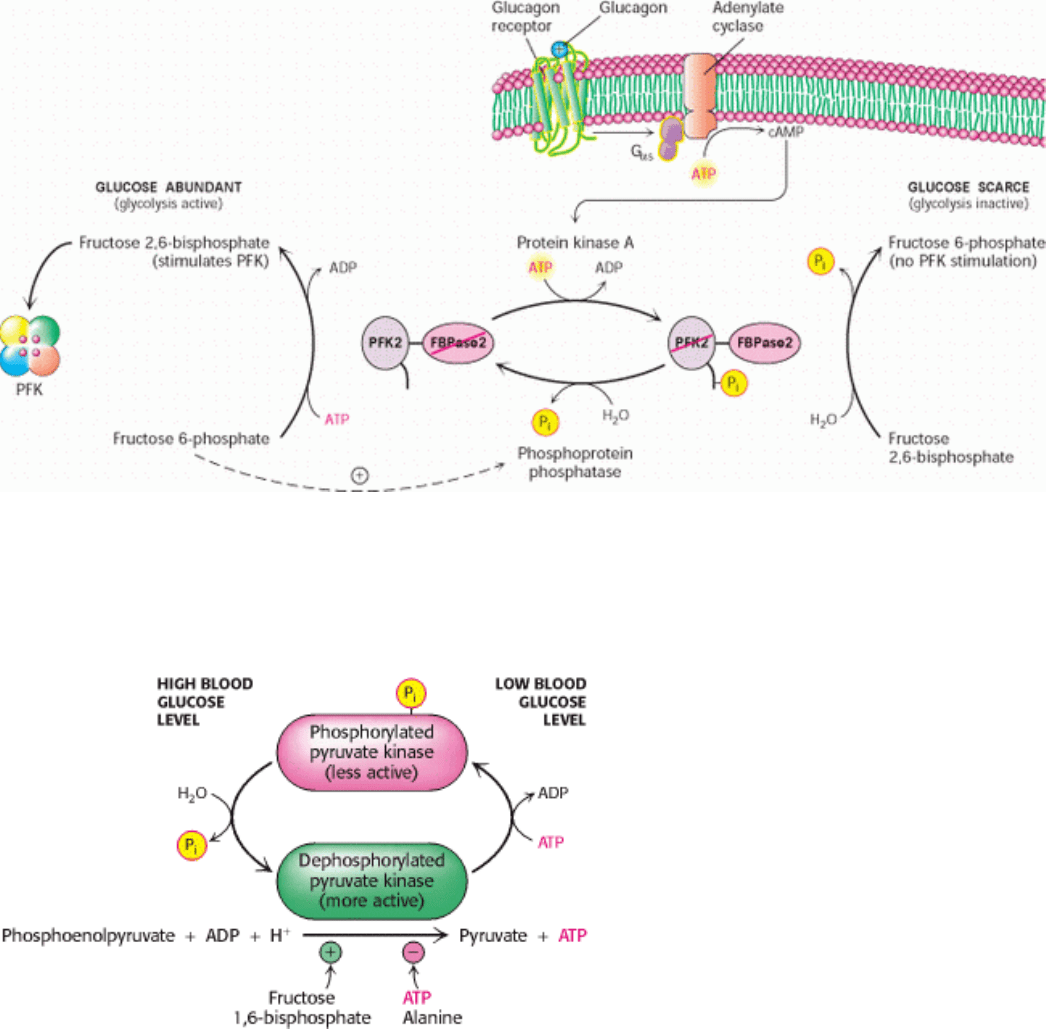
kinase to slow glycolysis when the energy charge is high. Finally, alanine (synthesized in one step from pyruvate,
Section 24.2.2) also allosterically inhibits the pyruvate kinases in this case, to signal that building blocks are abundant.
The isozymic forms differ in their susceptibility to covalent modification. The catalytic properties of the L form
but
not of the M form are also controlled by reversible phosphorylation (Figure 16.21). When the blood-glucose level is
low, the glucagon-triggered cyclic AMP cascade (Section 15.1.5) leads to the phosphorylation of pyruvate kinase, which
diminishes its activity. These hormone-triggered phosphorylations, like that of the bifunctional enzyme controlling the
levels of fructose 2,6-bisphosphate, prevent the liver from consuming glucose when it is more urgently needed by brain
and muscle (Section 30.3). We see here a clear-cut example of how isoenzymes contribute to the metabolic diversity of
different organs. We will return to the control of glycolysis after considering gluconeogenesis.
16.2.4. A Family of Transporters Enables Glucose to Enter and Leave Animal Cells
Several glucose transporters mediate the thermodynamically downhill movement of glucose across the plasma
membranes of animal cells. Each member of this protein family, named GLUT1 to GLUT5, consists of a single
polypeptide chain about 500 residues long (Table 16.4). The common structural theme is the presence of 12
transmembrane segments (Figure 16.22).
The members of this family have distinctive roles:
1. GLUT1 and GLUT3, present in nearly all mammalian cells, are responsible for basal glucose uptake. Their K
M
value
for glucose is about 1 mM, significantly less than the normal serum-glucose level, which typically ranges from 4 mM to
8 mM. Hence, GLUT1 and GLUT3 continually transport glucose into cells at an essentially constant rate.
