Berg J.M., Tymoczko J.L., Stryer L. Biochemistry
Подождите немного. Документ загружается.

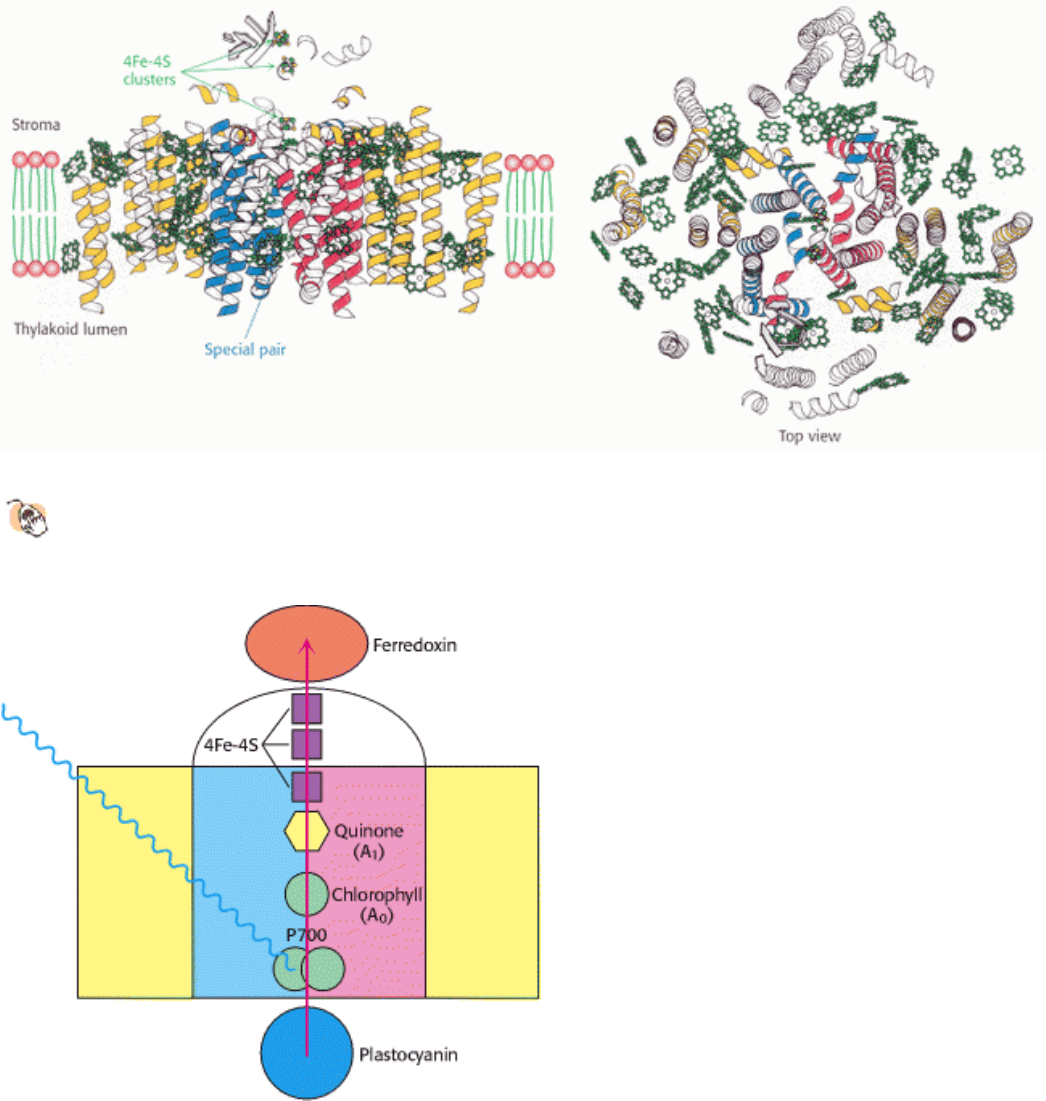
of the structure and absorb light maximally at 700 nm. This center, P700, initiates photoinduced charge separation
(Figure 19.20). The electron is transferred down a pathway through chlorophyll at site A
0
and quinone at site A
1
to a set
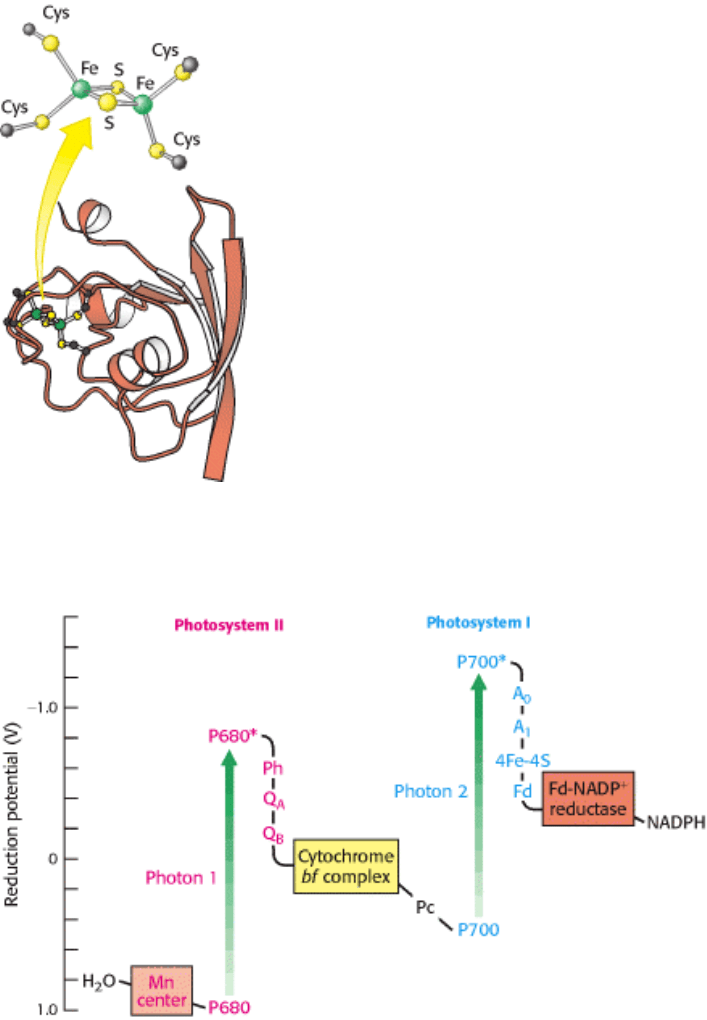
of 4Fe-4S clusters. From there, the electron is transferred to ferredoxin (Fd), a soluble protein containing a 2Fe-2S
cluster coordinated to four cysteine residues (Figure 19.21). The positive charge of P700
+
is neutralized by the transfer
of an electron from reduced plastocyanin. Thus, the overall reaction catalyzed by photosystem I is a simple one-electron
