Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.


714 34. UPDATES AND BIBLIOGRAPHY
and the coating is dried. Air knife coating is
limited to one side of the sheet at a time. It is
useful for carbonless papers where physical con-
tact with the paper is not possible. Some high-
end papers use two— or even three—pass coating.
Coating
pigments
The general properties of the most common
coating pigments (kaolin clay, calcium carbonate,
and titanium dioxide) were described earlier (pages
194-195 and 439-440). (Titanium dioxide absorbs
light in the near UV range and should not be used
with fluorescent whitening agents.) Particles for
coating formulations must have small size distribu-
tions,
generally below 1—2 fim, Delaminated clay
of ultrafme particle sizes are used in coatings to
give high gloss. Fine particle sizes give lower
gloss.
Coarse particles are used for matte finished
sheets. Of the additional pigments listed here,
aluminum trihydrate has appreciable use, but the
others enjoy relatively little use.
Aluminum
trihydrate,
ATH, is a high—bright-
ness pigment with a bluish color; the preferred
name is aluminum hydroxide, A1(0H)3. It is often
used as an extender for TiOj and acts as a spacer
between the TiOj particles. About 35,000 tons
were used by the world's paper industry in 1993.
Satin white, first used in the last quarter of
the 1800s, has appreciable use in premium grades
of paper in Europe, but has little use in the U.S.
It is made by reacting CaCOj with alum to give a
precipitate of needle—shaped particles consisting
of calcium sulfate. In paints it is called satin
white. Pearl filler is also calcium sulfate but is
prepared in a different manner.
Silica and silicate pigments have high bright-
ness,
small particle size, and low abrasion. The
composition is HjSiOj. They do not absorb UV
light and, therefore, work well with optical bright-
eners.
They are used on premium grades of paper
often with titanium dioxide.
Barium sulfate is an expensive filler with
good properties. Zinc oxide is occasionally used
as a coating material, but is more often used in
electrophotography.
Plastic pigments are usually spherical parti-
cles of polymers. Polystyrene is a hard plastic
and is the only material presently used for plastic
pigments. The size distribution is monodisperse,
although two (or more) different—sized particles
may be mixed together to give a high packing
density. Particle sizes are available from 0.2 to
1.0 fim. The cost is high, but high sheet gloss can
be obtained if the polystyrene is heated above its
glass transition temperature of about 100°C. The
density is about 1.05 (much lower than other
fillers and fibers, making it good for lightweight
papers) and the index of refraction is 1.60.
The rheology of the coating solutions is very
important. It is desired to have a relatively low
viscosity at high solids content so that drying costs
can be decreased. The solids content of coating
formulations is usually 50—75%. Low solids
content coatings with relatively high levels of
binders are used in the size press.
Coating
binders and adhesives
The use of coating binders must be limited so
that it does not fill in the voids between pigment
particles and, therefore, decrease the opacity of
the coating. Typical levels are 5 to 20% of the
dry solids of the coating formulation. Binder
formulations often consist of a soluble binder and
an emulsion binder. Binders must be particularly
strong for paper destined for offset printing.
Information on coating binder migration,
which causes ink mottling and poor adherence of
adhesive in many instances, is discussed on page
477.
Materials may be added to the coating
formulation to crosslink or precipitate the binder.
Starch (page 199) accounts for most of the
natural binders used in coating formulations.
Corn starch is most commonly used, but other
starches can be used. Starch is often modified to
improve its properties. Hydroxyethylated starch is
commonly used on premium grades of paper;
oxidized starch is also used and is less expensive.
Starch viscosity is decreased by enzymes (amylas-
es),
acid hydrolysis, or oxidative cleavage of
glycosidic linkages (called concersion or scission
in the paper industry). Proper cooking gives a
well—dispersed starch; cooking is accomplished
by the batch or continuous (jet) process. The
cooking process is sometimes monitored by ob-
serving the granules under a microscope with
polarized light; the granules are not polarized
after they are well dispersed. The starch is stabi-
lized by crosslinking on papers designed for offset
(lithographic) printing so that is does not dissolve
in the fountain solutions and picking is limited.

PAPER 715
Soy protein (commercially available since
1937) is used in some paper coatings and in
relatively large amounts in paperboard coatings.
The soy protein is received as a powder and is
often carboxylated, which is solubilized with the
addition of some alkali. Other formulations can
be dissolved in cold water. A wide variety of
viscosities are available. Soy protein replaced
casein in the 1950s. Casein (milk protein) has
been used widely in the past.
Synthetic binders are used as latexes (emul-
sions).
Latex originally was the term for emul-
sions of natural rubber, but the term now applies
to many emulsions of synthetic polymers. Sty-
rene—butadiene rubber (SBR) is the most
commonly used synthetic binder. Polystyrene is a
tough, strong, brittle material, and polybutadiene
is an elastic, rubbery material. The butadiene is
usually carboxylated to improve its bonding
ability. The relative amounts of these components
alters the properties of this copolymer. Styrene
content of 50% gives a tacky material with good
binding properties; styrene content of 70% gives
a material that is more brittle but gives good gloss.
