Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.


Finally, ribose-5-phosphate is converted into ribulose 5-phosphate by phosphopentose isomerase while xylulose 5-
phosphate is converted into ribulose 5-phosphate by phosphopentose epimerase. Ribulose 5-phosphate is converted into
ribulose 1,5-bisphosphate through the action of phosphoribulose kinase (Figure 20.11). The sum of these reactions is
This series of reactions completes the Calvin cycle (Figure 20.12). The sum of all the reactions results in the generation
of a hexose and the regeneration of the starting compound, ribulose 5-phosphate. In essence, ribulose 1,5-bisphosphate
acts catalytically, similarly to oxaloacetate in the citric acid cycle.
20.1.4. Three Molecules of ATP and Two Molecules of NADPH Are Used to Bring
Carbon Dioxide to the Level of a Hexose
What is the energy expenditure for synthesizing a hexose? Six rounds of the Calvin cycle are required, because one
carbon atom is reduced in each round. Twelve molecules of ATP are expended in phosphorylating 12 molecules of 3-
phosphoglycerate to 1,3-bisphosphoglycerate, and 12 molecules of NADPH are consumed in reducing 12 molecules of
1,3-bisphosphoglycerate to glyceraldehyde 3-phosphate. An additional six molecules of ATP are spent in regenerating
ribulose 1,5-bisphosphate. We can now write a balanced equation for the net reaction of the Calvin cycle.
Thus, three molecules of ATP and two molecules of NADPH are consumed in incorporating a single CO
2
molecule into
a hexose such as glucose or fructose.
20.1.5. Starch and Sucrose Are the Major Carbohydrate Stores in Plants
Plants contain two major storage forms of sugar: starch and sucrose. Starch, like its animal counterpart glycogen, is a
polymer of glucose residues, but it is less branched than glycogen because it contains a smaller proportion of α-1,6-
glycosidic linkages (Section 11.2.2). Another difference is that ADP-glucose, not UDP-glucose, is the activated
precursor. Starch is synthesized and stored in chloroplasts.
In contrast, sucrose (common table sugar), a disaccharide, is synthesized in the cytosol. Plants lack the ability to
transport hexose phosphates across the chloroplast membrane, but an abundant phosphate translocator mediates the
transport of triose phosphates from chloroplasts to the cytosol in exchange for phosphate. Fructose 6-phosphate formed
from triose phosphates joins the glucose unit of UDP-glucose to form sucrose 6-phosphate (Figure 20.13). Hydrolysis of
the phosphate ester yields sucrose, a readily transportable and mobilizable sugar that is stored in many plant cells, as in
sugar beets and sugar cane.

II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.1. Calvin Cycle. The Calvin cycle consists of three stages. Stage 1 is the fixation of carbon by the
carboxylation of ribulose 1,5-bisphosphate. Stage 2 is the reduction of the fixed carbon to begin the synthesis of hexose.
Stage 3 is the regeneration of the starting compound, ribulose 1,5-bisphosphate.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.2. Tracing the Fate of Carbon Dioxide. Radioactivity from
14
CO
2
is incorporated into 3-phosphoglycerate
within 5 s in irradiated cultures of algae. After 60 s, the radioactivity appears in many compounds, the intermediates
within the Calvin cycle. [Courtesy of Dr. J. A. Bassham.]

II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.3. Structure of Rubisco.
The enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase (rubisco) comprises
eight large subunits (one shown in red and the others in yellow) and eight small subunits (one shown in blue and
the others in white). The active sites lie in the large subunits.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.4. Role of the Magnesium Ion in the Rubisco Mechanism. Ribulose 1,5-bisphosphate binds to a magnesium
ion that is linked to rubisco through a glutamate residue, an aspartate residue, and the lysine carbamate. The coordinated

ribulose 1,5-bisphosphate gives up a proton to form a reactive enediolate species that reacts with CO
2
to form a new
carbon-carbon bond.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.5. Formation of 3-Phosphoglycerate. The overall pathway for the conversion of ribulose 1,5 bisphosphate
and CO
2
into two molecules of 3-phosphoglycerate. Although the free species are shown, these steps take place on the
magnesium ion.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.6. A Wasteful Side Reaction. The reactive enediolate intermediate on rubisco also reacts with molecular
oxygen to form a hydroperoxide intermediate, which then proceeds to form one molecule of 3-phosphoglycerate and one
molecule of phosphoglycolate.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water

