Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


Figure 16.24. Pathway of Gluconeogenesis. The distinctive reactions and enzymes of this pathway are shown in red.
The other reactions are common to glycolysis. The enzymes for gluconeogenesis are located in the cytosol, except for
pyruvate carboxylase (in the mitochondria) and glucose 6-phosphatase (membrane bound in the endoplasmic reticulum).
The entry points for lactate, glycerol, and amino acids are shown.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
Figure 16.25. Domain Structure of Pyruvate Carboxylase. The ATP-grasp domain activates HCO
3
-
and transfers
CO
2
to the biotin-binding domain. From there, the CO
2
is transferred to pyruvate generated in the central domain.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
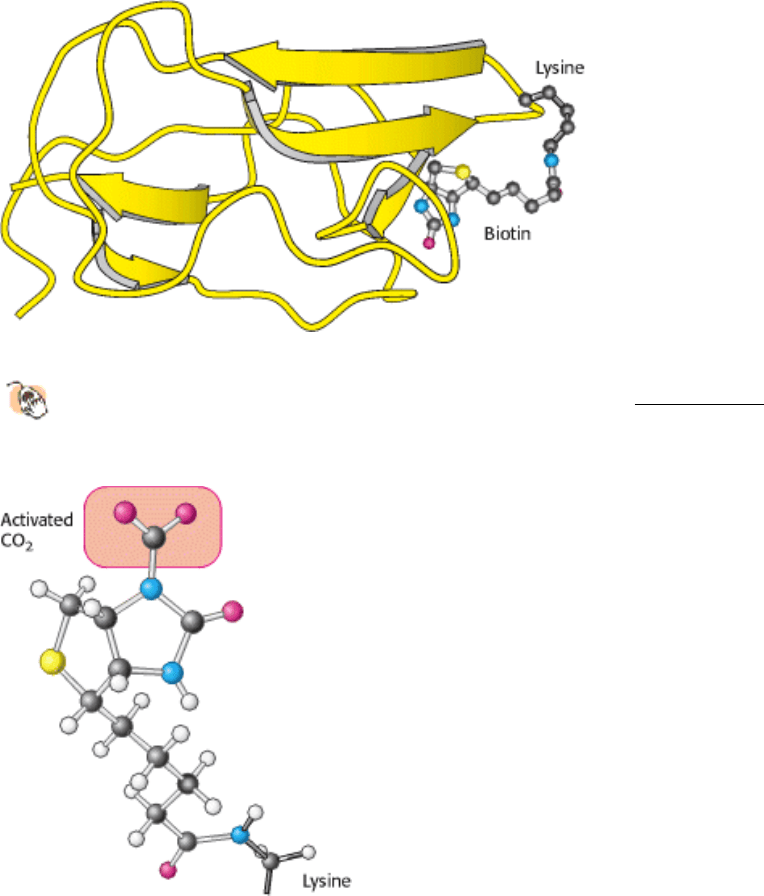
Figure 16.26. Biotin-Binding Domain of Pyruvate Carboxylase.