The latex particles are 0.1 to 0.3 /^m in diameter.
Synthetic binders often have poorer ink receptivity
than starch or protein binders, but give enhanced
ink gloss due to improved ink holdout.
Polyvinyl acetate
(PVA)
is the major compo-
nent used in white glues for wood. PVA may be
used with other natural or synthetic binders. It is
somewhat water sensitive and gives a hard surface.
It is useful for web offset coatings because it is
somewhat porous and imparts stiffness in the paper
(useful for improving the stiffness in the cross
machine direction); these properties decrease
paper blistering during printing. Vinyl acetate is
used with acrylic monomers to make copolymers.
Polyvinyl alcohol has use in specialty coatings.
Acrylic latexes are high—quality binders with
many advantages, but the SBR binders have
limited their use until recently. They provide
good stability against aging by light, heat, and
chemicals and fold strength. Polymethyl methacry-
late is one acrylic polymer (Lucite® or Plexiglas®).
Coating additives
A wide variety of materials are added to
improve and control coating formulations (see
Chapter 21, Colloid and Surface Chemistry).
Many are similar to the papermaking additives,
although retention and drainage are not consider-
ations. One must consider additives that are
already included with the pigments, such as
dispersants, and adhesives, such as surfactants.
Dispersants are used to keep pigment parti-
cles from agglomerating, which would decrease
their opacity. They are used at about
1 %
of the
pigment weight. Polyphosphates are used with
clay calcium carbonate, titanium dioxide, and
other mineral pigments. Polyphosphates, such as
tetrasodium pyrophosphate (Na4P207, also called
sodium diphosphate) are formed by dehydrating
sodium salts of phosphoric acid (such as disodium
phosphate, Na2HP04). Polyphosphates may
hydrolyze to low molecular forms in water with
heating or low pH; this process liberates acid,
which further catalyzes the reaction. Poly-
phosphate anions are effective at sequestering
multivalent cations. Aqueous solutions of sodium
silicates (water glass) are also used as dispersants.
Polyelectrolytes, such as low molecular
weight sulfonated materials or polyacrylates, may
be used as a second dispersant in case the
polyphosphate degrades or is used improperly.
Ethoxylated fatty alcohols are used as nonionic
dispersants, which prevent the pigments from
getting close enough to agglomerate (steric
hindrance).
Defoaming agents or foam control agents are
used to prevent entrained air from forming in the
coating mixture. Entrained air increases the
viscosity of the coating formulation and leads to
defects in the coated product.
Biocides are used especially when starch, a
favorite food of microorganisms everywhere, is
used as the binder. Good housekeeping, proper
machine design, and occasional sterilization help
control microorganisms. If problems are encoun-
tered, one should isolate the offending microorgan-
ism and use a suitable biocide.
Viscosity
control agents allow the rheological
(flow) characteristics to be regulated. The use of
most binders, and the desire to have a high solids
contents, usually means that most coating formula-
tions will have a high viscosity. Some chemicals
actually reduce the viscosity of coating formula-
tions,
including urea and polyvinyl pyrrolidone

716 34. UPDATES AND BffiLIOGRAPHY
with molecular weight below 40,000. Urea, used
at 5—20% of the weight of starch, stabilize the
viscosity behavior of modified starch.
It is relatively easy to increase viscosity by
using polymers with high molecular weight
(starch, alginates, or CMC).
Antistatic (electricity) agents work by ab-
sorbing water from the atmosphere (glycerides or
glycols), by adding conductivity to the coating
(potassium chloride or quaternary ammonium
compounds and other ionic surfactants), or by
putting a charge on the surface that is opposite to
the charge that otherwise tends to build.
Other additives include lubricants or
plasticizers, such as calcium or ammonium stea-
rate,
PEG, wax, or polyethylene, which are used
to prevent dusting. Specialized coatings contain
silicone or fluorocarbon release agents for paper to
which removable labels are attached, encapsulated
pigments for carbonless papers, etc.
Additives to control the appearance of the
final product include dyes and fluorescent whiten-
ers.
Additives that absorb harmful ultraviolet
radiation (salicylates, benzophenones, and other
aromatic compounds), which would otherwise
eventually cause fading or other photochemical
degradation, may be used.
Miscellaneous
The National Cash Register Company (NCR)
obtained the original patents for carbonless paper
in 1954. Commercial coated papers range from
grade 1 to 5. Grades 1 to 3 are "free sheets";
they contain no mechanical pulp. All number 5
grade contains some mechanical pulp.
Walter, J.C., The Coating Process, Tappi Press,
1993,
260 p. This is a useful, general reference
on paper coating including materials, the prepara-
tion of coating formulations, and the various
coating processes.
34.15 FLEXIBLE PACKAGING
Hammond, P.M. and M.W. Potts, An overview of
flexible—packaging markets and structures, Tappi
J, 72(1):81-88(1989).