oxidation-reduction reaction.
Given that the reduction potentials for plastocyanin and ferredoxin are +0.37 V and -0.45 V, respectively, the standard
free energy for the oxidation of reduced plastocyanin by oxidized ferredoxin is +18.9 kcal mol
-1
(+79.1 kJ mol
-1
). This
uphill reaction is driven by the absorption of a 700-nm photon which has an energy of 40.9 kcal mol
-1
(171 kJ mol
-1
).
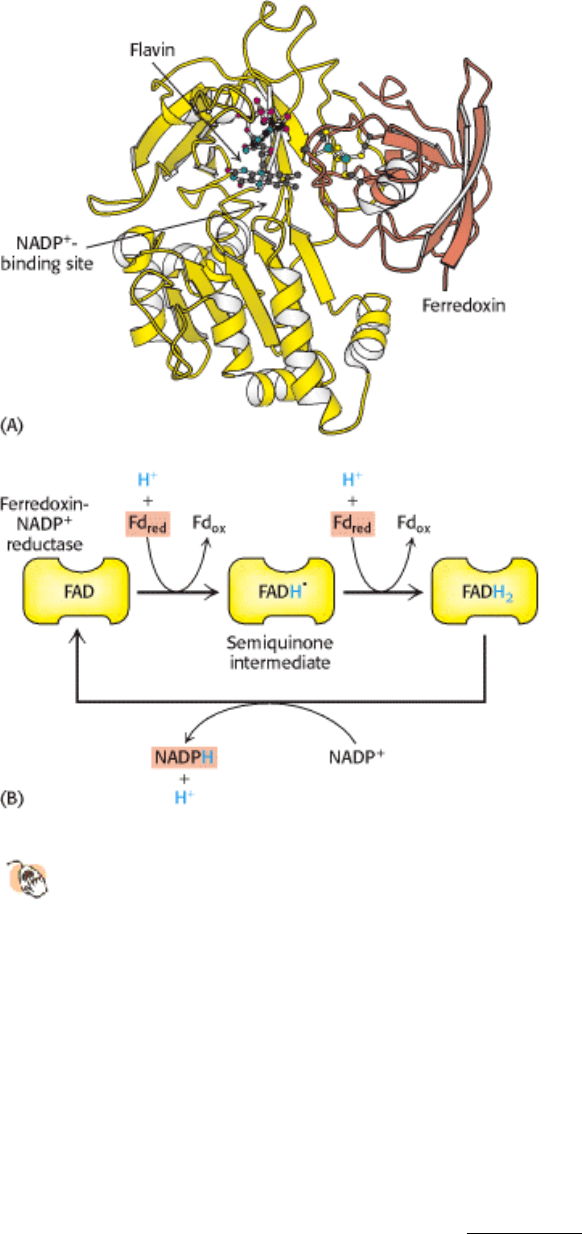
The cooperation between photosystem I and photosystem II creates electron flow from H
2
O to NADP
+
. The pathway of
electron flow is called the Z scheme of photosynthesis because the redox diagram from P680 to P700* looks like the
letter Z (Figure 19.22).