This likely structure is based on the structure of the
homologous domain from the enzyme acetyl CoA carboxylase (Section 22.4.1). The biotin is on a flexible tether,
allowing it to move between the ATP-bicarbonate site and the pyruvate site.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
Figure 16.27. Structure of Carboxybiotin.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
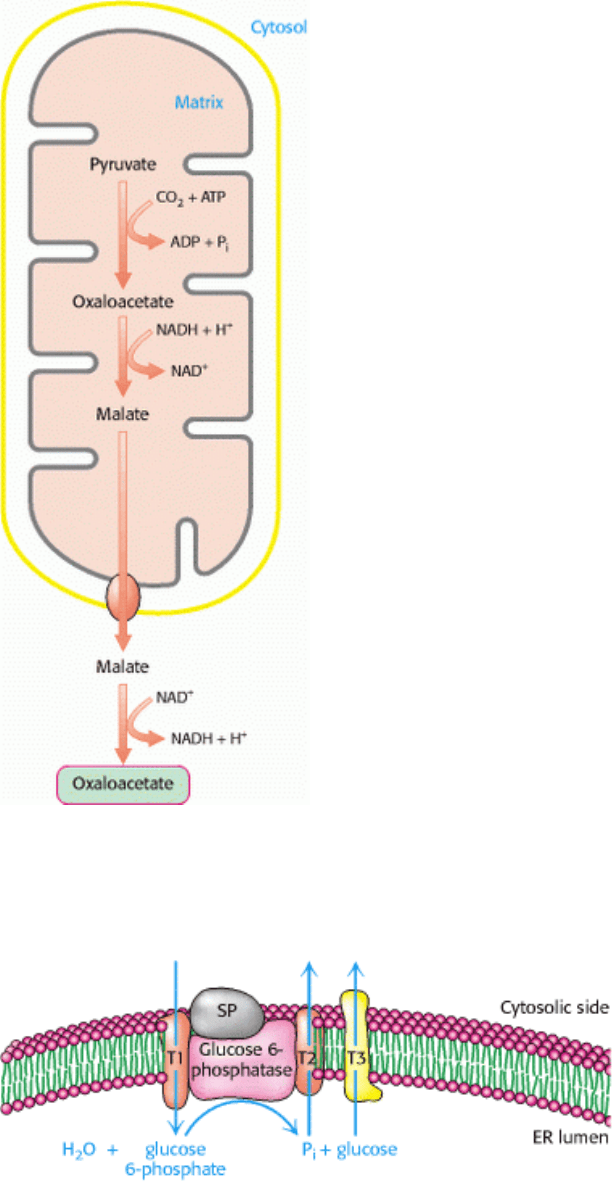
Figure 16.28. Compartmental Cooperation. Oxaloacetate utilized in the cytosol for gluconeogenesis is formed in the
mitochondrial matrix by carboxylation of pyruvate. Oxaloacetate leaves the mitochondrion by a specific transport system
(not shown) in the form of malate, which is reoxidized to oxaloacetate in the cytosol.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.3. Glucose Can Be Synthesized from Noncarbohydrate Precursors
Figure 16.29. Generation of Glucose from Glucose 6-Phosphate. Several endoplasmic reticulum (ER) proteins play a
role in the generation of glucose from glucose 6-phosphate. T1 transports glucose 6-phosphate into the lumen of the ER,
whereas T2 and T3 transport Pi and glucose, respectively, back into the cytosol. Glucose 6-phosphatase is stabilized by a
Ca
2+
-binding protein (SP). [After A. Buchell and I. D. Waddel. Biochem. Biophys. Acta 1092(1991):129.]

II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis
16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Gluconeogenesis and glycolysis are coordinated so that within a cell one pathway is relatively inactive while the other is
highly active. If both sets of reactions were highly active at the same time, the net result would be the hydrolysis of four
nucleotide triphosphates (two ATP plus two GTP) per reaction cycle. Both glycolysis and gluconeogenesis are highly
exergonic under cellular conditions, and so there is no thermodynamic barrier to such simultaneous activity. However,
the amounts and activities of the distinctive enzymes of each pathway are controlled so that both pathways are not highly
active at the same time. The rate of glycolysis is also determined by the concentration of glucose, and the rate of
gluconeogenesis by the concentrations of lactate and other precursors of glucose.
The interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate is stringently controlled (Figure 16.30). As
discussed in Section 16.2.1, AMP stimulates phosphofructokinase, whereas ATP and citrate inhibit it. Fructose 1,6-
bisphosphatase, on the other hand, is inhibited by AMP and activated by citrate. A high level of AMP indicates that the
energy charge is low and signals the need for ATP generation. Conversely, high levels of ATP and citrate indicate that
the energy charge is high and that biosynthetic intermediates are abundant. Under these conditions, glycolysis is nearly
switched off and gluconeogenesis is promoted.
Phosphofructokinase and fructose 1,6-bisphosphatase are also reciprocally controlled by fructose 2,6-bisphosphate in the
liver (Section 16.2.2). The level of F-2,6-BP is low during starvation and high in the fed state, because of the
antagonistic effects of glucagon and insulin on the production and degradation of this signal molecule. Fructose 2,6-
bisphosphate strongly stimulates phosphofructokinase and inhibits fructose 1,6-bisphosphatase. Hence, glycolysis is
accelerated and gluconeogenesis is diminished in the fed state. During starvation, gluconeogenesis predominates because
the level of F-2,6-BP is very low. Glucose formed by the liver under these conditions is essential for the viability of brain
and muscle.