Thompson, K.I., A look at 20th century film
development, Tappi J. 73(6)137-140(1990). This
is an interesting history of the development of
plastic films for packaging. Cellophane was the
material that started it in 1924 and was king until
the early 1960s, when polyethylene surpassed it.
Equipment technology and film markets are also
discussed. It is too bad more detail is not given.
34.16 ENVIRONMENTAL
Cluster rules
Swan, C.E., Cluster rules update, 58(11):23-
25(1995). In late 1993, the U.S. Environmental
Protection Agency (EPA) proposed that water and
air regulations would be combines for the U.S.
pulp and paper industry. The proposal was nick-
named the "Cluster Rules". The industry has said
these regulations are unnecessarily burdensome.
As of this writing, the situation is sfill unresolved,
and future reguations are still uncertain.
Sludge disposal:
incineration,
energy recovery
Solid waste disposal by landfilling is becom-
ing increasingly expensive (with a tipping fee on
the order of $75/ton) and of concern for many
reasons (such as the possibility of a particular
landfill being targeted as a hazardous waste site in
the future for reasons presently unidentified).
Sludge disposal by incineration is one area where
landfilling of solid wastes can be decreased. For
example, sludge from the primary clarifiers typi-
cally contains over 90% wood fiber, some calcium
salts,
etc. Most mills do not use this material as
fuel because it is difficult to dewater and they do
not require the fiiel.
Jacobson, W., Fluid—bed combustion: a solution
to increasing paper sludge problems. Paper Age
106(Recycling Annual):21-22(1990). Another
major source of sludge is that generated from
secondary fiber reclamation. Although quite
variable, paper sludges have similar heating values
to wood and about 8—10% ash content; deinking
sludges have about 11—18 MJ/kg (4600—7500
Btu/lb) (dry basis) and ash contents of 15—40%.
Fluid—bed combustion of these sludges is said to
be quite efficient when they are mixed with other
fuels such as wood.
Rude, J., Sludge disposal: a problem or opportuni-
ty, PaperAge 106(7):24(1990). The author consid-
ers drying of sludge for hog fuel by rotary driers.
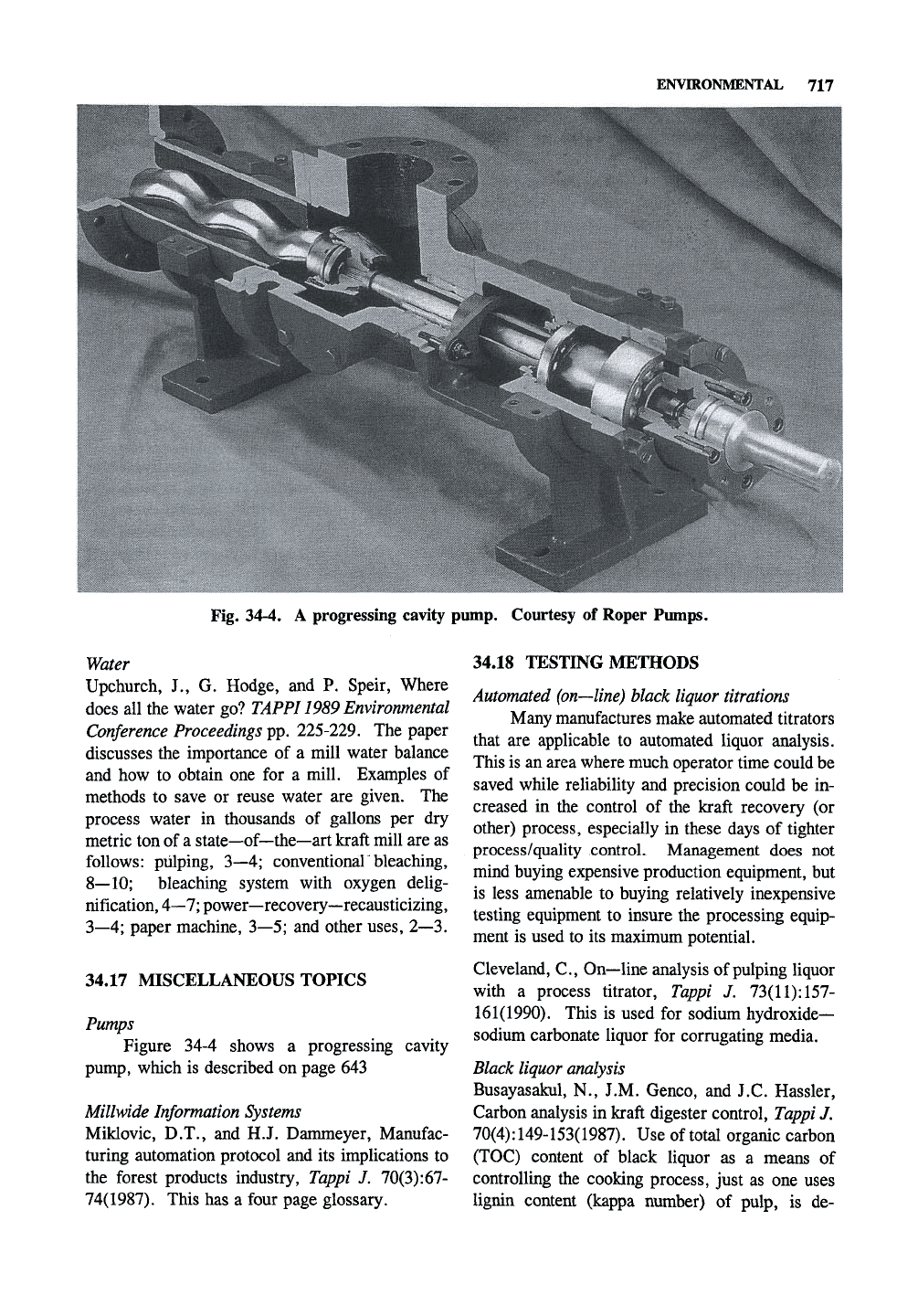
ENVIRONMENTAL 717
Fig. 34-4. A progressing cavity pump. Courtesy of Roper Pumps.
