Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Stage 2 of glycolysis. Two three-carbon fragments are produced from one six-carbon sugar.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
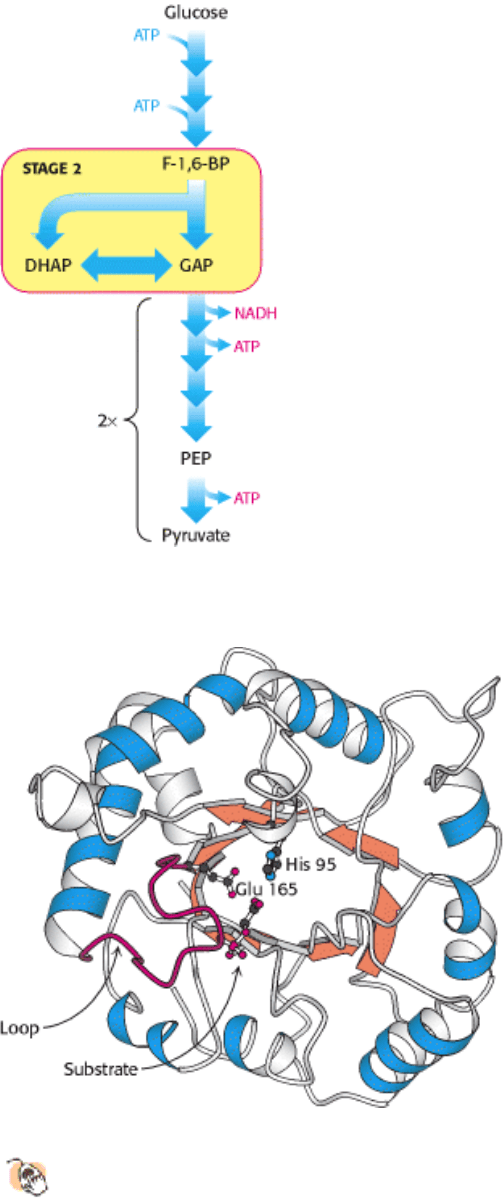
Figure 16.5. Structure of Triose Phosphate Isomerase.
This enzyme consists of a central core of eight parallel β
strands (orange) surrounded by eight α helices (blue). This structural motif, called an α β barrel, is also found in
the glycolytic enzymes aldolase, enolase, and pyruvate kinase. Histidine 95 and glutamate 165, essential
components of the active site of triose phosphate isomerase, are located in the barrel. A loop (red) closes off the active