The interconversion of phosphoenolpyruvate and pyruvate also is precisely regulated. Recall that pyruvate kinase is
controlled by allosteric effectors and by phosphorylation (Section 16.2.3). High levels of ATP and alanine, which signal
that the energy charge is high and that building blocks are abundant, inhibit the enzyme in liver. Conversely, pyruvate
carboxylase, which catalyzes the first step in gluconeogenesis from pyruvate, is activated by acetyl CoA and inhibited by
ADP. Likewise, ADP inhibits phosphoenolpyruvate carboxykinase. Hence, gluconeogenesis is favored when the cell is
rich in biosynthetic precursors and ATP.
The amounts and the activities of these essential enzymes also are regulated. The regulators in this case are hormones.
Hormones affect gene expression primarily by changing the rate of transcription, as well as by regulating the degradation
of mRNA. Insulin, which rises subsequent to eating, stimulates the expression of phosphofructokinase, pyruvate kinase,
and the bifunctional enzyme that makes and degrades F-2,6-BP. Glucagon, which rises during starvation, inhibits the
expression of these enzymes and stimulates instead the production of two key gluconeogenic enzymes,
phosphoenolpyruvate carboxykinase and fructose 1,6-bisphosphatase. Transcriptional control in eukaryotes is much
slower than allosteric control; it takes hours or days in contrast with seconds to minutes. The richness and complexity of
hormonal control are graphically displayed by the promoter of the phosphoenolpyruvate carboxykinase gene, which
contains regulatory sequences that respond to insulin, glucagon, glucocorticoids, and thyroid hormone (Figure 16.31).
16.4.1. Substrate Cycles Amplify Metabolic Signals and Produce Heat
A pair of reactions such as the phosphorylation of fructose 6-phosphate to fructose 1,6-bisphosphate and its hydrolysis
back to fructose 6-phosphate is called a substrate cycle. As already mentioned, both reactions are not simultaneously
fully active in most cells, because of reciprocal allosteric controls. However, the results of isotope-labeling studies have
shown that some fructose 6-phosphate is phosphorylated to fructose 1,6-bisphosphate in gluconeogenesis. There also is a
limited degree of cycling in other pairs of opposed irreversible reactions. This cycling was regarded as an imperfection in
metabolic control, and so substrate cycles have sometimes been called futile cycles. Indeed, there are pathological
conditions, such as malignant hyperthermia, in which control is lost and both pathways proceed rapidly with the

concomitant generation of heat by the rapid, uncontrolled hydrolysis of ATP.
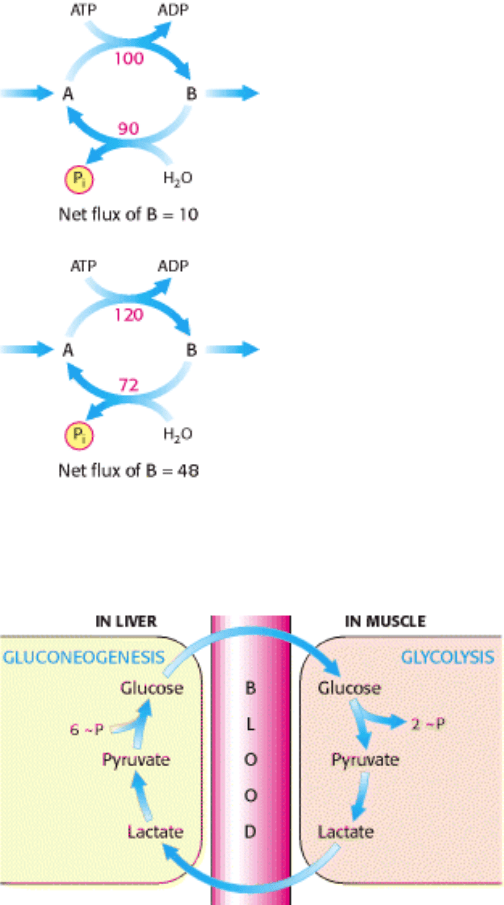
Despite such extraordinary circumstances, it now seems likely that substrate cycles are biologically important. One
possibility is that substrate cycles amplify metabolic signals. Suppose that the rate of conversion of A into B is 100 and
of B into A is 90, giving an initial net flux of 10. Assume that an allosteric effector increases the A
B rate by 20% to
120 and reciprocally decreases the B A rate by 20% to 72. The new net flux is 48, and so a 20% change in the rates
of the opposing reactions has led to a 380% increase in the net flux. In the example shown in Figure 16.32, this
amplification is made possible by the rapid hydrolysis of ATP. It has been suggested that the flux down the glycolytic
pathway may increase 1000-fold at the initiation of intense exercise. Because it seems unlikely that allosteric activation
of enzymes alone could explain this increased flux, the existence of substrate cycles may partly account for the rapid rise
in the rate of glycolysis.