^ater
Upchurch, J., G. Hodge, and P. Speir, Where
does all the water go? TAPPI1989
Environmental
Conference Proceedings pp. 225-229. The paper
discusses the importance of a mill water balance
and how to obtain one for a mill. Examples of
methods to save or reuse water are given. The
process water in thousands of gallons per dry
metric ton of a state—of—the—art kraft mill are as
follows: pulping, 3—4; conventional bleaching,
8—10; bleaching system with oxygen delig-
nification,
4—7;
power—recovery—recausticizing,
3—4; paper machine, 3—5; and other uses, 2—3.
34.17 MISCELLANEOUS TOPICS
Pumps
Figure 34-4 shows a progressing
pump, which is described on page 643
cavity
Millwide Information Systems
Miklovic, D.T., and H.J. Dammeyer, Manufac-
turing automation protocol and its implications to
the forest products industry, Tappi J. 70(3):67-
74(1987). This has a four page glossary.
34.18 TESTING METHODS
Automated
(on—line)
black liquor titrations
Many manufactures make automated titrators
that are applicable to automated liquor analysis.
This is an area where much operator time could be
saved while reliability and precision could be in-
creased in the control of the kraft recovery (or
other) process, especially in these days of tighter
process/quality control. Management does not
mind buying expensive production equipment, but
is less amenable to buying relatively inexpensive
testing equipment to insure the processing equip-
ment is used to its maximum potential.
Cleveland, C, On—line analysis of pulping liquor
with a process titrator, Tappi J.
73(11):
157-
161(1990). This is used for sodium hydroxide-
sodium carbonate liquor for corrugating media.
Black liquor analysis
Busayasakul, N., J.M. Genco, and J.C. Hassler,
Carbon analysis in kraft digester control, Tappi J.
70(4):
149-153(1987).
Use of total organic carbon
(TOC) content of black liquor as a means of
controlling the cooking process, just as one uses
lignin content (kappa number) of pulp, is de-

718 34. UPDATES AND BffiLIOGRAPHY
scribed. This method is not likely to be used
routinely in pulp and paper mills.
Lignin
structural analysis
Gardner, D.J., T.P Schultz, and G.D. McGinnis,
The pyrolytic behavior of selected lignin prepara-
tions,
/. Wood Chem. Tech, 5(1):85-110(1985).
Chemical analysis of lignin by a variety of meth-
ods such as nitrobenzene oxidation, thermal
analysis, pyrolysis, and IR were investigated.
This is a good introductory article describing the
tools that tell us what is known about lignin.
Hatfield, G.R., G.E. Maciel, O. Erbatur, and G.
Erbatur, Qualitative and quantitative analysis of
solid lignin samples by carbon—13 nuclear mag-
netic resonance spectrometry.
Anal.
Chem,
59:172-179(1987). Maciel's laboratory has stud-
ied numerous aspects of NMR of lignins.
Lignin
content
by
infrared
or
UV spectroscopy
The traditional method of
lignin
measurement
using permanganate ion can be supplanted by
optical spectroscopy methods like near—infrared
(NIR) spectroscopy or visible—ultraviolet (UV)
spectroscopy. Unlike permanganate titration of
pulp,
where only a single piece of data is ob-
tained, spectroscopy methods offer reflectance
curves that offer the potential for quantifying
several components by simultaneous equations at
several wavelengths. These methods are faster
than titrations and lend themselves to continuous,
on—line measurements. In the case of IR, infor-
mation about the polysaccharides is also available,
which, in turn, allows yield determinations with a
high degree of precision.
Angeus, L. and S.—A. Damlin, Optimal control
with on—line kappa number analysis. Pulp Paper
Can.
93(2):T32-T36(1992). These workers
measured the kappa number using UV absorbance
at 280 nm with success.