site on substrate binding.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
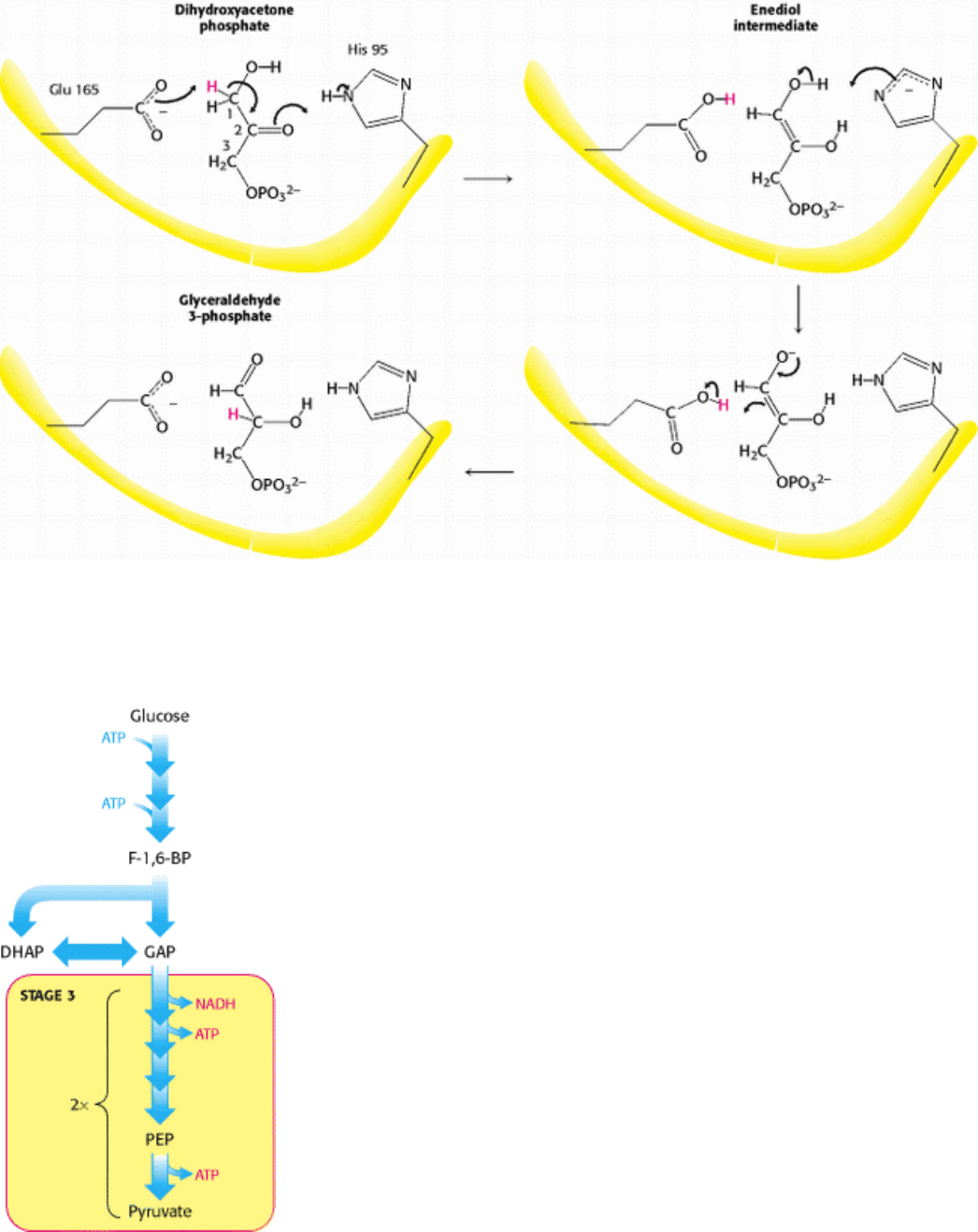
Figure 16.6. Catalytic Mechanism of Triose Phosphate Isomerase. Glutamate 165 transfers a proton between carbons
with the assistance of histidine 95, which shuttles between the neutral and relatively rare negatively charged form. The
latter is stabilized by interactions with other parts of the enzyme.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Stage 3 of Glycolysis. The oxidation of three-carbon fragments yields ATP.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
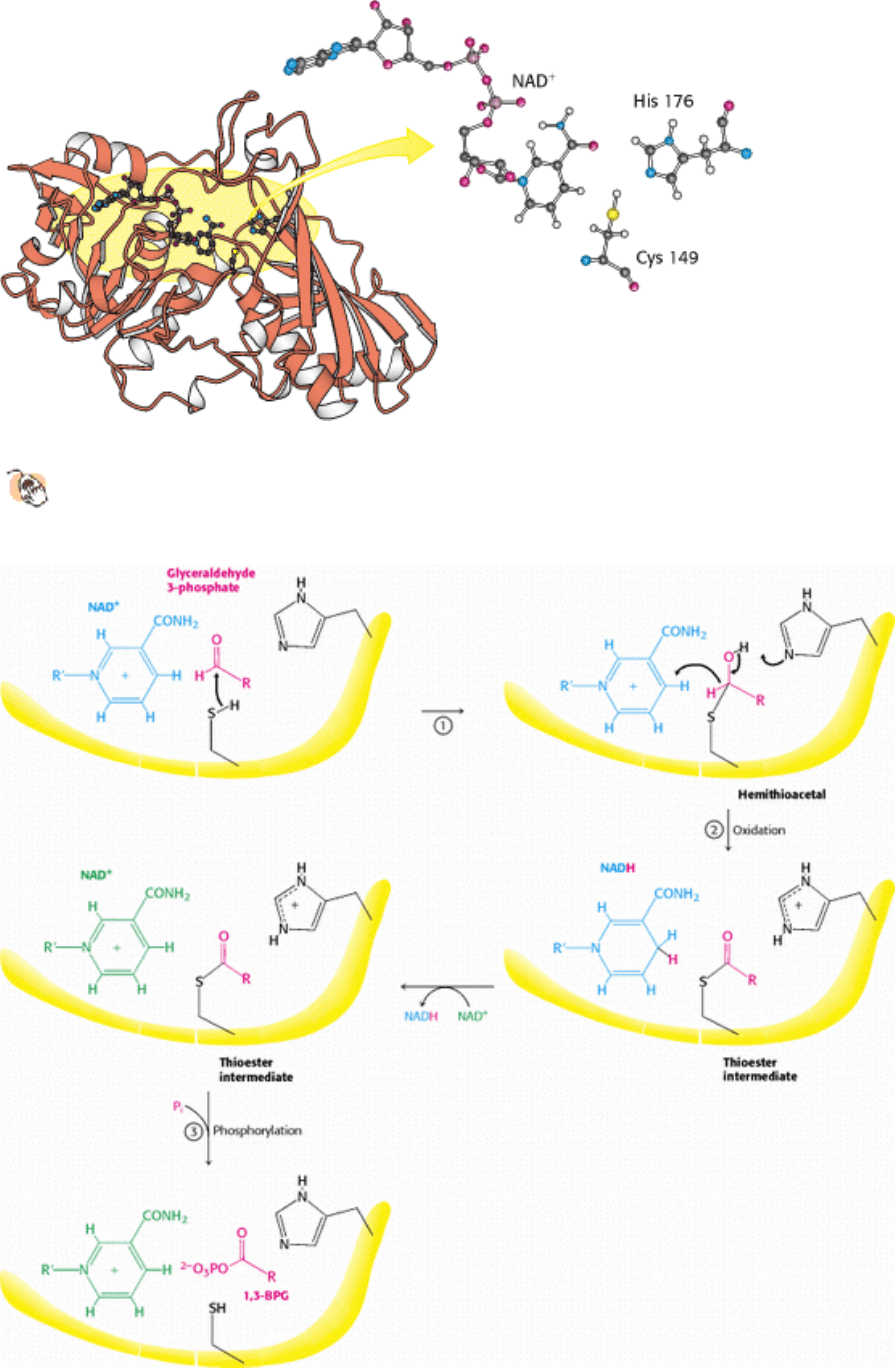
Figure 16.7. Structure of Glyceraldehyde 3-Phosphate Dehydrogenase.