The other potential biological role of substrate cycles is the generation of heat produced by the hydrolysis of ATP. A
striking example is provided by bumblebees, which must maintain a thoracic temperature of about 30°C to fly. A
bumblebee is able to maintain this high thoracic temperature and forage for food even when the ambient temperature is
only 10°C because phosphofructokinase and fructose 1,6-bisphosphatase in its flight muscle are simultaneously highly
active; the continuous hydrolysis of ATP generates heat. This bisphosphatase is not inhibited by AMP, which suggests
that the enzyme is specially designed for the generation of heat. In contrast, the honeybee has almost no fructose 1,6-
bisphosphatase activity in its flight muscle and consequently cannot fly when the ambient temperature is low.
16.4.2. Lactate and Alanine Formed by Contracting Muscle Are Used by Other Organs
Lactate produced by active skeletal muscle and erythrocytes is a source of energy for other organs. Erythrocytes lack
mitochondria and can never oxidize glucose completely. In contracting skeletal muscle during vigorous exercise, the rate
at which glycolysis produces pyruvate exceeds the rate at which the citric acid cycle oxidizes it. Under these conditions,
moreover, the rate of formation of NADH by glycolysis is greater than the rate of its oxidation by aerobic metabolism.
Continued glycolysis depends on the availability of NAD
+
for the oxidation of glyceraldehyde 3-phosphate. The
accumulation of both NADH and pyruvate is reversed by lactate dehydrogenase, which oxidizes NADH to NAD
+
as it
reduces pyruvate to lactate (Section 16.1.7). However, lactate is a dead end in metabolism. It must be converted back
into pyruvate before it can be metabolized. The only purpose of the reduction of pyruvate to lactate is to regenerate NAD
+
so that glycolysis can proceed in active skeletal muscle and erythrocytes. The formation of lactate buys time and shifts
part of the metabolic burden from muscle to other organs.
The plasma membrane of most cells contains carriers that render them highly permeable to lactate and pyruvate. Both
substances diffuse out of active skeletal muscle into the blood and are carried to the liver. Much more lactate than
pyruvate is transported out because the high NADH/NAD
+
ratio in contracting skeletal muscle favors the conversion of
pyruvate into lactate. The lactate that enters the liver is oxidized to pyruvate, a reaction favored by the low NADH/NAD
+
ratio in the cytosol of liver cells. Pyruvate in the liver is converted into glucose by the gluconeogenic pathway. Glucose
then enters the blood and is taken up by skeletal muscle. Thus, the liver furnishes glucose to contracting skeletal muscle,
which derives ATP from the glycolytic conversion of glucose into lactate. Contracting skeletal muscle supplies lactate to
the liver, which uses it to synthesize glucose. These reactions constitute the Cori cycle (Figure 16.33). Studies have
shown that alanine, like lactate, is a major precursor of glucose. In muscle, alanine is formed from pyruvate by
transamination (Section 24.2.2); the reverse reaction takes place in the liver. The interplay between glycolysis and
gluconeogenesis is summarized in Figure 16.34, which shows how these two pathways help to meet the energy needs of
different cell types.
Isozymic forms of lactate dehydrogenase in different tissues catalyze the interconversions of pyruvate and lactate.