Backa, S. and A. Brolin, Determination of pulp
characteristics by diffuse reflectance FTIR, Tappi
J. 74(5):218-226(1991).
Easty, D.B., S.A. Berben, F.A. DeThomas, and
P.J. Brimmer, Near—infrared spectroscopy
for
the
analysis of wood pulp: quantifying
hardwood—softwood mixtures and estimating
lignin content,
Tappi
J. 73(10):257-261 (1990).
Michell, A.J., Infra—red spectroscopy trans-
formed—new applications in wood and pulping
chemistry, Appita 41(5):375-380(1988).
Schultz, T.P. and D.A. Bums, Rapid secondary
analysis of lignocellulose: comparison of near
infrared (NIR) and Fourier transform infrared
(FTIR),
Tappi
J. 73(5):209-212 (1990).
Ion—selective
electrodes
Ion—selective electrodes (ISEs) are very
similar in use to pH electrodes. They are used for
chloride, potassium, calcium, carbon dioxide/
carbonate, oxygen, and a variety of other ions.
These methods are particularly suited for field
analysis and on—line measurements.
Lenz, B.L. and J.R. Mold, Ion—selective elec-
trode method compared to standard methods for
sodium determination in mill liquors, Tappi J.
54(12):
2051-2055(1971). Sodium ion can be
quickly measured directly with ion specific elec-
trodes, with about
1 %
error, over a wide range of
concentrations. A small sample is diluted with a
small amount of ionic strength adjuster (ISA, an
ammonium buffer solution) and the solution mea-
sured as if for pH, but in this case for p(Na'^).
This method would probably be useful for mea-
suring sodium loss in pulp by incubating the pulp
with ISA until ammonium ion has exchanged
sodium ion. It could also measure sodium concen-
trations at various stages of the recovery process.
Cooper, Jr., H.B.H., Continuous measurement of
sodium sulfide in black liquor, Tappi 58(6):59-
62(1975). This is a general article on the subject.
Schwartz, J.L. and T.S. Light, Analysis of alka-
line pulping liquor with sulfide ion—selective
electrode, Tappi J. 53(1):90-95(1970). New
electrodes often use a salicylate buffer with 1:3
sample :buffer dilution. This article has much
experimental detail.
Aluminum
ion
concentrations
Avery, L.P., Evaluation of retention aids, the
quantitative alum analysis of a papermaking
fiirnish and the effect of alum on retention, Tappi
62(2)43-46 (1979). The concentration of alum
was measured in an elegant fashion using a F'
ion-specific electrode. Fluoride complexes with
AP"^
and a titration with NaF using the ISE allows

TESTING METHODS 719
the determination of aluminum concentration
(complex formation values for F are available in
Lange).
This method can be used with poly-
aluminum chlorides (PAC), but low aluminum
values may be obtained as the polymer forms of
aluminum may react slowly. Perhaps a method
could be developed where excess fluoride is added
(if the approximate concentration of PAC is
known), the sample aged, and the fluoride back
titrated with alum (or measured directly).
Varriano—Marston, E. and H. Cheeseman,
8—Hydroxyquinoline as a stain for determining the
distribution of alum in paper, Tappi J.
69(6):
116-
117(1986).
Ion chromatography
Ion chromatography is a liquid chromatogra-
phy technique that separates ions for quantitative
and qualitative analysis. The procedure requires
a high—performance liquid chromatograph
(HPLC), usually available at corporate research
centers or university laboratories. The method is
applicable to pulping liquors, bleaching liquors,
and other process streams.
Cox, D., P. Jandik, and W. Jones, Applications of
single—column ion chromatography in the pulp
and paper industry. Pulp Paper
Can.
88(9):T318-
T321(1987).
Easty, D.B. and J.E. Johnson, Recent progress in
ion chromatographic analysis of pulping liquors:
determination of sulfide and sulfate, Tappi J.
70(3):
109-111(1987).
Murarka, S.K. and
N.S.
Fairchild, Ion chromatog-
raphy: Is it the *now' technique for monitoring
process streams in a paper mill? Part I: Principles
and instrumentation. Pulp Paper Can.
88(12):T438-T440(1987); ibid. Part II: Selected
applications. Pulp Paper Can. 88(12):T441-
T446(1987).
Bleaching chemicals
Fisher, R.P., M.D. Marks, and S.W. Jett, Mea-
surement methods for chlorine and chlorine diox-
ide emissions from bleach plants, Tappi J. 70(4):
97-102(1987). Chlorine and chlorine dioxide can
be captured from air by neutral potassium iodide
solution. A two—point titration is used to give
contents of both species.
Starch
in
paper
Biermann, C.J., Quantitative analysis of starch in
paper, Tappi J.
76(5):
12(1993).
A useful im-
provement over present TAPPI Standard methods
for starch analysis in paper would be a hybrid
method of enzymatic hydrolysis of starch followed
by specific detection of glucose. Specific detec-
tion of glucose could be done with glucose analyz-
ers available at drug stores or more sophisticated
ones.
Such a method would have the advantage of
taking very little operator time, being portable,
and being specific for glucose.
Imaging techniques
Paumi, J.D., Laser vs. camera inspection in the
paper industry, Tappi J.
71(11):
129-135(1988).