The active site includes a cysteine residue
and a histidine residue adjacent to a bound NAD
+
.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Figure 16.8. Catalytic Mechanism of Glyceraldehyde 3-Phosphate Dehydrogenase. The reaction proceeds through a
thioester intermediate, which allows the oxidation of glyceraldehyde to be coupled to the phosphorylation of 3-
phosphoglycerate.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
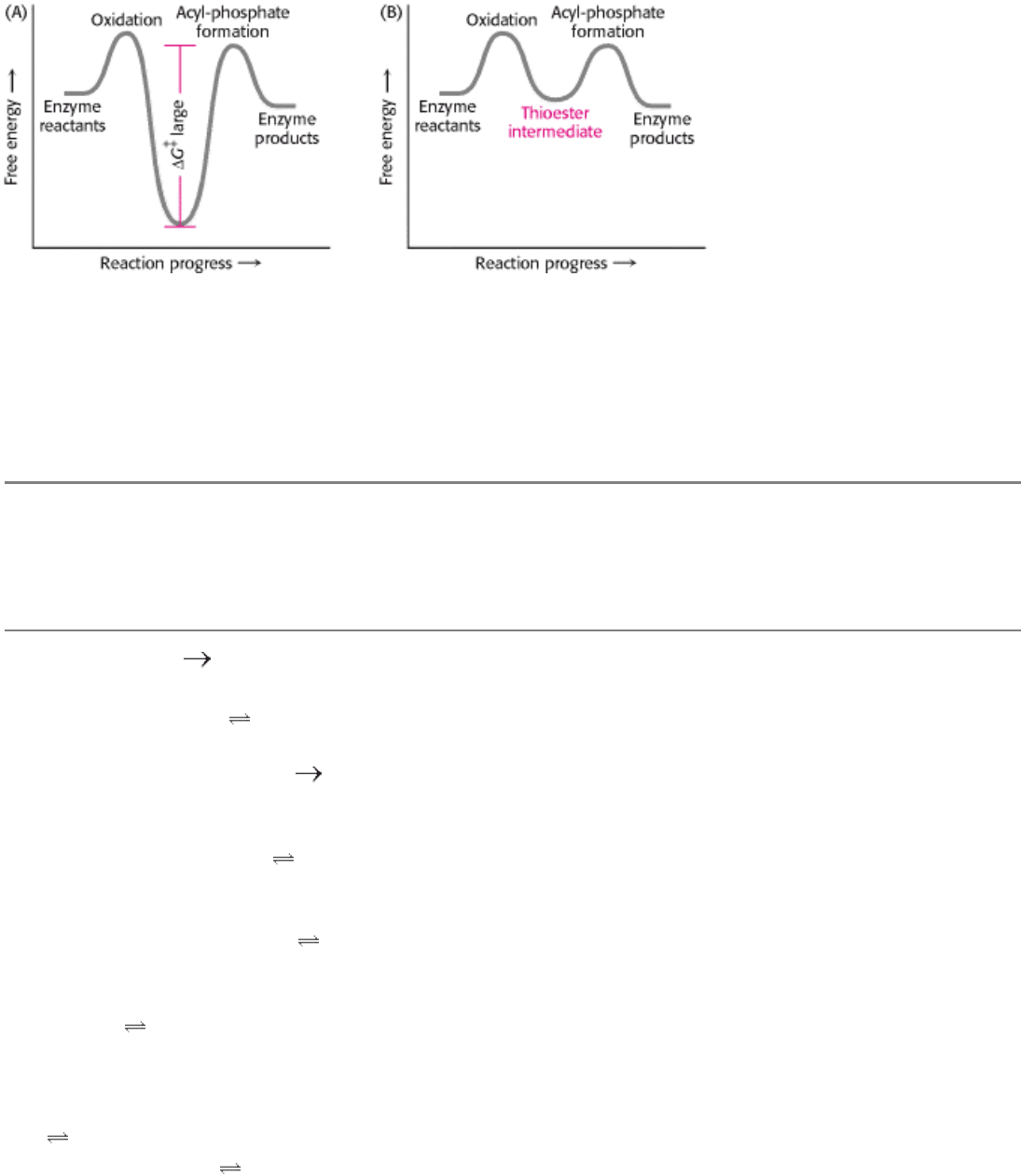
Figure 16.9. Free-Energy Profiles for Glyceraldehyde Oxidation Followed by Acyl-Phosphate Formation. (A) A
hypothetical case with no coupling between the two processes. The second step must have a large activation barrier,
making the reaction very slow. (B) The actual case with the two reactions coupled through a thioester intermediate.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Table 16.3. Reactions of glycolysis
Step Reaction Enzyme Reaction type
∆ G°´ in
kcal mol
-1
(kJ mol
-1
)
∆ G in
kcal mol
-
1
(kJ
mol
-1
)
1 Glucose + ATP glucose 6-
phosphate + ADP + H
+
Hexokinase Phosphoryl transfer -4.0 (-16.7) -8.0 (-
33.5)
2 Glucose 6-phosphate
fructose
6-phosphate
Phosphoglucose isomerase Isomerization +0.4 (+1.7) -0.6 (-
2.5)
3 Fructose 6-phosphate + ATP
fructose 1,6-bisphosphate +
ADP + H
+
Phosphofructokinase Phosphoryl transfer -3.4 (-14.2) -5.3 (-
22.2)
4 Fructose 1,6-bisphosphate
dihydroxyacetone phosphate +
glyceraldehyde 3-phosphate
Aldolase Aldol cleavage +5.7
(+23.8)
-0.3 (-
1.3)
