Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

mill to improve the flour color. One of the most
commonly used bleaching agents is benzoylperoxide.
Chlorine is another agent that is added as a gas
mainly to soft wheat flour, where another objective
is to control the flour pH level (Table 3).
0051 Enzymatic activity To compensate for any natural
deficiency of enzymes to convert starch to maltose,
usually malted barley flour or enzymes are added.
The treatment is approximately 0.25%, depending
on the strength of the malt and the requirements of
the flour treated.
Heat Treatment
0052 Heat treatment of flour is used to change its physical
and rheological properties. The heat reduces the elas-
ticity and even denatures the gluten. The starch can be
gelatinized and enzymatic activity reduced or elimin-
ated. The heat also causes a reduction in the bacterial
count of flour. In terms of amylograph values, a flour
viscosity of about 300 Brabender units (BU) could
be effected by heat treatment and reach 800 BU.
The new characteristics improve the water-holding
capacity of the treated flour. The heat-treated flour
is used for special end uses such as soups, sausage
filling material, infant foods, and other food and
nonfood products.
New Developments and Their Effect on
Product Characteristics
Debranning of Wheat Before Milling
0053 The newly applied process of debranning the wheat
outer layers from the kernels before milling yields
products that are different from those produced by a
conventional mill, in terms of quality and nutritional
values. The finely adjusted debranning machine
action on the wheat kernel allows the miller to
remove by abrasion individually each of the seven
bran layers before grinding the endosperm to flour.
From the outer to inner layer, these are: cuticle, epi-
carp, endocarp, testa, nucellar layer, and aleurone
layer. Each is significantly different in characteristics
from the other. While the nutritous aleurone layer is
high in protein content (28%), the epidermis, epicarp,
and endocarp have a very high level of insoluble
dietary fiber.
0054As mentioned earlier, the process of sprouting
softens the wheat kernel, and accordingly, when
milled on a conventional milling system, the bran is
pulverized somewhat more than sound wheat, and
the ash increases as a result of the fine bran particles.
Wheat debranning before milling removes most of the
kernel layers that contain high levels of a-amylase
such that the resulting flours have a reduced enzym-
atic activity and are more suitable for bread-baking
purposes.
See also: Air Classification: Uses in the Food Industry;
Bread: Dough Mixing and Testing Operations; Flour:
Roller Milling Operations; Analysis of Wheat Flours;
Dietary Importance; Milling: Principles of Milling; Types
of Mill and Their Uses; Starch: Structure, Properties, and
Determination; Wheat: Grain Structure of Wheat and
Wheat-based Products
Further Reading
Canadian International Grains Institute (1993) Grain &
Oilseeds Handling, Marketing, Processing. 4th edn,
vol. II.
Code of Federal Regulations (1991) Whole Wheat Flour.
No. 21, Part 137. Washington, DC: Food and Drug
Administration.
tbl0003 Table 3 Additives to wheat flour
Flour additive Physicalstate Rate (p.p.m.) Effect Maximumlevel (p.p.m.)
Nutritional additives
Thiamin Premix 4.4–7.7 Prevents beriberi Regulations
Riboflavin Premix 3.7–4.8 Eye and mouth tissue health Regulations
Niacin Premix 35–64 Prevents pellagra Regulations
Iron Premix 29–43 Forms hemoglobin Regulations
Folic acid Premix 1–2 Prevents birth defects Regulations
Calcium Powder 2116 Counter phytic acid Optional
Flour-improving additives
Potassium bromate Powder 10–20 Oxidation 50 p.p.m.
Azodicarbonamide Powder 2–20 Aging 45 p.p.m.
Chlorine Gas 1000–1400 Bleach/age As required
Benzoyl peroxide Powder 50 Bleach 150 p.p.m.
Ascorbic acid Powder 70 Mix-time reduction 200 p.p.m.
L-Cysteine (hydrochloride) Powder 30 Mix-time reduction 90 p.p.m.
Fungal a-amylase Powder 30 Diastatic supplement As required
4004 MILLING/Characteristics of Milled Products
Code of Federal Regulations (1996) Food and Drugs Stand-
ards of Identity. Parts 100–169, Sections 137.300 and
137.320. Revised 1 April 1996. Washington, DC: Food
and Drug Administration.