The title technique is used to scan paper webs
on—line at machine speeds up to 6000 ft/min for
detection of any defects.
Tomimasu, H., D. Kim, M. Suk, and P. Luner,
Comparison of four paper imaging techniques:
jS—radiography, electrography, light transmission,
and soft X—radiography, Tappi J.
74(7):
165-176
(1991).
These techniques are used for measuring
the uniformity of paper, i.e., its formation.
Pitch and
contaminant
analysis,
"stickles"
Buildup of pitch and polymer contaminants
on paper machine wires and clothing is a big prob-
lem. The stock temperature often determines
where the stickies will deposit. Often the source
is presumed to be the pulp or contaminants from
secondary fiber operations. These assumptions are
not always warranted; analysis of the residue is
instrumental to problem solving. Analytical tech-
niques include sample purification by extraction,
gas chromatography, NMR, FTIR, size—exclusion
(gel—filtration) chromatography, etc.
Biermann, C.J. and M.—K. Lee, Analytical tech-
niques for analyzing white pitch deposits,
Tappi
J.
73(1):
127-131(1990).
This is another general
paper on the subject.
Ekman, R. and B. Holmbom, Analysis by gas
chromatography of the wood extractives in pulp
and water samples from mechanical pulping of
spruce, Nordic Pulp Paper J.
(1):
16-24(1989).
Sithole, B.B., Modern methods for the analysis of
extractives from wood and pulp: a review, Appita

720 34. UPDATES AND BIBLIOGRAPHY
45(4):260-246(1992). This is a concise review
with a lot of information and 69 references.
Sjostrom, J. and B. Holmbom, Size—exclusion
chromatography of deposits in pulp and paper
mills,/.
Chromatogr, 411:363-370(1987).
Sjostrom, J. and B. Holmbom, A scheme for
chemical characterization of deposits in pulp and
paper production, Paperi ja Puu
(2):151-
156(1988). This is a useful, general paper on the
subject.
Sjostrom, J., B. Holmbom, and L. Wiklund,
Chemical characteristics of paper machine deposits
from impurities in deinked pulp, Nordic Pulp
Paper Res. J,
(4):
123-131(1987).
Suckling, I.D. and R.M. Ede, A quantitative ^^C
nuclear magnetic resonance method for the
analysis of wood extractives and pitch samples,
Appita, 43(1):77-80(1990). Stickles can be mea-
sured qualitatively by hot press tests and other
tests.
Gaseous
pollutant measurement
Barker, N.J and J.L. Siqueira, Continuous, remote
monitoring of specific reduced sulfur compounds
in ambient air by gas chromatog-
raphy—photoionizationdetector,79SSJ?«v/ro«m^nr
Conference Proceedings, CPPA, pp. 11—15
(1988).
Ambient TRS measurements 2 and 10 km
from a mill are described in this work.
Dirt in
wood,
pulp,
and
paper
Clark, J. d'A., R.S. Von Hazemburg, and R.J.
Knoll, Paper Trade Journal 96(5):40(1933). The
authors published a chart of dirt sizes. They
included 20 particles shapes for each of 17 sizes
from 0.01 nrni^ to 5 mm^. This is the basis of the
TAPPI dirt estimation chart used in the tests
described below.
TAPPI Standards are available for quantify-
ing dirt by light reflectance in pulp (Standard T
213) and paper and paperboard (Standard T 437).
In fact, both tests are very similar. With the
widespread availability of optical character recog-
nition (OCR) systems, these TAPPI Standards un-
doubtedly will become computerized or obsolete.
• 5.00
• 4.00
• 3.00
• 2.50
• 2.00
• 1.50
• 1.25
• 1.00
• 0.80
• 0.60
• 0.50
• 0.40
• 0.30
0.25
•
0.20 •
0.15 •
0.12 •
0.10 •
0.09 •
0.08 •
0.07 •
0.06 •
0.05 •
0.04 •
0.03
0.02
Fig. 34-5. A series of circles with areas in mm^.
[Standard T 537 is similar, but merely counts dirt
particles larger than a set threshold such as 0.02
mm^ eba (explained below). It is said to be de-
signed for OCR purposes of paper [OCR is used
to determine dirt, only that dirt is determined for
an (unspecified) OCR application.]
Dirt estimation is made with the TAPPI dirt
estimation chart. The chart consists of a series of
23 pairs of black circles and rectangles (each
rectangle has a length 20 times the width) com-
prising areas from 0.02 mm^ to 5 mm^. The
rectangles are not used but are included as a
leftover of Clark et al. (1933). The equivalent
black area (eba) of a dirt particle is defined as the
area of the dot from the dirt estimation chart that
most closely resembles the visual appearance (not
the actual size) of the dirt particle. A dark dirt
particle on a brown piece of paper would give a
smaller eba than the same particle on white paper.
The limiting eba (minimum size to be counted) of
pulp is 0.08 mm^ and in paper is 0.04 mm^. Dirt
is usually reported in
mmVm^,
which is ppm. The
dirt estimation chart is not exact, and correction
values must be used for many of the spots.