2. GLUT2, present in liver and pancreatic β cells, is distinctive in having a very high K
M
value for glucose (15 20
mM). Hence, glucose enters these tissues at a biologically significant rate only when there is much glucose in the blood.
The pancreas can thereby sense the glucose level and accordingly adjust the rate of insulin secretion. Insulin signals the
need to remove glucose from the blood for storage as glycogen or conversion into fat (Section 30.3). The high K
M
value
of GLUT2 also ensures that glucose rapidly enters liver cells only in times of plenty.
3. GLUT4, which has a K
M
value of 5 mM, transports glucose into muscle and fat cells. The presence of insulin, which
signals the fed state, leads to a rapid increase in the number of GLUT4 transporters in the plasma membrane. Hence,
insulin promotes the uptake of glucose by muscle and fat. The amount of this transporter present in muscle membranes
increases in response to endurance exercise training.
4. GLUT5, present in the small intestine, functions primarily as a fructose transporter.
This family of transporters vividly illustrates how isoforms of a single protein can significantly shape the
metabolic character of cells and contribute to their diversity and functional specialization. The transporters are
members of a superfamily of transporters called the major facilitator (MF) superfamily. Members of this family transport
sugars in organisms as diverse as E. coli, Trypansoma brucei (a parasitic protozoan that causes sleeping sickness), and
human beings. An elegant solution to the problem of fuel transport evolved early and has been tailored to meet the needs
of different organisms and even different tissues.
16.2.5. Cancer and Glycolysis
It has been known for decades that tumors display enhanced rates of glucose uptake and glycolysis. We now know
that these enhanced rates of glucose processing are not fundamental to the development of cancer, but we can ask
what selective advantage they might confer on cancer cells.
Cancer cells grow more rapidly than the blood vessels to nourish them; thus, as solid tumors grow, they are unable to