5 Dihydroxyacetone phosphate
glyceraldehyde 3-phosphate
Triose phosphate isomerase Isomerization +1.8 (+7.5) +0.6
(+2.5)
6 Glyceraldehyde 3-phosphate + P
i
+ NAD
+
1,3-
bisphosphoglycerate + NADH +
H
+
Glyceraldehyde 3-phosphate
dehydrogenase
Phosphorylation coupled
to oxidation
+1.5 (+6.3) -0.4 (-
1.7)
7 1,3-Bisphosphoglycerate + ADP
3-phosphoglycerate + ATP
Phosphoglycerate kinase Phosphoryl transfer -4.5 (-18.8) +0.3
(+1.3)
8 3-Phosphoglycerate
2-
phosphoglycerate
Phosphoglycerate mutase Phosphoryl shift +1.1 (+4.6) +0.2
(+0.8)
9 2-Phosphoglycerate
phosphoenolpyruvate + H
2
O
Enolase Dehydration +0.4 (+1.7) -0.8 (-
3.3)
10 Phosphoenolpyruvate + ADP + H
+
pyruvate + ATP
Pyruvate kinase Phosphoryl transfer -7.5 (-31.4) -4.0 (-
16.7)
Note: ∆ G, the actual free-energy change, has been calculated from ∆ G°´ and known concentrations of reactants under typical
physiologic conditions. Glycolysis can proceed only if the ∆ G values of all reactions are negative. The small positive ∆ G values
of three of the above reactions indicate that the concentrations of metabolites in vivo in cells undergoing glycolysis are not
precisely known.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Location of redox balance steps. The generation and consumption of NADH, located within the glycolytic pathway.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
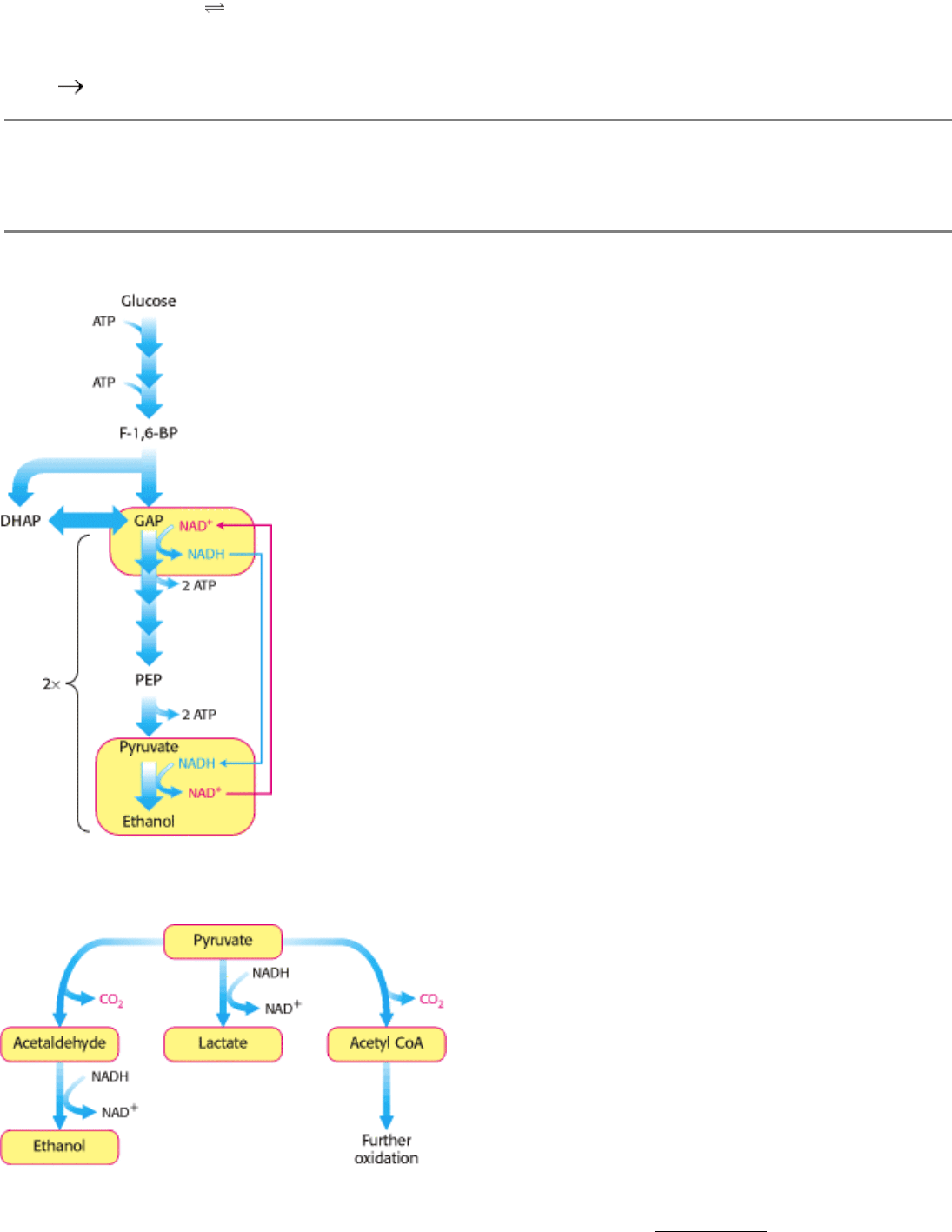
Figure 16.10. Diverse Fates of Pyruvate. Ethanol and lactate can be formed by reactions involving NADH.