Corrections are included in Standard T 213 only.
Fig. 34-4 shows a series of circles of various
approximate areas. A group of these are included
at the end of the book for practice use.

ANSWERS TO SELECTED PROBLEMS
CHAPllER 1
13.
The Abstract Bulletin of IPST for articles on
dioxins in pulp mill effluents. The Chemical
Abstracts (or medical abstracts) for the
long—term toxicity of dioxin since this is
independent of the source of dioxin.
CHAPTER 2
1.
False.
2.
Circle—brown, softwood, hardwood, soft-
wood, mechanical.
3.
a) Loss of extractives b) Wood decay
4.
1. Chip size distribution
2.
Chip rot content
3.
Extractives content
4.
Age (from pith) of wood in chip
5.
Wood species
6. Wood density
7.
Moisture content
5.
Fines—overcook to give a low yield and they
tend to plug the digester's liquor circulation
system. Fines tend to contain high percent-
ages of dirt and grit.
Oversize—undercook, give a large amount of
screen rejects and shives; also, larger
amounts of bleaching chemicals will be used.
6. Typically 20-30% depending on the process,
chip price, etc. Sometimes as high as 50%.
7.
Cellulose, hemicellulose, and lignin.
8. Circle cellulose then hemicelluloses.
9. Lignin.
13.
Briefly, dissolving pulp (relatively pure cellu-
lose) is swelled in alkali and then treated with
monochloroacetic acid. The cellulosate ion
(R—0~) is a nucleophile and attacks the pri-
mary chloride of monochloroacetic acid. The
result is:
Cell-0-
+ CI-CH2-COO- ->
Cell-O-CH.COO- Na+ + Cl"
14.
The fibril angle of the S—2 cell wall layer.
15.
They adsorb and desorb water from the air
around them depending upon the temperature
and relative humidity.
16.
Hydrogen bonding.
18.
The maximum level of recycling paper on a
sustained basis is considered to be 50—60%.
At rates higher than this a significant portion
of fibers have been through one, two, or
several more uses and have lost most of their
strength.
19.
$20—40/ton. $400, or more than half the
cost of virgin pulp.
CHAPTER 3
1.
Mechanical separation by softening and
fatigue of the compound middle lamella or
chemical dissolution of the lignin that binds
the fibers together.
3.
Chemical pulp has higher tensile strength and
sheet density; mechanical pulp has higher
yield, unbleached brightness, and opacity.
4.
The lignin softens to the point that fiber sepa-
ration occurs at the middle lamella (instead of
at the primary cell wall) and results in fibers
that are "coated" with lignin. Since hydro-
gen bonding does not occur with lignin,
strength is greatly reduced in the final sheet.
The higher temperature darkens the pulp.
5.
TMP, or especially CTMP, is much stronger
than SOW since fiber liberation is much
more specific in the TMP process, leading to
longer fibers. This increase in strength
means lower amounts of chemical pulp are
required.
7.
Batch and continuous digesters.
8. The kappa number is a measure of residual
lignin; consequently, this is a measure of
lignin content as a function of yield. The
lower the lignin content at a given yield, the
more selective (good), the pulping process.
721

722 ANSWERS TO SELECTED PROBLEMS
Other factors such as cellulose viscosity, pulp
strength, and bleachability must ultimately be
determined to make a final judgment regard-
ing the efficacy of a particular pulping pro-
cess.
9. No. If the resultant pulp has a low cellulose
viscosity and strength, it will be unsuitable
for making paper.
10.
Anthraquinone is a pulping additive used in
some kraft and alkaline sulfite pulping meth-
ods.
It undergoes a cyclic redox process.
Lignin is reduced while cellulose and hemi-
celluloses are oxidized. The oxidation of the
carbohydrates is of particular interest since
the reducing end groups (the free anomeric
carbon atoms) are oxidized from aldehyde to
carboxylic acids that prevents the alkaline
peeling reaction. (This is the same end from
which the peeling reaction occurs.)
11.
NaOHandNa2S.
12.
The pulping time and temperature are com-
bined into the H-factor. Only those two vari-
ables are considered in the H-factor; other
variables such as sulfidity and active alkali
will also influence the degree of cook.
13.
The spent liquor cannot be recovered for
reuse in the pulping process. Since there is
only a limited market for the spent liquor for
drilling muds, dispersants, and other uses,
the spent liquor is a large disposal problem
for large—scale pulp production based on this
method.
14.
Free (sulfurous acid, H2SO3) and combined
(sulfite ion, SO^^-) SO2.
CHAPTER 4
1.
The kraft recovery process allows 1) recov-
ery of inorganic chemicals for reuse, 2)
recovery of the intrinsic energy of the waste
organic compounds to supply energy for mill
processes, 3) the disposal of the organic
materials, which would otherwise be an
environmental problem.
2.
a) 2NaOH + CO2 -^ Na2C03 + H2O
b) NaCOs + Ca(0H)2 ^ 2NaOH + CaCOj
lOOCC
c) CaC03 -* CaO + CO2
heat
d) H20(l) - H20(g)
3.