obtain oxygen efficiently. In other words, they begin to experience hypoxia. Under these conditions, glycolysis leading
to lactic acid fermentation becomes the primary source of ATP. Glycolysis is made more efficient in hypoxic tumors by
the action of a transcription factor, hypoxia-inducible transcription factor (HIF-1). In the absence of oxygen, HIF-1
increases the expression of most glycolytic enzymes and the glucose transporters GLUT1 and GLUT3 (Table 16.5). In
fact, glucose uptake correlates with tumor aggressiveness and a poor prognosis. These adaptations by the cancer cells
enable the tumor to survive until vascularization can occur. HIF-1 also stimulates the growth of new tumors by
increasing the expression of signal molecules, such as vascular endothelial growth factor (VEGF), that facilitate the
growth of blood vessels (Figure 16.23). Without such vascularization, the tumor would cease to grow and either die or
remain harmlessly small. Efforts are underway to develop drugs that inhibit vascularization of tumors.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Figure 16.16. Structure of Phosphofructokinase.
Phosphofructokinase in the liver is a tetramer of four identical
subunits. The positions of the catalytic and allosteric sites are indicated.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
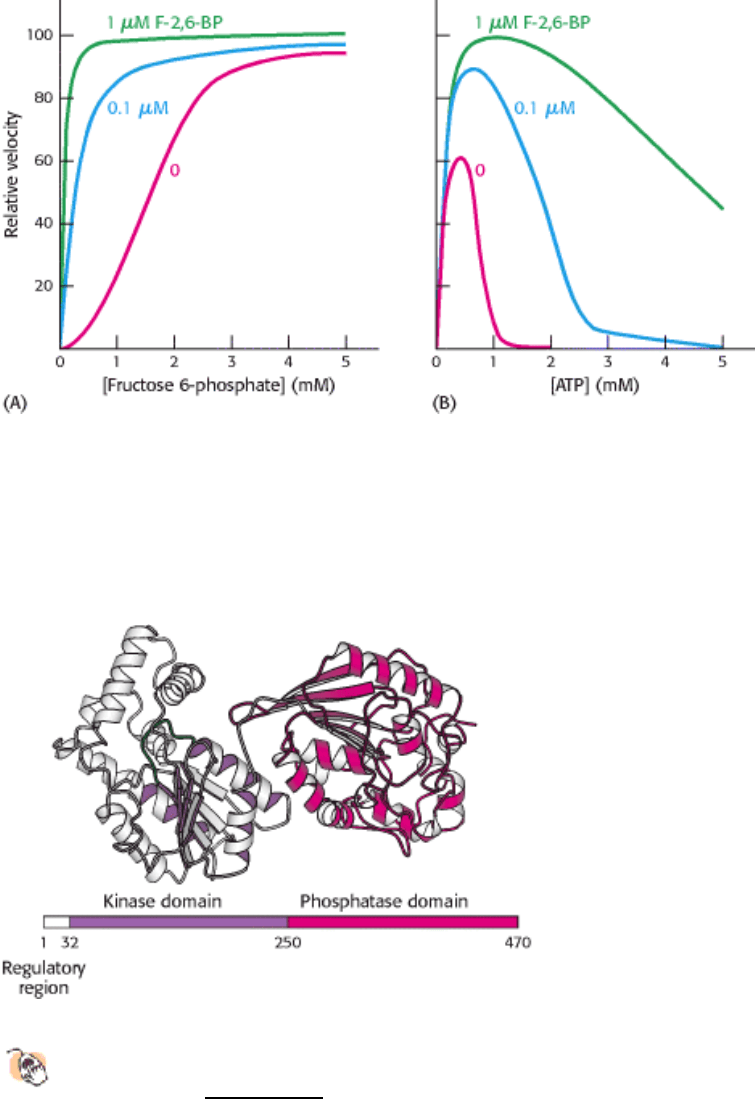
Figure 16.17. Allosteric Regulation of Phosphofructokinase. A high level of ATP inhibits the enzyme by decreasing
its affinity for fructose 6-phosphate. AMP diminishes and citrate enhances the inhibitory effect of ATP.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
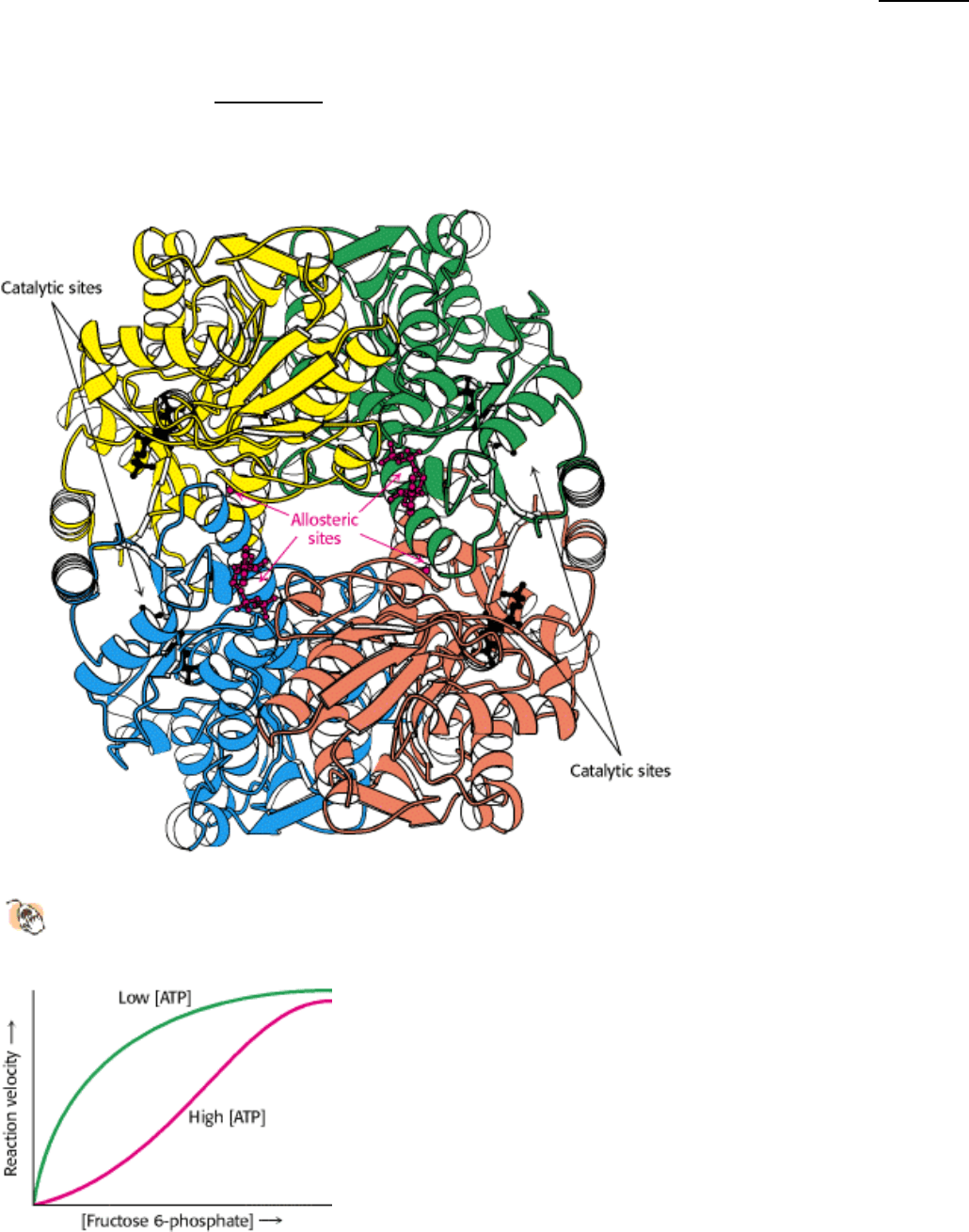
Figure 16.18. Activation of Phosphofructokinase by Fructose 2,6-Bisphosphate. (A) The sigmoidal dependence of
velocity on substrate concentration becomes hyperbolic in the presence of 1 µM fructose 2,6-bisphosphate. (B) ATP,
acting as a substrate, initially stimulates the reaction. As the concentration of ATP increases, it acts as an allosteric
inhibitor. The inhibitory effect of ATP is reversed by fructose 2,6-bisphosphate. [After E. Van Schaftingen, M.F. Jett, L.