19.3.4. Ferredoxin-NADP
+
Reductase Converts NADP
+
into NADPH
Although reduced ferredoxin is a strong reductant, it is not useful for driving many reactions, in part because ferredoxin
carries only one available electron. In contrast, NADPH, a two-electron reductant, is widely used in biosynthetic
processes, including the reactions of the Calvin cycle (Chapter 20). How can reduced ferredoxin be used to drive the
reduction of NADP
+
to NADPH? This reaction is catalyzed by ferredoxin-NADP
+
reductase, a flavoprotein (Figure
19.23A). The bound FAD moiety in this enzyme accepts electrons, one at a time, from two molecules of reduced
ferredoxin as it proceeds from its oxidized form, through a semiquinone intermediate, to its fully reduced form (Figure
19.23B). The enzyme then transfers a hydride ion to NADP
+
to form NADPH. Note that this reaction takes place on the
stromal side of the membrane. Hence, the uptake of a proton in the reduction of NADP
+
further contributes to the proton
gradient across the thylakoid membrane.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
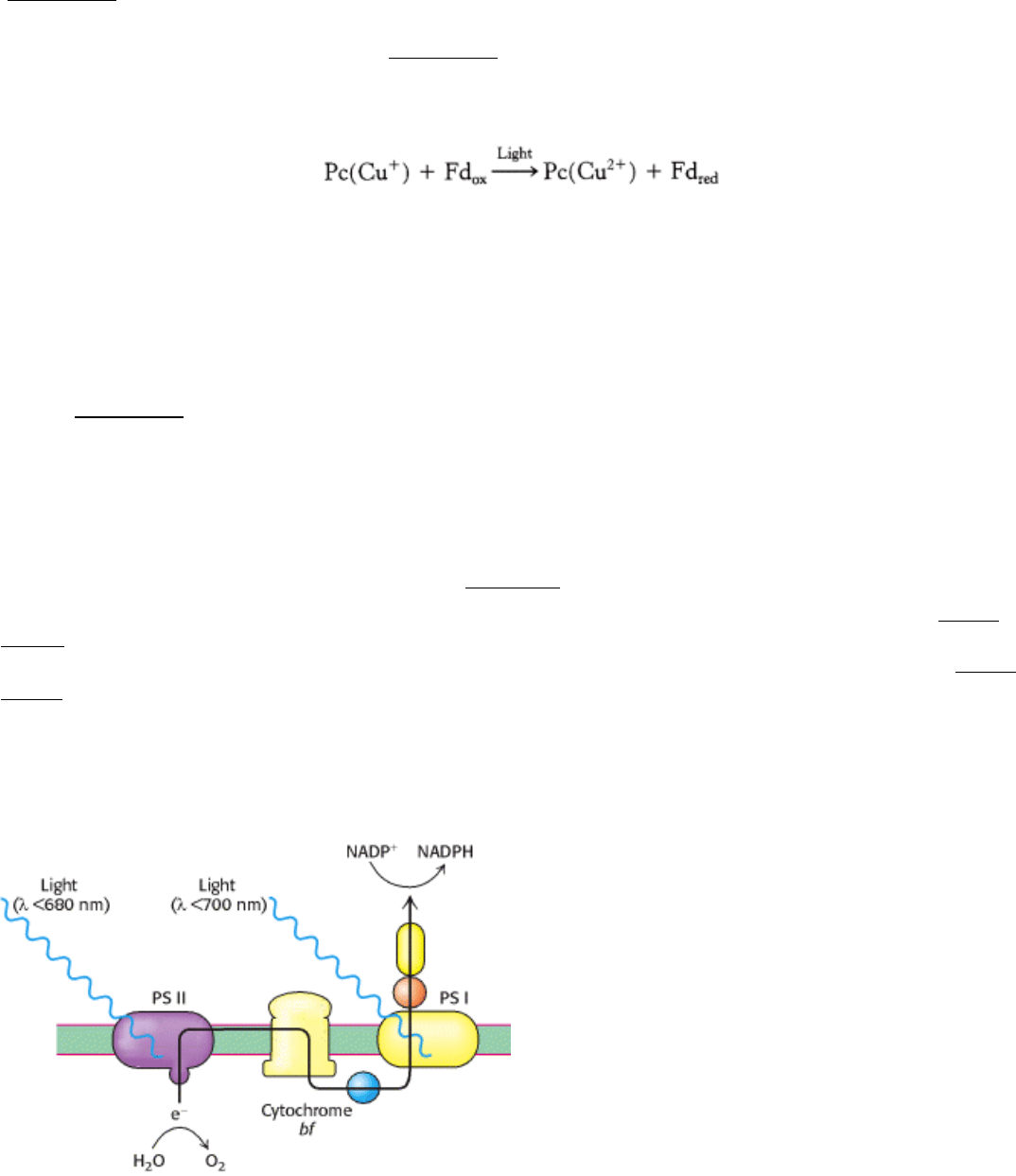
Figure 19.11. Two Photosystems. The absorption of photons by two distinct photosytems (PS I and PS II) is required
for complete electron flow from water to NADP
+
.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
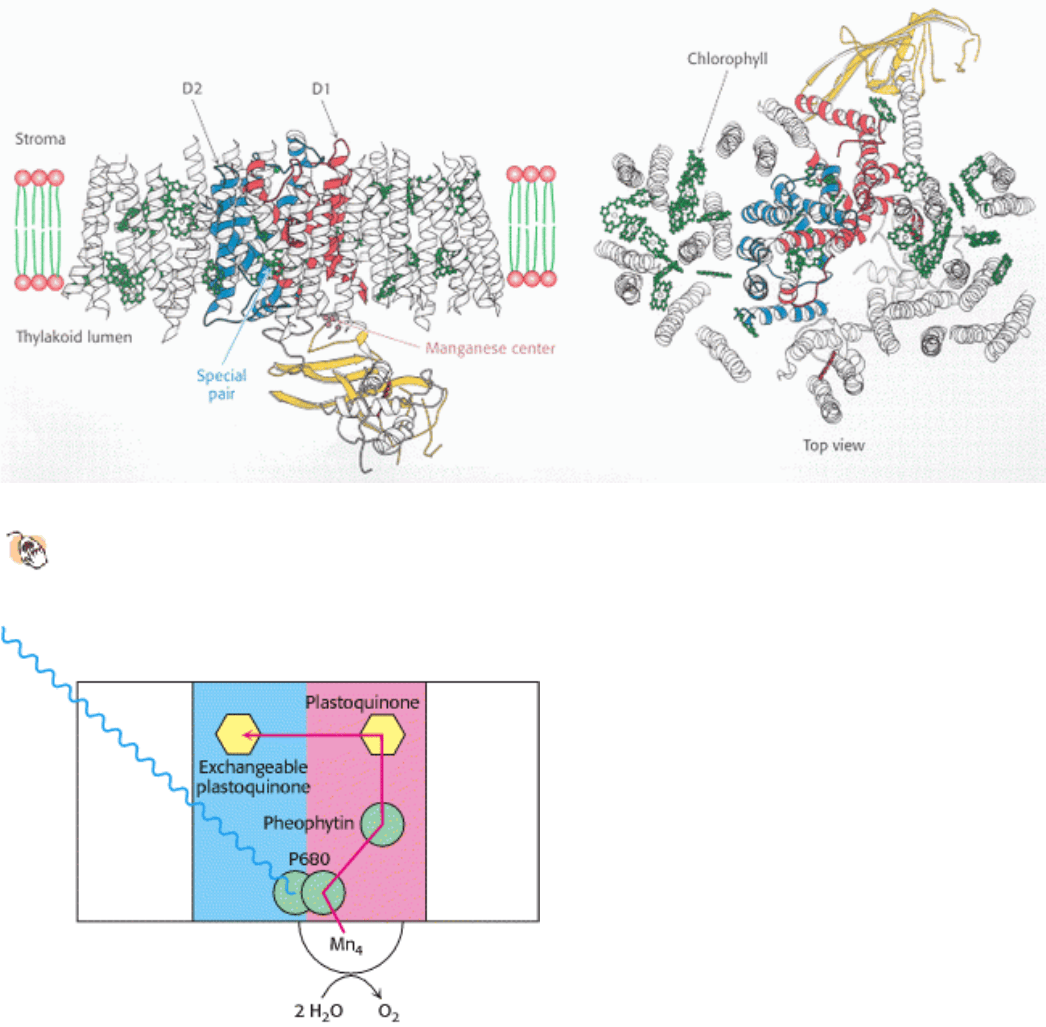
Figure 19.12. The Structure of Photosystem II.