Turpentine is recovered from the digester
relief gases during digester heating. Tall oil
is collected by skimming the surface of the
partially concentrated black liquor.
4.
Spent NSSC cooking liquor from the semi-
chemical pulping process is added to the
black liquor of the kraft pulping cycle to the
extent it can be used as make—up chemical.
5.
High dilution will result in less sodium loss
through the pulp, increase the evaporation
costs as more water must be removed, and
decrease the bleaching costs as more of the
soluble, residual lignin is removed.
6. One extra ton of water per ton of pulp is
produced, and each ton of water requires
4.24""^ ton of steam, 472 additional pounds of
steam will be required per ton of pulp.
2000 lb water lib steam .«/^« , u
X
=
472 lb steam/ton
1
ton pulp
4.24 lb water
7.
Long tube vertical evaporators and falling
film evaporators.
8. Concentration of black liquor by direct con-
tact with the hot flue gases of the recovery
boiler strips significant quantities of sulfur
compounds from the black liquor, causing
extremely odorous emissions, wasting sulfur,
and contributing to acid rain. Preoxidation
of black liquor prior to combustion decreases
the problem but decreases the fuel value of
the black liquor. Indirect evaporators (con-
centrators) are now used, as they decrease
sulfur emissions without the need for black
liquor preoxidation.
9. As the solid content increases beyond 65%
the viscosity increases exponentially, making
the liquor difficult to pump. On the other
hand, removal of water prior to combustion
of the black liquor increases the theoretical
amount of recoverable energy.

CHAPTER 4 723
10.
Reduction occurs in the bottom of the recov-
ery boiler and these conditions alow sulfur
chemicals to be recovered in their reduced
form (Na2S). Oxidation occurs in the top of
the recovery boiler and limits CO emissions.
11.
The ESP is used to capture particulate mat-
ter, especially Na2S04 and soot particles, for
reuse in the recovery boiler thereby decreas-
ing pollution.
12.
The sodium sulfide would be partly converted
to sodium sulfite, sulfate, and similar com-
pounds. This would precipitate as calcium
sulfite or sulfate during causticizing.
13.
The lime travels from the top of the kiln to
the bottom. Unless the lime mud is dried
separately, the lime mud first dries, is then
heated to temperature, and then undergoes
calcining as it travels through the kiln.
14.
The dregs accumulate in the lime mud, there-
by decreasing the lime availability and caus-
ing problems in lime mud settling.
15.
It is a measure of the extent of conversion of
sodium carbonate (inactive chemical) to sodi-
um hydroxide (active pulping chemical) in
the causticizing process.
CHAPTER 5
1.
Mechanical pulps are high in lignin content.
During their bleaching the lignin is pre-
served, but the color-bearing groups
(chromophores) are altered to decrease their
light absorbance. Bleaching of chemical
pulps involves lignin removal as the primary
goal—no lignin, no lignin color.
2.
Color reversion
is
a consideration in mechan-
ical pulps since large amounts of lignin are
still present.
3.
The lignin content can be measured by
permanganate ion titration (kappa number or
k number) or by light absorption in the UV
or IR range. The latter may be converted to
an equivalent kappa or k number as if the
pulp were titrated with permanganate. This
is done since titration with permanganate is
still the most common method.
4.
30 X 0.147 = 4.4% lignin (approximately).
5.
Cellulose viscosity is the viscosity of cellu-
lose in solution. It is an indicator of the
degree of polymerization of the cellulose and
the strength of chemical pulps. Overly harsh
pulping and bleaching treatments decrease the
DP of cellulose and pulp strength.
6. Treatment of pulps with chlorine does not
remove lignin since this is necessarily done
under acidic conditions where the phenolic
groups are not in the salt form that contrib-
utes to water solubility. Alkali extraction
produces more phenolic groups and converts
the phenolic groups to phenolate groups that
are water soluble. In a nutshell, chlorine
"predisposes" the lignin to removal by alkali
extraction. In fact, about 75% of the lignin
present before chlorination is removed by the
subsequent alkali extraction.
If additional bleaching chemicals were used
prior to alkali extraction, most of them would
be consumed by the lignin that would other-
wise have been removed by the inexpensive
extraction stage.
7.
Chlorine (followed by alkali extraction) is not
overly specific for lignin removal. This
treatment would not be done in the later
stages of bleaching where the lignin content
is very low.
8. If the pH is too low the cellulose degrades by
acid hydrolysis. If the pH is too high, the
cellulose degrades by oxidation. Overchlo-
rination results in cellulose degradation and
the production of large amounts of chlorinat-
ed organic compounds including dioxins.
10.
Bleaching in several stages allows different
parts of the lignin molecule to be attacked.
It is more efficient in that lower amounts of
chemical are used overall.
11.
If the pH drops to low then hypochlorite is in
equilibrium with hypochlorous acid which
degrades the pulp by oxidation.
12.
The high NaCl content increase the dead load
and corrosion in the recovery boiler.