Hue, and H. G. Hers. Proc. Natl. Acad. Sci. 78(1981):3483.]
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Figure 16.19. Domain Structure of the Bifunctional Enzyme Phosphofructokinase 2.
The kinase domain (purple) is
fused to the phosphatase domain (red). The kinase domain is a P-loop NTP hydrolase domain, as indicated by the
purple shading (Section 9.4.4). The bar represents the amino acid sequence of the enzyme.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Figure 16.20. Control of the Synthesis and Degradation of Fructose 2,6-Bisphosphate. A low blood-glucose level as
signaled by glucagon leads to the phosphorylation of the bifunctional enzyme and hence to a lower level of fructose 2,6-
bisphosphate, slowing glycolysis. High levels of fructose 6-phosphate accelerate the formation of fructose 2,6-
bisphosphate by facilitating the dephosphorylation of the bifunctional enzyme.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Figure 16.21. Control of the Catalytic Activity of Pyruvate Kinase. Pyruvate kinase is regulated by allosteric
effectors and covalent modification.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Table 16.4. Family of glucose transporters
Name Tissue location K
m
Comments
GLUT1 All mammalian tissues 1 mM Basal glucose uptake
GLUT2
Liver and pancreatic β cells
15
20 mM In the pancreas, plays a role in regulation of insulin
In the liver, removes excess glucose from the blood
GLUT3 All mammalian tissues 1 mM Basal glucose uptake
GLUT4 Muscle and fat cells 5 mM Amount in muscle plasma membrane increases with endurance
training
GLUT5 Small intestine
Primarily a fructose transporter
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
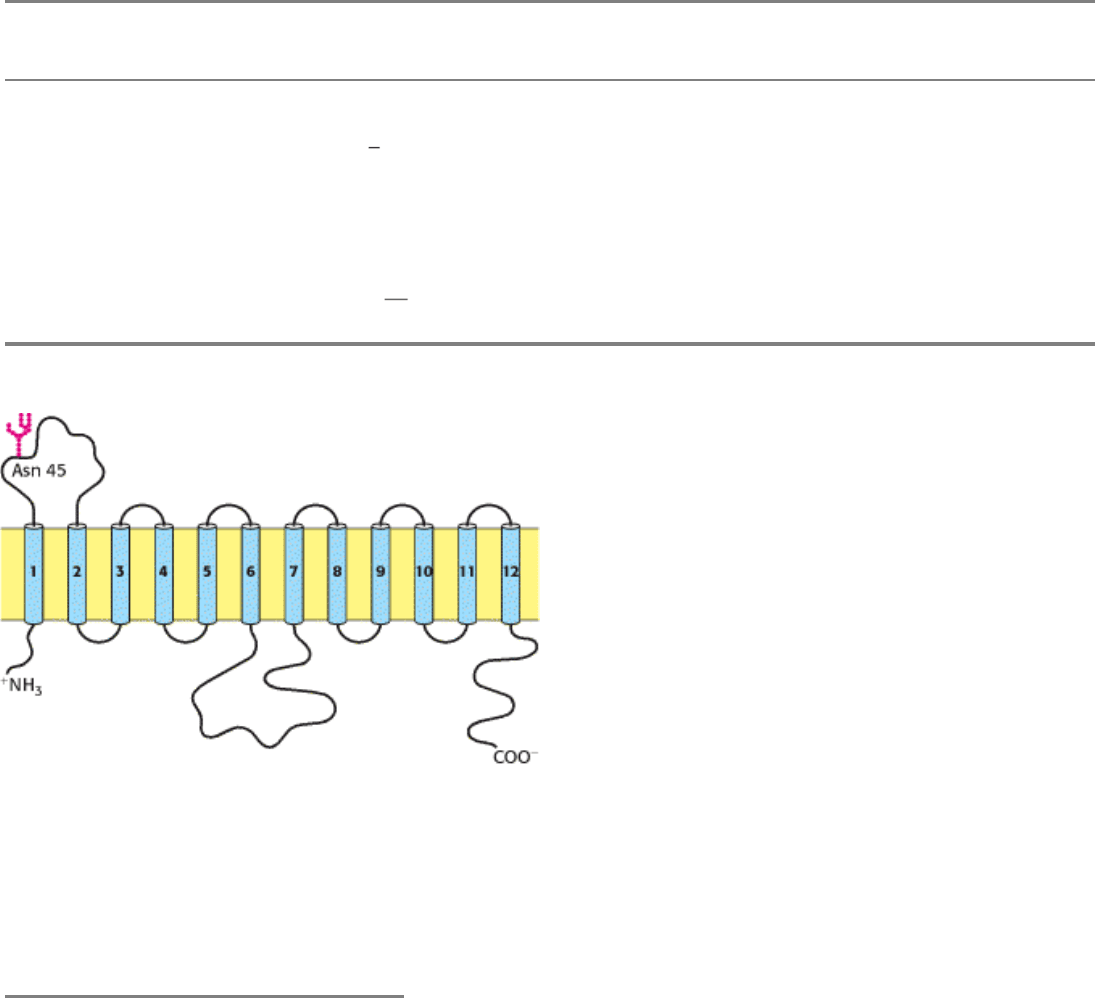
Figure 16.22. Model of a Mammalian Glucose Transporter. The hydrophobicity profile of the protein indicates 12
transmembrane α helices. [From M. Muekler, C. Caruso, S. A. Baldwin, M. Panico, M. Blench, H. R. Morris, W. J.