Lactate dehydrogenase is a tetramer of two kinds of 35-kd subunits encoded by similar genes: the H type
predominates in the heart, and the homologous M type in skeletal muscle and the liver. These subunits associate to form
five types of tetramers: H
4
, H
3
M
1
, H
2
M
2
, H
1
M
3
, and M
4
. The H
4
isozyme (type 1) has higher affinity for substrates than
does the M
4
isozyme (type 5) and, unlike M
4
, is allosterically inhibited by high levels of pyruvate. The other isozymes
have intermediate properties, depending on the ratio of the two kinds of chains. H
4
is designed to oxidize lactate to

pyruvate, which is then utilized as a fuel by the heart through aerobic metabolism. Indeed, heart muscle never functions
anaerobically. In contrast, M
4
is optimized to operate in the reverse direction, to convert pyruvate into lactate to allow
glycolysis to proceed under anaerobic conditions. We see here an example of how gene duplication and divergence
generate a series of homologous enzymes that foster metabolic cooperation between organs.
16.4.3. Glycolysis and Gluconeogenesis Are Evolutionarily Intertwined
The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic
generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. The fact that
glycolytic enzymes with similar properties do not have similar amino acid sequences also provides a clue to how the
pathway originated. Although there are four kinases and two isomerases in the pathway, both sequence and structural
comparisons do not suggest that these sets of enzymes are related to one another by divergent evolution. The absence of
such similarities implies that glycolytic enzymes were derived independently rather than by gene duplication. The
common dinucleotide-binding domain found in the dehydrogenases (Section 16.1.10) and the α β barrels are the only
major recurring elements.
We can speculate on the relationship between glycolysis and gluconeogenesis if we think of glycolysis as consisting of
two segments: the metabolism of hexoses (the upper segment) and the metabolism of trioses (the lower segment). The
enzymes from the upper segment are different in some species and are missing entirely in some archaea, whereas
enzymes from the lower segment are quite conserved. In fact, four enzymes of the lower segment are present in all
species. This lower part of the pathway is common to glycolysis and gluconeogenesis. This common part of the two
pathways may be the oldest part, constituting the core to which the other steps were added. The upper part would vary
according to the sugars that were available to evolving organisms in particular niches. Interestingly, this core part of
carbohydrate metabolism can generate triose precursors for ribose sugars, a component of RNA and a critical
requirement for the RNA world. Thus, we are left with the unanswered question, Was the original core pathway used for
energy conversion or biosynthesis?
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated

Figure 16.30. Reciprocal Regulation of Gluconeogenesis and Glycolysis in the Liver. The level of fructose 2,6-
bisphosphate is high in the fed state and low in starvation. Another important control is the inhibition of pyruvate kinase
by phosphorylation during starvation.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Figure 16.31. The Promoter of the Phosphoenolpyruvate Carboxykinase Gene. This promoter is approximately 500
bp in length and contains regulatory sequences (response elements) that mediate the action of several hormones. IRE,
insulin response element; GRE, glucocorticoid response element; TRE, thyroid hormone response element; CREI and
CREII, cAMP response elements. [After M. M. McGrane, J. S Jun, Y. M. Patel, and R. W. Hanson. Trends Biochem. Sci.
17(1992):40.]
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Figure 16.32. Substrate Cycle. This ATP-driven cycle operates at two different rates. A small change in the rates of the
two opposing reactions results in a large change in the net flux of product B.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Figure 16.33. The Cori Cycle. Lactate formed by active muscle is converted into glucose by the liver. This cycle shifts
part of the metabolic burden of active muscle to the liver.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.4. Gluconeogenesis and Glycolysis Are Reciprocally Regulated

Figure 16.34. Cooperation between Glycolysis and Gluconeogenesis. Glycolysis and gluconeogenesis are
coordinated, in a tissue-specific fashion, to ensure that the glucose-dependent energy needs of all cells are met.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis
Summary
Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Glycolysis is the set of reactions that converts glucose into pyruvate. The 10 reactions of glycolysis take place in the
cytosol. In the first stage, glucose is converted into fructose 1,6-bisphosphate by a phosphorylation, an isomerization,
and a second phosphorylation reaction. Two molecules of ATP are consumed per molecule of glucose in these reactions,
which are the prelude to the net synthesis of ATP. In the second stage, fructose 1,6-bisphosphate is cleaved by aldolase
into dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, which are readily interconvertible. In the third stage,
ATP is generated. Glyceraldehyde 3-phosphate is oxidized and phosphorylated to form 1,3-bisphosphoglycerate, an acyl
phosphate with a high phosphoryl-transfer potential. This molecule transfers a phosphoryl group to ADP to form ATP
and 3-phosphoglycerate. A phosphoryl shift and a dehydration form phosphoenolpyruvate, a second intermediate with a
high phosphoryltransfer potential. Another molecule of ATP is generated as phosphoenolpyruvate is converted into
pyruvate. There is a net gain of two molecules of ATP in the formation of two molecules of pyruvate from one molecule
of glucose.