Current Good Manufacturing Practices (1986) Federal
Food and Drug Administration, Food, Drug, and Cos-
metics, Act Sections 402(a)(3) and 402(a)(4). Washing-
ton, DC: Federal Food and Drug Administration.
Evers T and McRitchie F (2000) Ash who needs it? World
Grain 18(6): 56–63.
Farrand EA (1972) The influence of particle size and starch
damage on the characteristics of bread flours. The
Bakers Digest, February, 22–26.
Gruber FJ (1964) Getting the most from a bread score.
American Society of Bakery Engineers Bulletin 174.
September, 702–708.
Manser J (1985) Feinheitsgrad von Durum-Mahlerzeugnis-
sen aus der Sicht der Teigwarenindustrie. Getreide Mehl
& Brot 39(4): 117–123.
Panter A (1988) Divide milling of Canadian spring wheat
flour. Association of Operative Millers Technical Bul-
letin December: 5347–5353.
Posner ES (1991) Wheat and flour ash as a measure of
millability. Cereal Foods World 36(8): 626–629.
Posner ES (2000) Wheat. In: Kulp K and Ponte JG, Jr. (eds)
Handbook of Cereal Science and Technology. New
York: Marcel Dekker.
Posner ES and Hibbs AN (1997) Wheat Flour Milling.
St. Paul, MN: American Association of Cereal Chemists.
Pyler EJ (1982) Baking Science and Technology. Chicago,
IL: Siebel.
Mincing See Meat: Sausages and Comminuted Products
MINERALS – DIETARY IMPORTANCE
J H Freeland-Graves, The University of Texas at
Austin, Austin, TX, USA
P J Trotter, Augustana College, Rock Island, Illinois,
USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Minerals are of critical importance in the diet even
though they comprise only 4–6% of the human
body. Major (macro-) minerals are those required in
amounts greater than 100 mg day
1
and represent 1%
or less of body weight (Figure 1). These include cal-
cium, phosphorus, magnesium, sulfur, potassium,
chloride, and sodium (Table 1). Trace (micro-)
minerals are essential in much smaller amounts, less
than 100 mg day
1
, and make up less than 0.01% of
body weight. Essential trace elements are chromium,
copper, fluoride, iodine, iron, manganese, silicon, and
zinc (Table 2). Ultratrace minerals are a subcategory
of trace minerals that are required in amounts less
than 50 ng g
1
in the diets of animals. These include
arsenic, boron, molybdenum, nickel, selenium, and
vanadium (Table 3). Other inorganic elements which
may contribute to biological processes, but which
have not been established as essential, are barium,
bromine, cadmium, lead, lithium, and tin. Nonnutri-
tive metals such as aluminum, bismuth, gallium, gold,
mercury, and silver may also be present in small
amounts in foods. These metals have no known func-
tion and may contaminate wholesome food and create
toxic symptoms. (See Heavy Metal Toxicology; Trace
Elements; Refer to individual minerals.)
Food Sources
0002A mineral is an inorganic homogeneous substance.
When food is burned, the organic portions oxidize;
the ashes remaining are minerals. The ash can be ana-
lyzed to determine the specific quantity and type of
minerals present. The level of some minerals, such as
iodine and selenium, in foods is particularly dependent
upon the amount of the mineral in the soil of the region
where the food is produced. Avariety of common food
sources of minerals are listed in Tables 1–3.
0003The natural occurrence of minerals in food is not
always desirable. In vegetable oils, miniscule amounts
of iron and copper contribute to the development of
rancidity. Sequestrants, such as ethylenediaminetetra-
acetic acid, are added to render them ineffective.
Other foods are enriched by the addition of minerals.
Some examples are the addition of iodine to salt, iron
to flour and bread, calcium to orange juice, and iron
and other minerals to breakfast cereals. (See Food
Fortification.)