Allard, G. E. Lienhard, and H. F. Lodish. Science 229(1985):941.]
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
Table 16.5. Proteins in glucose metabolism encoded by genes regulated by hypoxia-inducible factor
GLUT1
GLUT3
Hexokinase
Phosphofructokinase
Aldolase
Glyceraldehyde 3-phosphate dehydrogenase
Phosphoglycerate kinase
Enolase
Pyruvate kinase
Lactate dehydrogenase
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.2. The Glycolytic Pathway Is Tightly Controlled
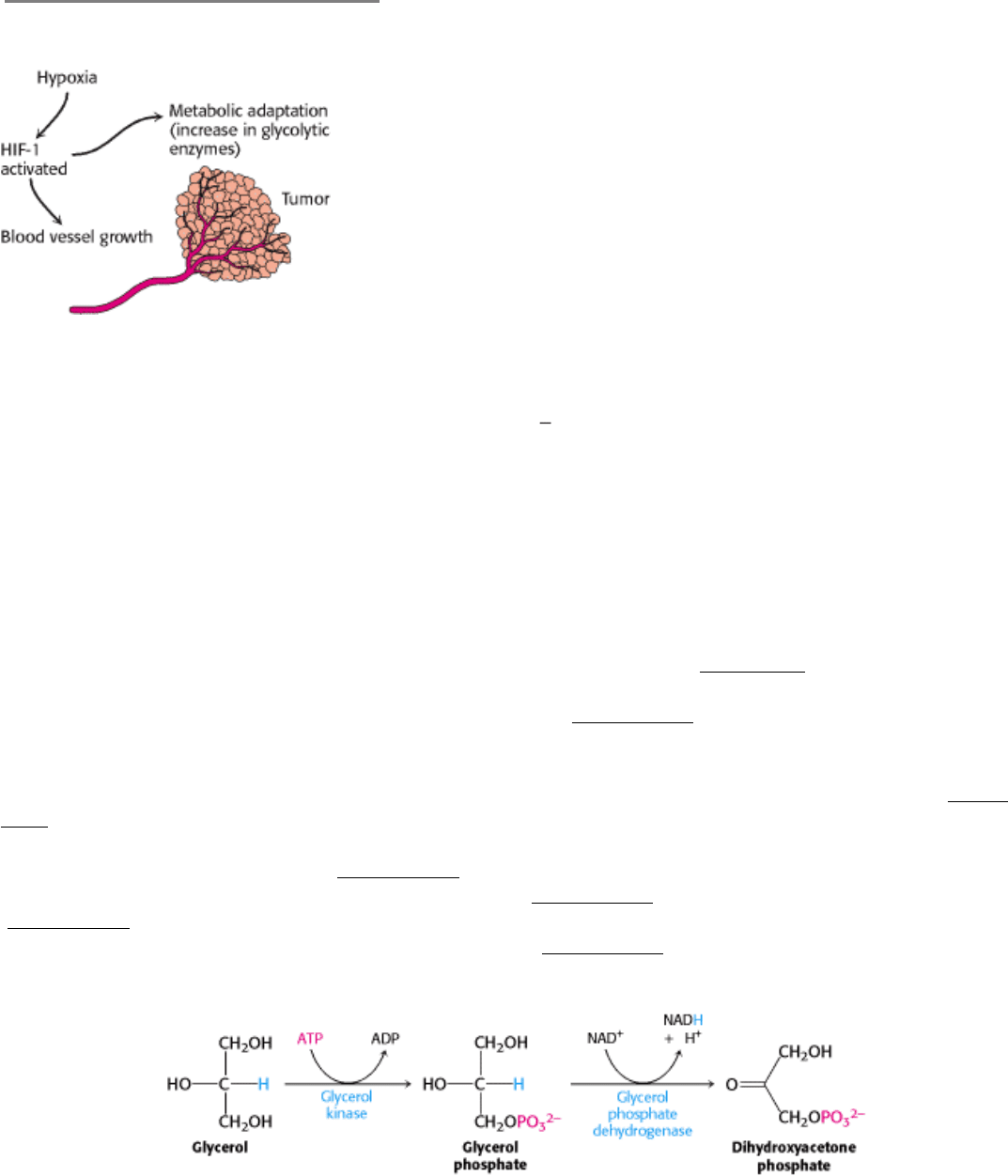
Figure 16.23. Alteration of Gene Expression in Tumors Due to Hypoxia. The hypoxic conditions inside a tumor mass
lead to the activation of the hypoxia-inducible transcription factor (HIF-1), which induces metabolic adaptation (increase
in glycolytic enzymes) and activates angiogenic factors that stimulate the growth of new blood vessels. [Adapted from C.