Figure 20.7. Photorespiratory Reactions. Phosphoglycolate is formed as a product of the oxygenase reaction in
chloroplasts. After dephosphorylation, glycolate is transported into peroxisomes where it is converted into glyoxylate
and then glycine. In mitochondria, two glycines are converted into serine, after losing a carbon as CO
2
and ammonia.
The ammonia is salvaged in chloroplasts.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.8. Electron Micrograph of a Peroxisome Nestled between Two Chloroplasts. [Courtesy of Dr. Sue Ellen
Frederick.]
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
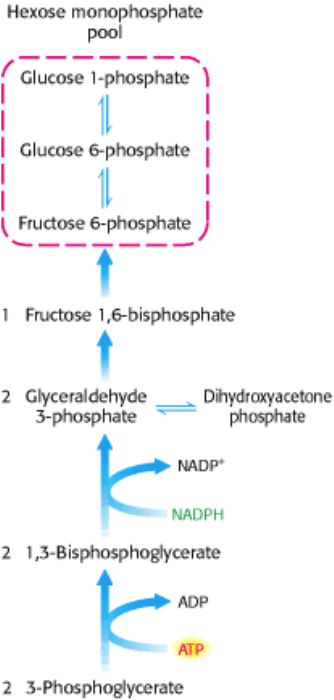
Figure 20.9. Hexose Phosphate Formation. 3-Phosphoglycerate is converted into fructose 6-phosphate in a pathway
parallel to that of glyconeogenesis.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water

Figure 20.10. Formation of Five-Carbon Sugars. First, transketolase converts a six-carbon sugar and a three-carbon
sugar into a four-carbon sugar and a five-carbon sugar. Then, aldolase combines the four-carbon product and a three-
carbon sugar to form a seven-carbon sugar. Finally, this seven-carbon fragment combines with another three-carbon
fragment to form two additional five-carbon sugars.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water

Figure 20.11. Regeneration of Ribulose 1,5-Bisphosphate. Both ribose 5-phosphate and xylulose 5-phosphate are
converted into ribulose 5-phosphate, which is then phosphorylated to complete the regeneration of ribulose 1,5-
bisphosphate.
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water
Figure 20.12. Calvin Cycle. The diagram shows the reactions necessary with the correct stoichiometry to convert three
molecules of CO
2
into one molecule of DHAP. The cycle is not as simple as presented in Figure 20.1; rather, it entails
many reactions that lead ultimately to the synthesis of glucose and the regeneration of ribulose 1,5-bisphosphate. [After
J. R. Bowyer and R. C. Leegood. "Photosynthesis," in Plant Biochemistry, P. M. Dey and J. B. Harborne, Eds.
(Academic Press, 1997), p. 85.]
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway 20.1. The Calvin Cycle Synthesizes Hexoses from Carbon Dioxide and Water

Figure 20.13. Synthesis of Sucrose. Sucrose 6-phosphate is formed by the reaction between fructose 6-phosphate and
the activated intermediate uridine diphosphate glucose (UDP-glucose).
II. Transducing and Storing Energy 20. The Calvin Cycle and the Pentose Phosphate Pathway
20.2. The Activity of the Calvin Cycle Depends on Environmental Conditions
Carbon dioxide assimilation by the Calvin cycle operates during the day, whereas carbohydrate degradation to yield
energy takes place primarily at night. How are synthesis and degradation coordinately controlled? The light reactions
lead to changes in the stroma
namely, an increase in pH and in Mg
2+
, NADPH, and reduced ferredoxin
concentration all of which contribute to the activation of certain Calvin cycle enzymes (Figure 20.14).
20.2.1. Rubisco Is Activated by Light-Driven Changes in Proton and Magnesium Ion
Concentrations
As stated earlier, the rate-limiting step in the Calvin cycle is the carboxylation of ribulose 1,5-bisphosphate to form two
molecules of 3-phosphoglycerate. The activity of rubisco increases markedly on illumination. The addition of CO
2
to
lysine 201 of rubisco to form the carbamate is essential for Mg
2+
coordination and, hence, catalytic activity (Section
20.1.1). Carbamate formation is favored by alkaline pH and high concentrations of Mg
2+
ion in the stroma, both of
which are consequences of the light-driven pumping of protons from the stroma into the thylakoid space. Magnesium ion
concentration rises because Mg
2+
ions from the thylakoid space are released into the stroma to compensate for the influx
of protons.
20.2.2. Thioredoxin Plays a Key Role in Regulating the Calvin Cycle
Light-driven reactions lead to electron transfer from water to ferredoxin and, eventually, to NADPH. Both reduced
ferredoxin and NADPH regulate enzymes from the Calvin cycle. One key protein in these regulatory processes is
thioredoxin, a 12-kd protein containing neighboring cysteine residues that cycle between a reduced sulfhydryl and an
oxidized disulfide form (Figure 20.15). The reduced form of thioredoxin activates many biosynthetic enzymes by
reducing disulfide bridges that control their activity and inhibits several degradative enzymes by the same means (Table