Alternatively, a two-carbon unit from pyruvate can be coupled to coenzyme A (see Section 17.1.1) to form acetyl CoA.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
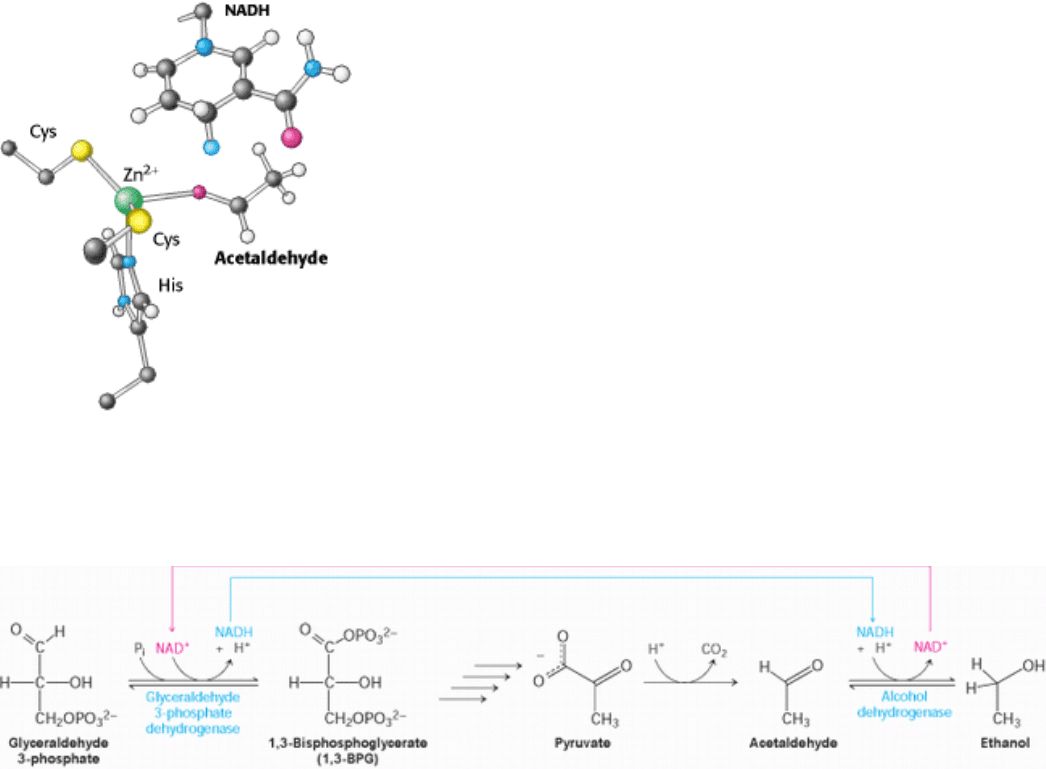
Figure 16.11. Active Site of Alcohol Dehydrogenase. The active site contains a zinc ion bound to two cysteine residues
and one histidine residue. The zinc ion binds the acetaldehyde substrate through its oxygen atom, polarizing it so that it
more easily accepts a hydride (light blue) from NADH. Only the nicotinamide ring of NADH is shown.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Figure 16.12. Maintaining Redox Balance. The NADH produced by the glyceraldehyde 3-phosphate dehydrogenase
reaction must be reoxidized to NAD
+
for the glycolytic pathway to continue. In alcoholic fermentation, alcohol