V. Dang and G. L. Semenza. Trends Biochem. Sci. 24(1999):68
72.]
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis
16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
We now turn to the synthesis of glucose from noncarbohydrate precursors, a process called gluconeogenesis. This
metabolic pathway is important because the brain depends on glucose as its primary fuel and red blood cells use only
glucose as a fuel. The daily glucose requirement of the brain in a typical adult human being is about 120 g, which
accounts for most of the 160 g of glucose needed daily by the whole body. The amount of glucose present in body fluids
is about 20 g, and that readily available from glycogen, a storage form of glucose (Section 21.1), is approximately 190 g.
Thus, the direct glucose reserves are sufficient to meet glucose needs for about a day. During a longer period of
starvation, glucose must be formed from noncarbohydrate sources (Section 30.3.1).
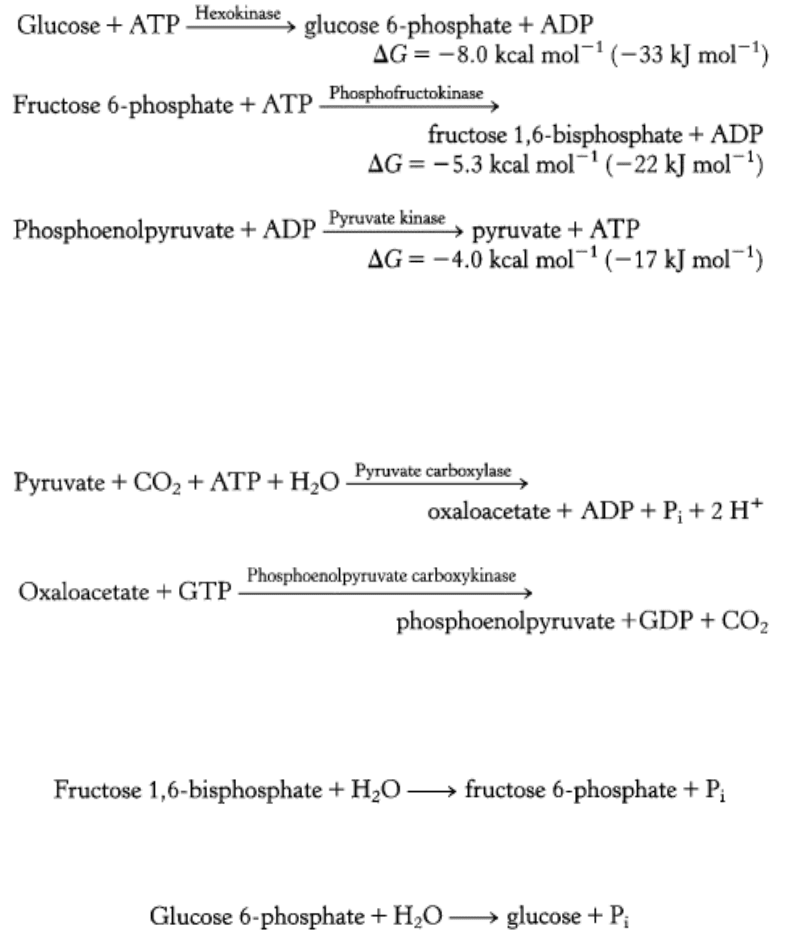
The gluconeogenic pathway converts pyruvate into glucose. Noncarbohydrate precursors of glucose are first converted
into pyruvate or enter the pathway at later intermediates such as oxaloacetate and dihydroxyacetone phosphate (Figure
16.24). The major noncarbohydrate precursors are lactate, amino acids, and glycerol. Lactate is formed by active skeletal
muscle when the rate of glycolysis exceeds the rate of oxidative metabolism. Lactate is readily converted into pyruvate
by the action of lactate dehydrogenase (Section 16.1.9). Amino acids are derived from proteins in the diet and, during
starvation, from the breakdown of proteins in skeletal muscle (Section 30.3.1). The hydrolysis of triacylglycerols
(Section 22.2.1) in fat cells yields glycerol and fatty acids. Glycerol is a precursor of glucose, but animals cannot convert
fatty acids into glucose, for reasons that will be discussed later (Section 22.3.7). Glycerol may enter either the
gluconeogenic or the glycolytic pathway at dihydroxyacetone phosphate.
The major site of gluconeogenesis is the liver, with a small amount also taking place in the kidney. Little
gluconeogenesis takes place in the brain, skeletal muscle, or heart muscle. Rather, gluconeogenesis in the liver and
kidney helps to maintain the glucose level in the blood so that brain and muscle can extract sufficient glucose from it to
meet their metabolic demands.