The electron acceptor in the oxidation of glyceraldehyde 3-phosphate is NAD
+
, which must be regenerated for glycolysis
to continue. In aerobic organisms, the NADH formed in glycolysis transfers its electrons to O
2
through the electron-
transport chain, which thereby regenerates NAD
+
. Under anaerobic conditions and in some microorganisms, NAD
+
is
regenerated by the reduction of pyruvate to lactate. In other microorganisms, NAD
+
is regenerated by the reduction of
pyruvate to ethanol. These two processes are examples of fermentations.
The Glycolytic Pathway Is Tightly Controlled
The glycolytic pathway has a dual role: it degrades glucose to generate ATP, and it provides building blocks for the
synthesis of cellular components. The rate of conversion of glucose into pyruvate is regulated to meet these two major
cellular needs. Under physiologic conditions, the reactions of glycolysis are readily reversible except for the ones
catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase. Phosphofructokinase, the most important control
element in glycolysis, is inhibited by high levels of ATP and citrate, and it is activated by AMP and fructose 2,6-
bisphosphate. In the liver, this bisphosphate signals that glucose is abundant. Hence, phosphofructokinase is active when
either energy or building blocks are needed. Hexokinase is inhibited by glucose 6-phosphate, which accumulates when
phosphofructokinase is inactive. ATP and alanine allosterically inhibit pyruvate kinase, the other control site, and
fructose 1,6-bisphosphate activates the enzyme. Consequently, pyruvate kinase is maximally active when the energy
charge is low and glycolytic intermediates accumulate.
Glucose Can Be Synthesized from Noncarbohydrate Precursors
Gluconeogenesis is the synthesis of glucose from noncarbohydrate sources, such as lactate, amino acids, and glycerol.
Several of the reactions that convert pyruvate into glucose are common to glycolysis. Gluconeogenesis, however,
requires four new reactions to bypass the essential irreversibility of three reactions in glycolysis. In two of the new
reactions, pyruvate is carboxylated in mitochondria to oxaloacetate, which in turn is decarboxylated and phosphorylated
in the cytosol to phosphoenolpyruvate. Two high-energy phosphate bonds are consumed in these reactions, which are
catalyzed by pyruvate carboxylase and phosphoenolpyruvate carboxykinase. Pyruvate carboxylase contains a biotin
prosthetic group. The other distinctive reactions of gluconeogenesis are the hydrolyses of fructose 1,6-bisphosphate and
glucose 6-phosphate, which are catalyzed by specific phosphatases. The major raw materials for gluconeogenesis by the
liver are lactate and alanine produced from pyruvate by active skeletal muscle. The formation of lactate during intense
muscular activity buys time and shifts part of the metabolic burden from muscle to the liver.
Gluconeogenesis and Glycolysis Are Reciprocally Regulated
Gluconeogenesis and glycolysis are reciprocally regulated so that one pathway is relatively inactive while the other is
highly active. Phosphofructokinase and fructose 1,6-bisphosphatase are key control points. Fructose 2,6-bisphosphate, an
intracellular signal molecule present at higher levels when glucose is abundant, activates glycolysis and inhibits
gluconeogenesis by regulating these enzymes. Pyruvate kinase and pyruvate carboxylase are regulated by other effectors
so that both are not maximally active at the same time. Allosteric regulation and reversible phosphorylation, which are
rapid, are complemented by transcriptional control, which takes place in hours or days.
Key Terms
glycolysis
lactic acid fermentation
alcoholic fermentation
gluconeogenesis
obligate anaerobe
facultative anaerobe
hexokinase
kinase
phosphofructokinase (PFK)
thioester intermediate