The D1 and D2 subunits are shown in red and blue and the structure of
a bound cytochrome molecule is shown in yellow. Chlorophyll molecules are shown in green.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.13. Electron Flow Through Photosystem II. Light absorption induces electron transfer from P680 down an
electron-transfer pathway to an exchangeable plastoquinone. The positive charge on P680 is neutralized by electron flow
from water molecules bound at the manganese center.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
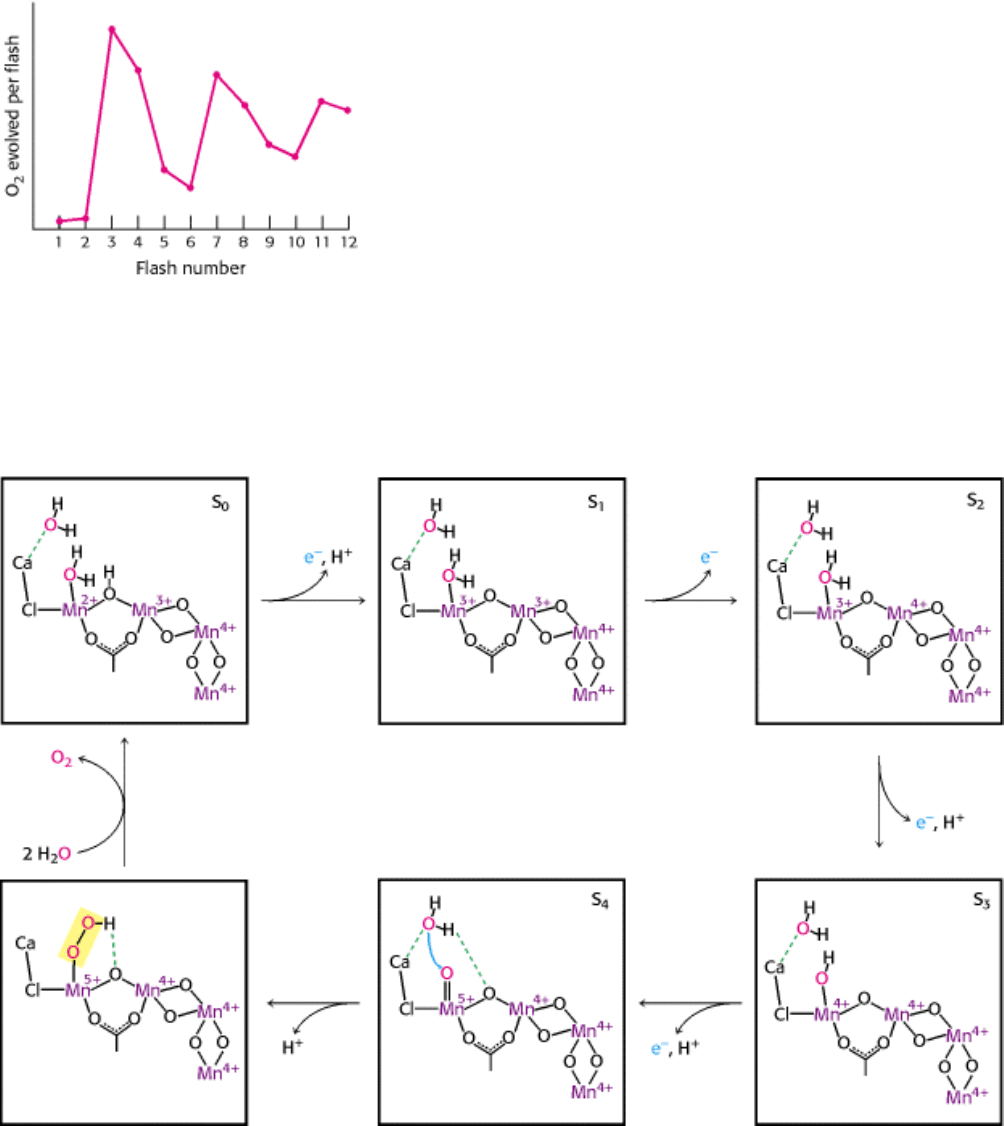
Figure 19.14. Four Photons Are Required to Generate One Oxygen Molecule. When dark-adapted chloroplasts are
exposed to a brief flash of light, one electron passes through photosystem II. Monitoring the O
2
released after each flash
reveals that four flashes are required to generate each O
2
molecule. The peaks in O
2
release occur after the 3rd, 7th, and
11th flashes because the dark-adapted chloroplasts start in the S
1
state-that is, the one-electron reduced state.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.15. A Plausible Scheme for Oxygen Evolution from the Manganese Center. A possible partial structure
for the manganese center is shown. The center is oxidized, one electron at a time, until two bound H
2
O molecules are
linked to form a molecule of O
2
, which is then released from the center. A tyrosine residue (not shown) also participates
in the coupled proton-electron transfer steps. The structures are designated S
0
through S
4
to indicate the number of
electrons that have been removed.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
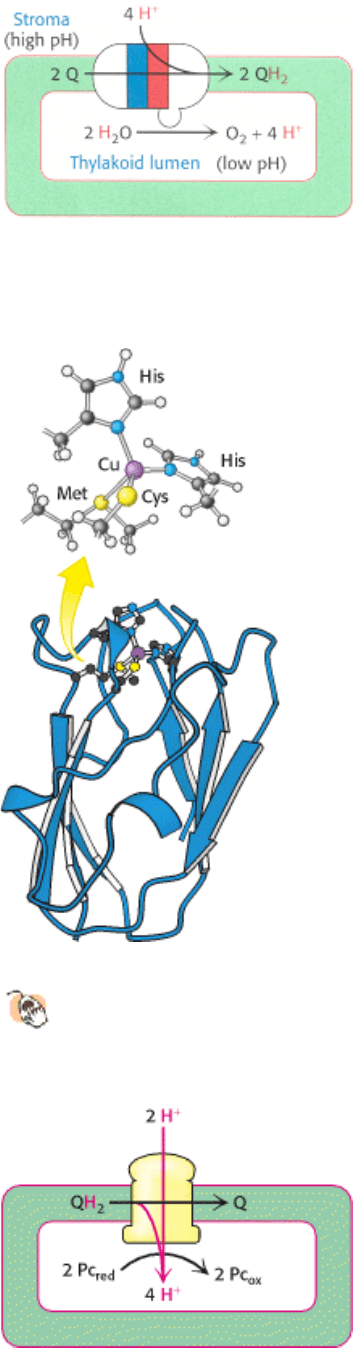
Figure 19.16. Proton-Gradient Direction. Photosystem II releases protons into the thylakoid lumen and takes them up
from the stroma. The result is a pH gradient across the thylakoid membrane with an excess of protons (low pH) inside.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.17. Structure of Plastocyanin.