dehydrogenase oxidizes NADH and generates ethanol. In lactic acid fermentation (not shown), lactate dehydrogenase
oxidizes NADH while generating lactic acid.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
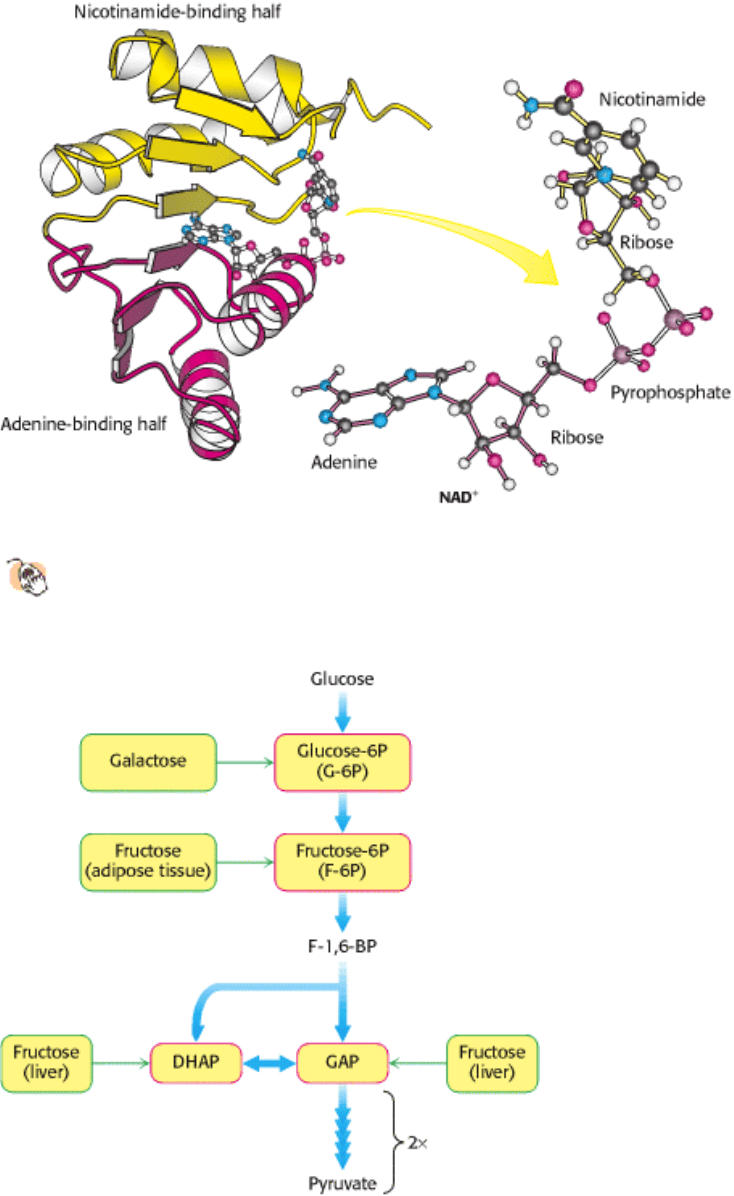
Figure 16.13. NAD
+
-binding region in dehydrogenases. The nicotinamide-binding half (yellow) is structurally similar
to the adenine-binding half (red). The two halves together form a structural motif called a Rossmann fold. The
NAD
+
molecule binds in an extended conformation.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Figure 16.14. Entry Points in Glycolysis for Galactose and Fructose
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
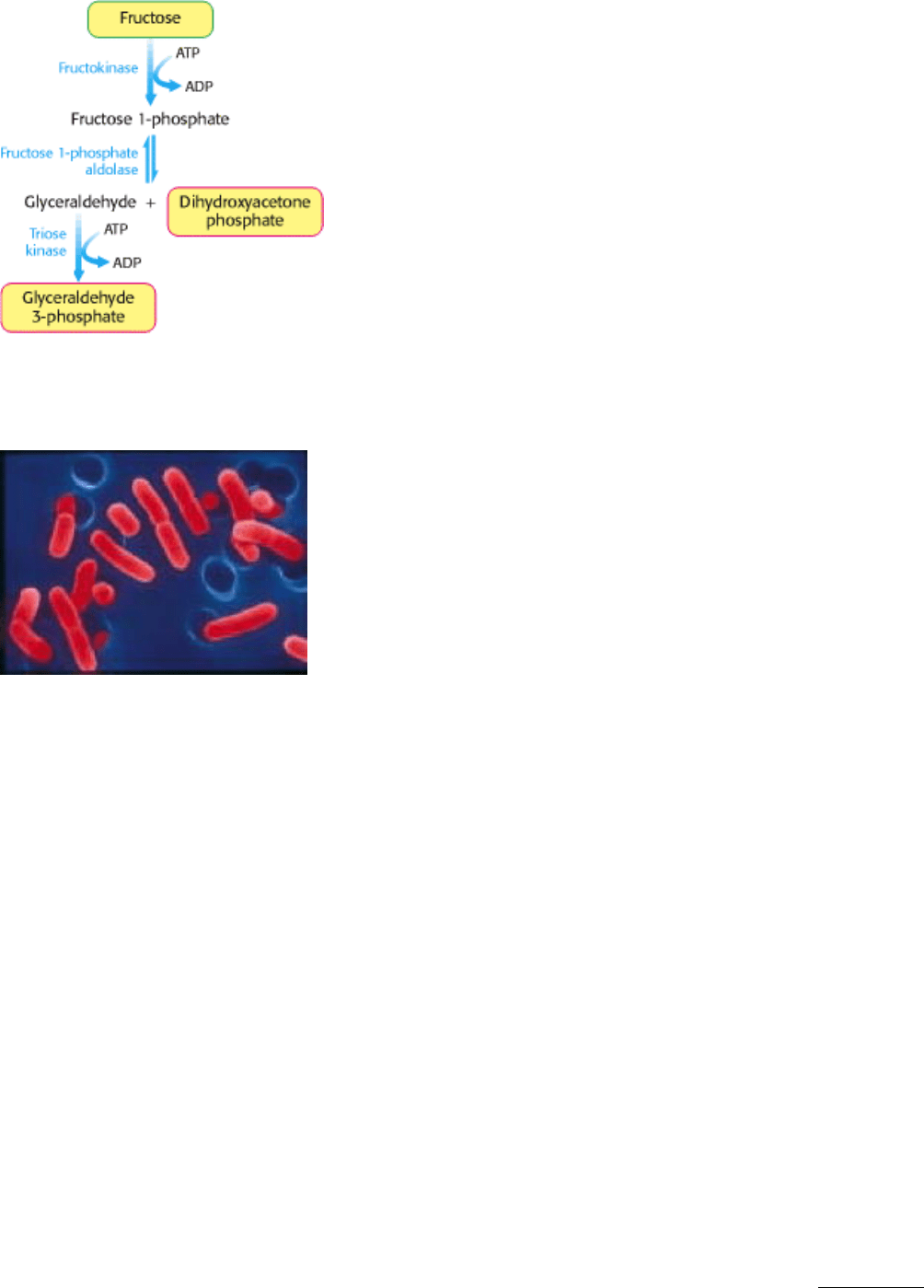
Figure 16.15. Fructose Metabolism. Fructose enters the glycolytic pathway in the liver through the fructose 1-
phosphate pathway.