MINERALS – DIETARY IMPORTANCE 4005
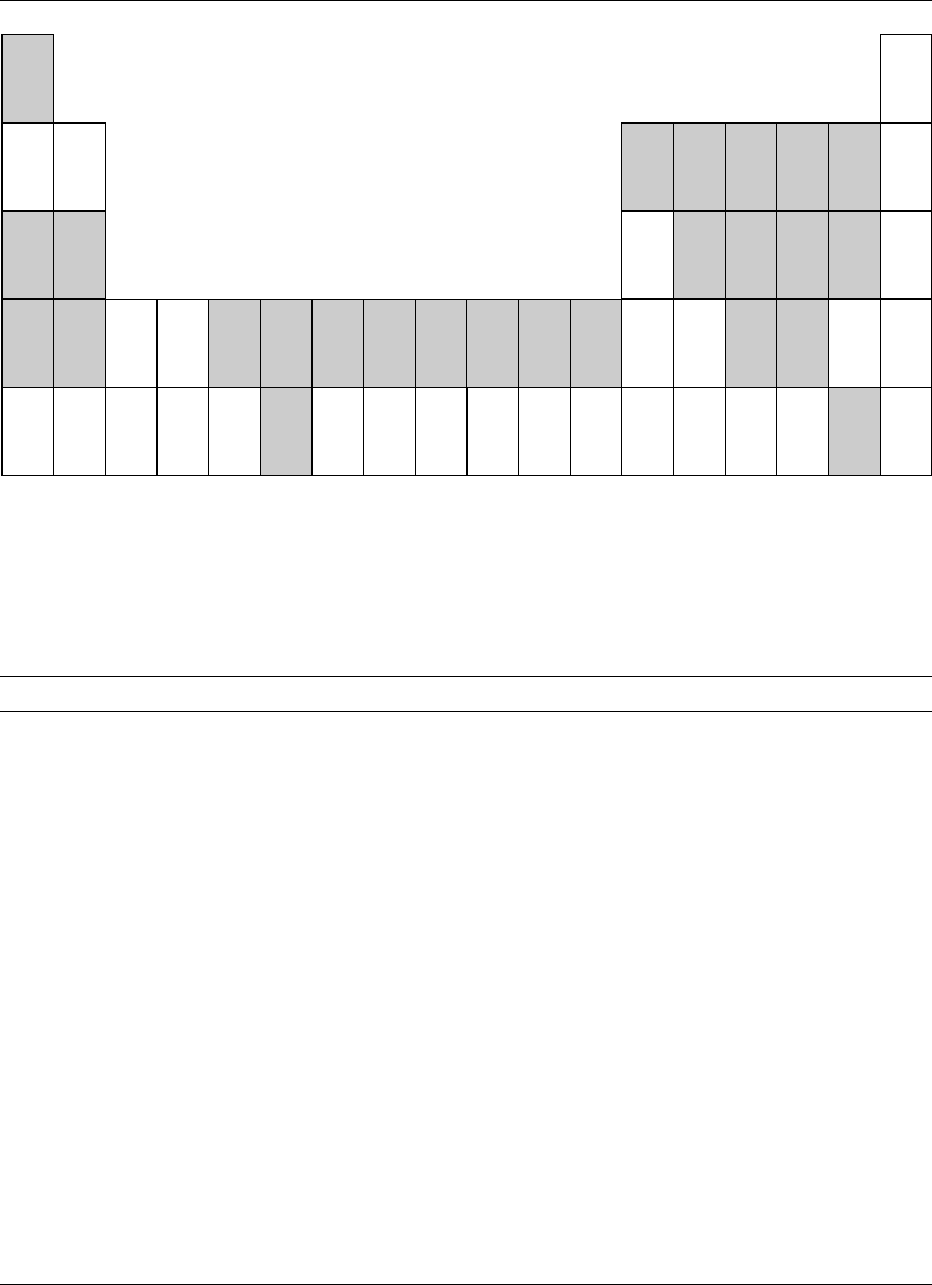
1
H
3
Li
11
Na
19
K
37
Rb
4
B
12
Mg
20
Ca
38
Sr
21
Sc
39
Y
22
Ti
40
Zr
23
V
41
Nb
24
Cr
42
Mo
25
Mn
43
Tc
26
Fe
44
Ru
27
Co
45
Rh
28
Ni
46
Pd
29
Cu
47
Ag
30
Zn
48
Cd
5
B
13
A1
31
Ga
49
In
6
C
14
Si
32
Ge
50
Sn
7
N
15
P
33
As
51
Sb
8
O
16
S
34
Se
52
Te
9
?