Two histidine residues, a cysteine residue, and a methionine residue
coordinate a copper ion in a distorted tetrahedral manner in this protein, which carries electrons from cytochrome
bf complex to photosystem I.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.18. Cytochrome BF Contribution to Proton Gradient. The cytochrome bf complex oxidizes QH
2
to Q
through the Q cycle. Four protons are released into the thylakoid lumen in each cycle.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.19. Structure of Photosystem I.
The psaA and psaB subunits are shown in yellow, with the regions similar to
those in the core of photosytem II shown in red and blue. Chlorophyll molecules are shown in green and three 4Fe-
4S clusters are indicated.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.20. Electron Flow Through Photosystem I to Ferredoxin. Light absorption induces electron transfer from
P700 down an electron-transfer pathway that includes a chlorophyll molecule, a quinone molecule, and three 4Fe-4S
clusters to reach ferredoxin. The positive charge left on P700 is neutralized by electron transfer from reduced
plastocyanin.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.21. Structure of Ferredoxin. In plants, ferredoxin contains a 2Fe-2S cluster. This protein accepts electrons
from photosystem I and carries them to ferredoxin-NADP
+
reductase.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.22. Pathway of Electron Flow From H
2
O to NADP
+
in Photosynthesis. This endergonic reaction is made
possible by the absorption of light by photosystem II (P680) and photosystem I (P700). Abbreviations: Ph, pheophytin;
Q
A
and Q
B
, plastoquinone-binding proteins; Pc, plastocyanin; A
0
and A
1
, acceptors of electrons from P700*; Fd,
ferredoxin; Mn, manganese.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.3. Two Photosystems Generate a Proton Gradient and NADPH in Oxygenic Photosynthesis
Figure 19.23. Structure and Function of Ferredoxin-NADP
+
Reductase. (A) Structure of ferredoxin-NADP
+
reductase. This enzyme accepts electrons, one at a time, from ferredoxin (shown in orange). (B) Ferredoxin-NADP
+
reductase first accepts one electron from reduced ferredoxin to form a flavin semiquinone intermediate. The
enzyme then accepts a second electron to form FADH
2
, which then transfers two electrons and a proton to NADP
+
to
form NADPH.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis
19.4. A Proton Gradient Across the Thylakoid Membrane Drives ATP Synthesis
In 1966, André Jagendorf showed that chloroplasts synthesize ATP in the dark when an artificial pH gradient is imposed
across the thylakoid membrane. To create this transient pH gradient, he soaked chloroplasts in a pH 4 buffer for several
hours and then rapidly mixed them with a pH 8 buffer containing ADP and P
i
. The pH of the stroma suddenly increased
to 8, whereas the pH of the thylakoid space remained at 4. A burst of ATP synthesis then accompanied the disappearance