16.3.1. Gluconeogenesis Is Not a Reversal of Glycolysis
In glycolysis, glucose is converted into pyruvate; in gluconeogenesis, pyruvate is converted into glucose. However,
gluconeogenesis is not a reversal of glycolysis. Several reactions must differ because the equilibrium of glycolysis lies
far on the side of pyruvate formation. The actual ∆ G for the formation of pyruvate from glucose is about -20 kcal mol
-1
(-84 kJ mol
-1
) under typical cellular conditions. Most of the decrease in free energy in glycolysis takes place in the three
essentially irreversible steps catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase.
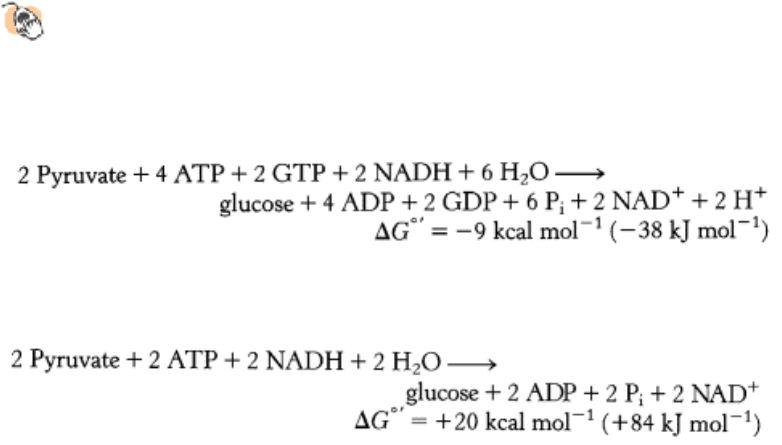
In gluconeogenesis, the following new steps bypass these virtually irreversible reactions of glycolysis:
1. Phosphoenolpyruvate is formed from pyruvate by way of oxaloacetate through the action of pyruvate carboxylase and
phosphoenolpyruvate carboxykinase.
2. Fructose 6-phosphate is formed from fructose 1,6-bisphosphate by hydrolysis of the phosphate ester at carbon 1.
Fructose 1,6-bisphosphatase catalyzes this exergonic hydrolysis.
3. Glucose is formed by hydrolysis of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphatase.
We will examine each of these steps in turn.
16.3.2. The Conversion of Pyruvate into Phosphoenolpyruvate Begins with the
Formation of Oxaloacetate
The first step in gluconeogenesis is the carboxylation of pyruvate to form oxaloacetate at the expense of a molecule of
ATP. Then, oxaloacetate is decarboxylated and phosphorylated to yield phosphoenolpyruvate, at the expense of the high

phosphoryl-transfer potential of GTP. Both of these reactions take place inside the mitochondria.
The first reaction is catalyzed by pyruvate carboxylase and the second by phosphoenolpyruvate carboxykinase. The sum
of these reactions is:
Pyruvate carboxylase is of special interest because of its structural, catalytic, and allosteric properties. The N-terminal
300 to 350 amino acids form an ATP-grasp domain (Figure 16.25), which is a widely used ATP-activating domain to be
discussed in more detail when we investigate nucleotide biosynthesis (Section 25.1.1). The C-terminal 80 amino acids
constitute a biotin-binding domain (Figure 16.26) that we will see again in fatty acid synthesis (Section 22.4.1). Biotin is
a covalently attached prosthetic group, which serves as a carrier of activated CO
2
. The carboxylate group of biotin is
linked to the ε-amino group of a specific lysine residue by an amide bond (Figure 16.27). Note that biotin is attached to
pyruvate carboxylase by a long, flexible chain.
The carboxylation of pyruvate takes place in three stages:
Recall that, in aqueous solutions, CO
2
exists as HCO
3
-
with the aid of carbonic anhydrase (Section 9.2). The HCO
3
-
is
activated to carboxyphosphate. This activated CO
2
is subsequently bonded to the N-1 atom of the biotin ring to form the
carboxybiotin-enzyme intermediate (see Figure 16.27). The CO
2
attached to the biotin is quite activated. The ∆ G°´ for
its cleavage
is -4.7 kcal mol
-1
(-20 kJ mol
-1
). This negative ∆ G°´ indicates that carboxybiotin is able to transfer CO
2
to acceptors
without the input of additional free energy.
The activated carboxyl group is then transferred from carboxybiotin to pyruvate to form oxaloacetate. The long, flexible
link between biotin and the enzyme enables this prosthetic group to rotate from one active site of the enzyme (the ATP-
bicarbonate site) to the other (the pyruvate site).
The first partial reaction of pyruvate carboxylase, the formation of carboxybiotin, depends on the presence of acetyl
CoA. Biotin is not carboxylated unless acetyl CoA is bound to the enzyme. Acetyl CoA has no effect on the second
partial reaction. The allosteric activation of pyruvate carboxylase by acetyl CoA is an important physiological control
mechanism that will be discussed in Section 17.3.1.
16.3.3. Oxaloacetate Is Shuttled into the Cytosol and Converted into
Phosphoenolpyruvate

Pyruvate carboxylase is a mitochondrial enzyme, whereas the other enzymes of gluconeogenesis are cytoplasmic.