F
17
Cl
35
Br
53
I
2
He
10
Ne
18
Ar
36
Kr
54
Xe
fig0001 Figure 1 A portion of the periodic table of elements. Elements thought to be essential are shaded; fluoride may be beneficial rather
than essential.
tbl0001 Table 1 Food sources, physiological functions, deficiency symptoms, and requirement of major minerals
Mineral Foodsource Physiological functions Deficiencysymptoms Toxicity symptoms Requirements
Calcium Milk, cheese,
yogurt, turnip,
greens
Bone calcification, blood
clotting, muscle
contraction, nerve
transmission
Rickets, osteoporosis,
osteomalacia, tetany
Hypercalcemia, kidney
stones, constipation,
depression, confusion
UL 2.5 g day
1a
1000 mg
b
Chloride Salt, processed
foods
Electrolyte in fluid balance,
gastric acidity, acid–
base balance
Hypochloremic metabolic
alkalosis, hypotension
Hypertension (in
conjunction with excess
sodium)
750 mg
c
Magnesium Spices, nuts, coffee,
cocoa, vegetables
Cellular metabolism,
muscle relaxation, nerve
transmission
Nervous disorders, muscle
weakness, tetany,
arrhythmia,
hypertension
Diarrhea, UL 350 mg
nonfood magnesium per
day
a
310–420 mg
b
Phosphorus Cheese, meats,
peanuts, soft
drinks
Bone calcification, energy
release, membrane
structure, acid–base
balance
Fatigue, anorexia, bone
demineralization,
muscle weakness
Metastatic calcification and
skeletal porosity (in
animals) UL 4 g day
1a
700 mg
b
Potassium Fresh foods,
molasses, milk,
legumes,
bananas
Electrolyte in fluid balance,
nerve transmission,
muscle contraction,
blood pressure
Weakness, anorexia,
cardiac arrhythmia,
irrational behavior
Muscle weakness and
paralysis, cardiac
arrhythmia or arrest
2000 mg
c
Sodium Salt, cured meats,
processed foods
Electrolyte in fluid balance,
membrane potential of
cells, active transport,
blood pressure
Hyponatremia, nausea,
anorexia, weakness,
confusion, convulsions
Edema, hypertension,
congestive heart failure
in elderly
500 mg
c
Sulfur Meat, fish, eggs,
cheese, legumes
Energy transfer
(constituent of sulfur-
containing amino acids,
insulin and some
vitamins)
Unknown Depressed growth
(animals)
a
Tolerable upper intake level (UL) recommended for adults from the Institute of Medicine (1999): see Further Reading.
b
Daily recommendation for an adult man from the Institute of Medicine (1999): see Further Reading.
c
Daily recommendation for an adult man from the National Research Council (1989): see Further Reading.
4006 MINERALS – DIETARY IMPORTANCE

Bioavailability
0004 The total amount of a mineral in a food does not
necessarily reflect the amount that is available for
absorption into the body. The presence of dietary
fibers and mineral-binding ligands, such as phytates
and oxalates, may substantially diminish the bioavail-
ability of minerals. Phytates are abundant in bran,
whole grains, and oil-seed legumes; oxalates are
found in dark-green leafy vegetables, such as spinach.
Diets rich in these compounds, found in strict vege-
tarian diets, may have the potential to affect mineral
status adversely. (See Bioavailability of Nutrients;
Vegetarian Diets.)
0005 Polyphenolic compounds, formerly called tannins,
are other dietary components that bind minerals. In
tea, large concentrations of polyphenolic compounds
can reduce the absorption of iron in a meal by as
much as 87%. (See Tannins and Polyphenols.)