of the pH gradient across the thylakoid membrane (Figure 19.24). This incisive experiment was one of the first to
unequivocally support the hypothesis put forth by Peter Mitchell that ATP synthesis is driven by proton-motive force.
The principles by which ATP synthesis takes place in chloroplasts are nearly identical with those for oxidative
phosphorylation. We have seen how light induces electron transfer through photosystems II and I and the cytochrome bf
complex. At various stages in this process, protons are released into the thylakoid lumen or taken up from the stroma,
generating a proton gradient. Such a gradient can be maintained because the thylakoid membrane is essentially
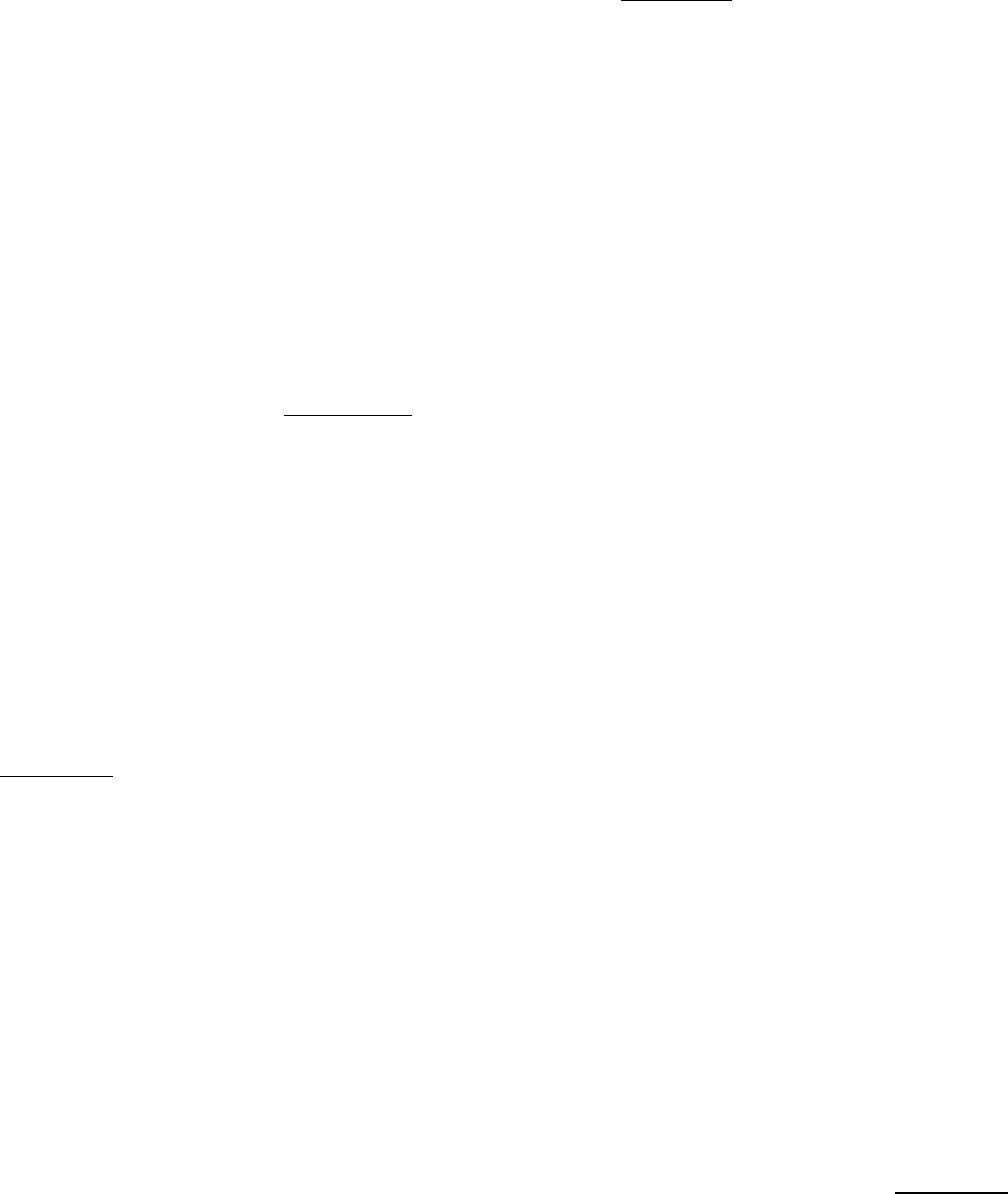
impermeable to protons. The thylakoid space becomes markedly acidic, with the pH approaching 4. The light-induced
transmembrane proton gradient is about 3.5 pH units. As discussed in Section 18.4, energy inherent in the proton
gradient, called the proton-motive force (∆p), is described as the sum of two components: a charge gradient and a
chemical gradient. In chloroplasts, nearly all of ∆ p arises from the pH gradient, whereas, in mitochondria, the
contribution from the membrane potential is larger. The reason for this difference is that the thylakoid membrane is quite
permeable to Cl
-
and Mg
2+
. The light-induced transfer of H
+
into the thylakoid space is accompanied by the transfer of
either Cl
-
in the same direction or Mg
2+
(1 Mg
2+
per 2 H
+
) in the opposite direction. Consequently, electrical neutrality
is maintained and no membrane potential is generated. A pH gradient of 3.5 units across the thylakoid membrane
corresponds to a proton-motive force of 0.20 V or a ∆ G of -4.8 kcal mol
-1
(-20.0 kJ mol
-1
).
19.4.1. The ATP Synthase of Chloroplasts Closely Resembles Those of Mitochondria
and Prokaryotes
The proton-motive force generated by the light reactions is converted into ATP by the ATP synthase of chloroplasts, also
called the CF
1
-CF
0
complex (C stands for chloroplast and F for factor). CF
1
-CF
0
ATP synthase closely resembles the
F
1
-F
0
complex of mitochondria (Section 18.4.1). CF
0
conducts protons across the thylakoid membrane, whereas CF
1
catalyzes the formation of ATP from ADP and P
i
.
CF
0
is embedded in the thylakoid membrane. It consists of four different polypeptide chains known as I (17 kd), II (16.5
kd), III (8 kd), and IV (27 kd) having an estimated stoichiometry of 1:2:12:1. Subunits I, II, and III correspond to
subunits a, b, and c, respectively, of the mitochondrial F
0
subunit, and subunit IV is similar in sequence to subunit a.
CF
1
, the site of ATP synthesis, has a subunit composition α
3
β
3
γ δ ε. The β subunits contain the catalytic sites, similar
to the F
1
subunit of mitochondrial ATP synthase. Remarkably, β subunits of corn chloroplast ATP synthase are more
than 60% identical in amino acid sequence with those of human ATP synthase, despite the passage of approximately 1
billion years since the separation of the plant and animal kingdoms.