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis 16.1. Glycolysis Is an Energy-Conversion Pathway in Many Organisms
Scanning electron micrograph of Lactobacillus. The anaerobic bacteria Lactobacillus is shown here (artificially
colored) at a magnification of 22, 245×. As suggested by its name, this genus of bacteria ferments glucose into lactic
acid, and is widely used in the food industry. Lactobacillus is also a component of the normal human bacterial flora of
the urogenital tract where, because of its ability to generate an acidic environment, it prevents growth of harmful
bacteria. [Dr. Dennis Kunkel/PhotoTake.]
II. Transducing and Storing Energy 16. Glycolysis and Gluconeogenesis
16.2. The Glycolytic Pathway Is Tightly Controlled
The flux through the glycolytic pathway must be adjusted in response to conditions both inside and outside the cell. The
rate of conversion of glucose into pyruvate is regulated to meet two major cellular needs: (1) the production of ATP,
generated by the degradation of glucose, and (2) the provision of building blocks for synthetic reactions, such as the
formation of fatty acids. In metabolic pathways, enzymes catalyzing essentially irreversible reactions are potential sites
of control. In glycolysis, the reactions catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase are virtually
irreversible; hence, these enzymes would be expected to have regulatory as well as catalytic roles. In fact, each of them
serves as a control site. Their activities are regulated by the reversible binding of allosteric effectors or by covalent
modification. In addition, the amounts of these important enzymes are varied by the regulation of transcription to meet
changing metabolic needs. The time required for reversible allosteric control, regulation by phosphorylation, and
transcriptional control is typically in milliseconds, seconds, and hours, respectively.
16.2.1. Phosphofructokinase Is the Key Enzyme in the Control of Glycolysis
Phosphofructokinase is the most important control element in the mammalian glycolytic pathway (Figure 16.16). High

levels of ATP allosterically inhibit the enzyme in the liver (a 340-kd tetramer), thus lowering its affinity for fructose 6-
phosphate. A high concentration of ATP converts the hyperbolic binding curve of fructose 6-phosphate into a sigmoidal
one (Figure 16.17). ATP elicits this effect by binding to a specific regulatory site that is distinct from the catalytic site.
AMP reverses the inhibitory action of ATP, and so the activity of the enzyme increases when the ATP/AMP ratio is
lowered. In other words, glycolysis is stimulated as the energy charge falls. A fall in pH also inhibits
phosphofructokinase activity. The inhibition of phosphofructokinase by H
+
prevents excessive formation of lactic acid
(Section 16.1.9) and a precipitous drop in blood pH (acidosis).
Why is AMP and not ADP the positive regulator of phosphofructokinase? When ATP is being utilized rapidly, the
enzyme adenylate kinase (Section 9.4) can form ATP from ADP by the following reaction:
Thus, some ATP is salvaged from ADP, and AMP becomes the signal for the low-energy state. Moreover, the use of
AMP as an allosteric regulator provides an especially sensitive control. We can understand why by considering, first,
that the total adenylate pool ([ATP], [ADP], [AMP]) in a cell is constant over the short term and, second, that the
concentration of ATP is greater than that of ADP and the concentration of ADP is, in turn, greater than that of AMP.
Consequently, small-percentage changes in [ATP] result in larger-percentage changes in the concentrations of the other
adenylate nucleotides. This magnification of small changes in [ATP] to larger changes in [AMP] leads to tighter control
by increasing the range of sensitivity of phosphofructokinase.
Glycolysis also furnishes carbon skeletons for biosyntheses, and so a signal indicating whether building blocks are
abundant or scarce should also regulate phosphofructokinase. Indeed, phosphofructokinase is inhibited by citrate, an
early intermediate in the citric acid cycle (Section 17.1.3). A high level of citrate means that biosynthetic precursors are
abundant and additional glucose should not be degraded for this purpose. Citrate inhibits phosphofructokinase by
enhancing the inhibitory effect of ATP.
In 1980, fructose 2,6-bisphosphate (F-2,6-BP) was identified as a potent activator of phosphofructokinase. Fructose 2,6-
bisphosphate activates phosphofructokinase by increasing its affinity for fructose 6-phosphate and diminishing the
inhibitory effect of ATP (Figure 16.18). In essence, Fructose 2,6-bisphosphate is an allosteric activator that shifts the
conformational equilibrium of this tetrameric enzyme from the T state to the R state.