Oxaloacetate, the product of the pyruvate carboxylase reaction, is reduced to malate inside the mitochondrion for
transport to the cytosol. The reduction is accomplished by an NADH-linked malate dehydrogenase. When malate has
been transported across the mitochondrial membrane, it is reoxidized to oxaloacetate by an NAD
+
-linked malate
dehydrogenase in the cytosol (Figure 16.28).
Finally, oxaloacetate is simultaneously decarboxylated and phosphorylated by phosphoenolpyruvate carboxykinase in
the cytosol. The CO
2
that was added to pyruvate by pyruvate carboxylase comes off in this step. Recall that, in
glycolysis, the presence of a phosphoryl group traps the unstable enol isomer of pyruvate as phosphoenolpyruvate
(Section 16.1.7). In gluconeogenesis, the formation of the unstable enol is driven by decarboxylation the oxidation of
the carboxylic acid to CO
2
and trapped by the addition of a phosphate to carbon 2 from GTP. The two-step pathway
for the formation of phosphoenolpyruvate from pyruvate has a ∆ G°´ of +0.2 kcal mol
-1
(+0.13 kJ mol
-1
) in contrast with
+7.5 kcal mol
-1
(+31 kJ mol
-1
) for the reaction catalyzed by pyruvate kinase. The much more favorable ∆ G°´ for the
two-step pathway results from the use of a molecule of ATP to add a molecule of CO
2
in the carboxylation step that can
be removed to power the formation of phosphoenolpyruvate in the decarboxylation step. Decarboxylations often drive
reactions otherwise highly endergonic. This metabolic motif is used in the citric acid cycle (Section 17.1), the pentose
phosphate pathway (Section 20.3.1), and fatty acid synthesis (Section 22.4.3).
16.3.4. The Conversion of Fructose 1,6-bisphosphate into Fructose 6-phosphate and
Orthophosphate Is an Irreversible Step
On formation, phosphoenolpyruvate is metabolized by the enzymes of glycolysis but in the reverse direction. These
reactions are near equilibrium under intracellular conditions; so, when conditions favor gluconeogenesis, the reverse
reactions will take place until the next irreversible step is reached. This step is the hydrolysis of fructose 1,6-
bisphosphate to fructose 6-phosphate and P
i
.
The enzyme responsible for this step is fructose 1,6-bisphosphatase. Like its glycolytic counterpart, it is an allosteric
enzyme that participates in the regulation of gluconeogenesis. We will return to its regulatory properties later in the
chapter.
16.3.5. The Generation of Free Glucose Is an Important Control Point
The fructose 6-phosphate generated by fructose 1,6-bisphosphatase is readily converted into glucose 6-phosphate. In
most tissues, gluconeogenesis ends here. Free glucose is not generated; rather, the glucose 6-phosphate is processed in
some other fashion, notably to form glycogen. One advantage to ending gluconeogenesis at glucose 6-phosphate is that,
unlike free glucose, the molecule cannot diffuse out of the cell. To keep glucose inside the cell, the generation of free
glucose is controlled in two ways. First, the enzyme responsible for the conversion of glucose 6-phosphate into glucose,
glucose 6-phosphatase, is regulated. Second, the enzyme is present only in tissues whose metabolic duty is to maintain
blood-glucose homeostasis
tissues that release glucose into the blood. These tissues are the liver and to a lesser extent
the kidney.
This final step in the generation of glucose does not take place in the cytosol. Rather, glucose 6-phosphate is transported
into the lumen of the endoplasmic reticulum, where it is hydrolyzed to glucose by glucose 6-phosphatase, which is
bound to the membrane (Figure 16.29). An associated Ca
2+
-binding stabilizing protein is essential for phosphatase
activity. Glucose and P
i
are then shuttled back to the cytosol by a pair of transporters. The glucose transporter in the
endoplasmic reticulum membrane is like those found in the plasma membrane (Section 16.2.4). It is striking that five
proteins are needed to transform cytosolic glucose 6-phosphate into glucose.
16.3.6. Six High Transfer Potential Phosphoryl Groups Are Spent in Synthesizing
Glucose from Pyruvate
Conceptual Insights, Energetics of Glucose Metabolism. See the section on
gluconeogenesis in the Conceptual Insights module to review why and how
gluconeogenesis must differ from the reversal of glycolysis.
The stoichiometry of gluconeogenesis is:
In contrast, the stoichiometry for the reversal of glycolysis is:
Note that six nucleotide triphosphate molecules are hydrolyzed to synthesize glucose from pyruvate in gluconeogenesis,
whereas only two molecules of ATP are generated in glycolysis in the conversion of glucose into pyruvate. Thus, the
extra cost of gluconeogenesis is four high phosphoryl-transfer potential molecules per molecule of glucose synthesized
from pyruvate. The four additional high phosphoryl-transfer potential molecules are needed to turn an energetically
unfavorable process (the reversal of glycolysis, ∆ G°´ = + 20 kcal mol
-1
[+84 kJ mol
-1
]) into a favorable one
(gluconeogenesis, ∆ G°´ = -9 kcal mol
-1
[-38 kJ mol
-1
]). This is a clear example of the coupling of reactions: ATP
hydrolysis is used to power an energetically unfavorable reaction.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