Significantly, the membrane orientation of CF
1
-CF
0
is reversed compared with that of the mitochondrial ATP synthase
(Figure 19.25). Thus, protons flow out of the thylakoid lumen through ATP synthase into the stroma. Because CF
1
is on
the stromal surface of the thylakoid membrane, the newly synthesized ATP is released directly into the stromal space.
Recall that NADPH formed through the action of photosystem I and ferredoxin-NADP
+
reductase also is released into
the stromal space. Thus, ATP and NADPH, the products of the light reactions of photosynthesis, are appropriately
positioned for the subsequent dark reactions, in which CO
2
is converted into carbohydrate.
19.4.2. Cyclic Electron Flow Through Photosystem I Leads to the Production of ATP
Instead of NADPH
An alternative pathway for electrons arising from P700, the reaction center of photosystem I, contributes to the
versatility of photosynthesis. The electron in reduced ferredoxin can be transferred to the cytochrome bf complex rather
than to NADP
+
. This electron then flows back through the cytochrome bf complex to reduce plastocyanin, which can
then be reoxidized by P700
+
to complete a cycle. The net outcome of this cyclic flow of electrons is the pumping of
protons by the cytochrome bf complex. The resulting proton gradient then drives the synthesis of ATP. In this process,
called cyclic photophosphorylation, ATP is generated without the concomitant formation of NADPH (Figure 19.26).
Photosystem II does not participate in cyclic photophosphorylation, and so O
2
is not formed from H
2
O. Cyclic
photophosphorylation takes place when NADP
+
is unavailable to accept electrons from reduced ferredoxin, because of a
very high ratio of NADPH to NADP
+
.
19.4.3. The Absorption of Eight Photons Yields One O
2
, Two NADPH, and Three ATP
Molecules
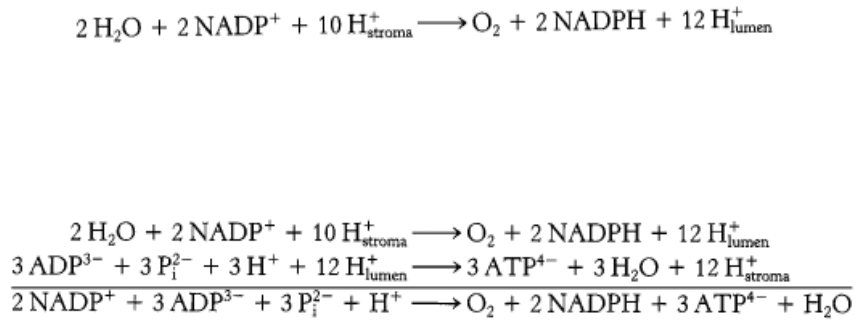
We can now estimate the overall stoichiometry for the light reactions. The absorption of 4 photons by photosystem II
generates 1 molecule of O
2
and releases 4 protons into the thylakoid lumen. The 2 molecules of plastoquinol are
oxidized by the Q cycle of the cytochrome bf complex to release 8 protons into the lumen. Finally, the electrons from 4
molecules of reduced plastocyanin are driven to ferredoxin by the absorption of 4 additional photons. The 4 molecules of
reduced ferredoxin generate 2 molecules of NADPH. Thus, the overall reaction is:
The 12 protons released in the lumen can then flow through ATP synthase. Given the apparent stoichiometry of 12
subunit III components in CF
0
, we expect that 12 protons must pass through CF
0
to complete one full rotation of CF
1
and, hence, generate and release 3 molecules of ATP. Given this ratio, the overall reaction is
Cyclic photophosphorylation is somewhat more productive with regard to ATP synthesis. The absorption of 4 photons
by photosystem I results in the release of 8 protons into the lumen by the cytochrome bf system. These protons flow
through ATP synthase to yield 2 molecules of ATP (assuming the same ratio of ATP molecules generated per proton).
Thus, each 2 absorbed photons yield 1 molecule of ATP. No NADPH is produced.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.4. A Proton Gradient Across the Thylakoid Membrane Drives ATP Synthesis
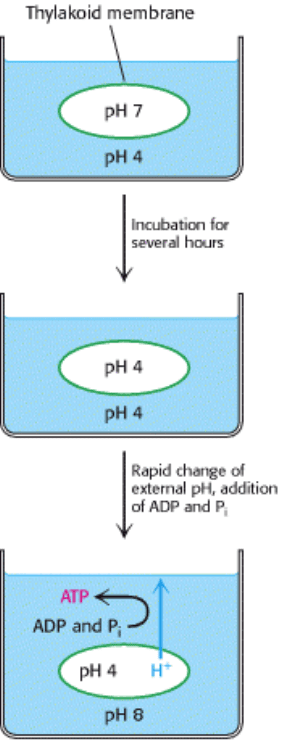
Figure 19.24. Jagendorf's Demonstration. Chloroplasts synthesize ATP after the imposition of a pH gradient.
II. Transducing and Storing Energy 19. The Light Reactions of Photosynthesis 19.4. A Proton Gradient Across the Thylakoid Membrane Drives ATP Synthesis