20.1). In chloroplasts, oxidized thioredoxin is reduced by ferredoxin in a reaction catalyzed by ferredoxin-thioredoxin
reductase. This enzyme contains a 4Fe-4S cluster that couples two one-electron oxidations of reduced ferredoxin to the
two-electron reduction of thioredoxin. Thus, the activities of the light and dark reactions of photosynthesis are
coordinated through electron transfer from reduced ferredoxin to thioredoxin and then to component enzymes
containing regulatory disulfide bonds (Figure 20.16). We shall return to thioredoxin when we consider the reduction of
ribonucleotides (Section 25.3).
Other means of control also exist. For instance, phosphoribulose kinase and glyceraldehyde 3-phosphate dehydrogenase
also are regulated by NADPH directly. In the dark, these enzymes associate with a small protein called CP12 to form a
large complex in which the enzymes are inactivated. NADPH generated in the light reactions binds to this complex,
leading to the release of the enzymes. Thus, the activity of these enzymes depends first on reduction by thioredoxin and
then on the NADPH-mediated release from CP12.
20.2.3. The C
4
Pathway of Tropical Plants Accelerates Photosynthesis by
Concentrating Carbon Dioxide
Recall that the oxygenase activity of rubisco increases more rapidly with temperature than does its carboxylase activity.
How then do plants, such as sugar cane, that grow in hot climates prevent very high rates of wasteful photorespiration?
Their solution to this problem is to achieve a high local concentration of CO
2
at the site of the Calvin cycle in their
photosynthetic cells. The essence of this process, which was elucidated by M. D. Hatch and C. R. Slack, is that four-
carbon (C
4
) compounds such as oxaloacetate and malate carry CO
2
from mesophyll cells, which are in contact with air,
to bundle-sheath cells, which are the major sites of photosynthesis (Figure 20.17). Decarboxylation of the four-carbon
compound in a bundle-sheath cell maintains a high concentration of CO
2
at the site of the Calvin cycle. The three-carbon
compound pyruvate returns to the mesophyll cell for another round of carboxylation.
The C
4
pathway for the transport of CO
2
starts in a mesophyll cell with the condensation of CO
2
and
phosphoenolpyruvate to form oxaloacetate, in a reaction catalyzed by phosphoenolpyruvate carboxylase. In some
species, oxaloacetate is converted into malate by an NADP
+
-linked malate dehydrogenase. Malate goes into the bundle-
sheath cell and is oxidatively decarboxylated within the chloroplasts by an NADP
+
-linked malate dehydrogenase. The
released CO
2
enters the Calvin cycle in the usual way by condensing with ribulose 1,5-bisphosphate. Pyruvate formed in
this decarboxylation reaction returns to the mesophyll cell. Finally, phosphoenolpyruvate is formed from pyruvate by
pyruvate-P
i
dikinase.
The net reaction of this C
4
pathway is
Thus, the energetic equivalent of two ATP molecules is consumed in transporting CO
2
to the chloroplasts of the bundle-
sheath cells. In essence, this process is active transport: the pumping of CO
2
into the bundle-sheath cell is driven by the
hydrolysis of one molecule of ATP to one molecule of AMP and two molecules of orthophosphate. The CO
2
concentration can be 20-fold as great in the bundle-sheath cells as in the mesophyll cells.
When the C
4
pathway and the Calvin cycle operate together, the net reaction is