16.2.2. A Regulated Bifunctional Enzyme Synthesizes and Degrades Fructose 2,6 -
bisphosphate
How is the concentration of fructose 2,6-bisphosphate appropriately controlled? Two enzymes regulate the concentration
of this important regulator of glycolysis by phosphorylating fructose 6-phosphate and dephosphorylating fructose 2,6-
bisphosphate. Fructose 2,6-bisphosphate is formed in a reaction catalyzed by phosphofructokinase 2 (PFK2), a different
enzyme from phosphofructokinase. Fructose 2,6-bisphosphate is hydrolyzed to fructose 6-phosphate by a specific
phosphatase, fructose bisphosphatase 2 (FBPase2). The striking finding is that both PFK2 and FBPase2 are present in a
single 55-kd polypeptide chain (Figure 16.19). This bifunctional enzyme contains an N-terminal regulatory domain,
followed by a kinase domain and a phosphatase domain. PFK2 resembles adenylate kinase in having a P-loop NTPase
domain (Sections 9.4.1 and 9.4.3), whereas FBPase2 resembles phosphoglycerate mutase (Section 16.1.7). Recall that
the mutase is essentially a phosphatase. In the bifunctional enzyme, the phosphatase activity evolved to become specific

for F-2,6-BP. The bifunctional enzyme itself probably arose by the fusion of genes encoding the kinase and phosphatase
domains.
The bifunctional enzyme exists in five isozymic forms (isoforms) that differ in size and kinetics as well as
immunological and regulatory properties. Recall that isoenzymes, or isozymes, have essentially the same architectural
plan and catalytic properties but differ in how they are regulated. The L isoform, which predominates in the liver, and the
M isoform, found in muscle are generated by alternative splicing (Section 28.3.6) of the transcription product of a single
gene. The L isoform helps to maintain blood-glucose homeostasis. In the liver, the concentration of fructose 6-phosphate
rises when blood-glucose concentration is high, and the abundance of fructose 6-phosphate accelerates the synthesis of F-
2,6-BP. Hence, an abundance of fructose 6-phosphate leads to a higher concentration of F-2,6-BP, which in turn
stimulates phosphofructokinase. Such a process is called feedforward stimulation. What controls whether PFK2 or
FBPase2 dominates the bifunctional enzyme's activities in the liver? The activities of PFK2 and FBPase2 are
reciprocally controlled by phosphorylation of a single serine residue. When glucose is scarce, a rise in the blood level of
the hormone glucagon triggers a cyclic AMP cascade, through its 7TM receptor and G
α
s
(Section 15.1), leading to the
phosphorylation of this bifunctional enzyme by protein kinase A (Figure 16.20). This covalent modification activates
FBPase2 and inhibits PFK2, lowering the level of F-2,6-BP. Thus, glucose metabolism by the liver is curtailed.
Conversely, when glucose is abundant, the enzyme loses its attached phosphate group. This covalent modification
activates PFK2 and inhibits FBPase2, raising the level of F-2,6-BP and accelerating glycolysis. This coordinated control
is facilitated by the location of the kinase and phosphatase domains on the same polypeptide chain as the regulatory
domain. We shall return to this elegant switch when we consider the integration of carbohydrate metabolism (Section
16.4).
16.2.3. Hexokinase and Pyruvate kinase Also Set the Pace of Glycolysis
Phosphofructokinase is the most prominent regulatory enzyme in glycolysis, but it is not the only one. Hexokinase, the
enzyme catalyzing the first step of glycolysis, is inhibited by its product, glucose 6-phosphate. High concentrations of
this molecule signal that the cell no longer requires glucose for energy, for storage in the form of glycogen, or as a
source of biosynthetic precursors, and the glucose will be left in the blood. For example, when phosphofructokinase is
inactive, the concentration of fructose 6-phosphate rises. In turn, the level of glucose 6-phosphate rises because it is in
equilibrium with fructose 6-phosphate. Hence, the inhibition of phosphofructokinase leads to the inhibition of
hexokinase. However, the liver, in keeping with its role as monitor of blood-glucose levels, possesses a specialized
isozyme of hexokinase called glucokinase that is not inhibited by glucose 6-phosphate. Glucokinase phosphorylates
glucose only when it is abundant because it has about a 50-fold affinity for glucose than does hexokinase. The role of
glucokinase is to provide glucose 6-phosphate for the synthesis of glycogen, a storage form of glucose (Section 21.4),
and for the formation of fatty acids (Section 22.1). The low glucose affinity of glucokinase in the liver gives the brain
and muscles first call on glucose when its supply is limited, whereas it ensures that glucose will not be wasted when it is
abundant.
Why is phosphofructokinase rather than hexokinase the pacemaker of glycolysis? The reason becomes evident on noting
that glucose 6-phosphate is not solely a glycolytic intermediate. Glucose 6-phosphate can also be converted into
glycogen or it can be oxidized by the pentose phosphate pathway (Section 20.3) to form NADPH. The first irreversible
reaction unique to the glycolytic pathway, the committed step, (Section 10.2), is the phosphorylation of fructose 6-
phosphate to fructose 1,6-bisphosphate. Thus, it is highly appropriate for phosphofructokinase to be the primary control
site in glycolysis. In general, the enzyme catalyzing the committed step in a metabolic sequence is the most important
control element in the pathway.
Pyruvate kinase, the enzyme catalyzing the third irreversible step in glycolysis, controls the outflow from this pathway.
This final step yields ATP and pyruvate, a central metabolic intermediate that can be oxidized further or used as a
building block. Several isozymic forms of pyruvate kinase (a tetramer of 57-kd subunits) encoded by different genes are
present in mammals: the L type predominates in liver, and the M type in muscle and brain. The L and M forms of
pyruvate kinase have many properties in common. Both bind phosphoenolpyruvate cooperatively. Fructose 1,6-
bisphosphate, the product of the preceding irreversible step in glycolysis, activates both isozymes to enable them to keep
pace with the oncoming high flux of intermediates. ATP allosterically inhibits both the L and the M forms of pyruvate