0006The amount of available mineral in a food may be
further reduced by the large quantities of other min-
erals. For instance, the amount of oral manganese
absorbed may be greatly decreased by the concomitant
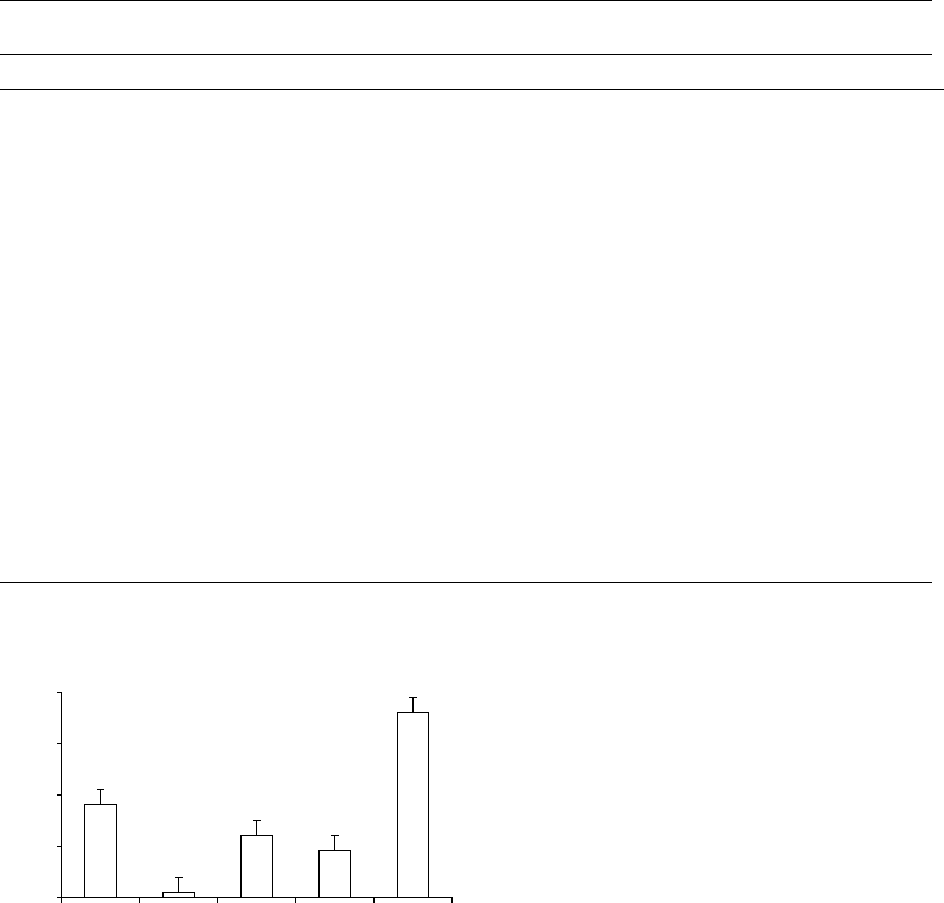
ingestion of calcium (Figure 2). Phosphates are known
to diminish the absorption of zinc. Food sources that
are high in fiber, phytates, oxalates, polyphenolic com-
pounds, and other minerals should therefore be used in
moderation in order to insure optimal mineral ad-
equacy of the diet. (See Dietary Fiber: Effects of Fiber
on Absorption; Phytic Acid: Nutritional Impact.)
0007In contrast, some dietary factors improve the
bioavailability of minerals. Ascorbic acid, sugars,
and amino acids enhance the absorption of iron and
amino acids; citrate, phosphate, gluconate, and high
tbl0002 Table 2 Food sources, physiological functions, deficiency symptoms, and requirement of essential trace minerals
Mineral Food sources Physiological functions Deficiency symptoms Toxicity symptoms Requirements
a
Chromium Mushrooms, yeast,
prunes, nuts
Glucose metabolism,
enhanced insulin
sensitivity, nucleic acid
stability
Glucose intolerance,
neuropathy, elevated
serum insulin and lipids
Cancer (hexavalent form) 35 mg
Copper Nuts, shellfish, liver,
raisins, grains,
chocolate
Iron utilization, nervous
system, pigmentation,
immune defense,
neovascularization
Neutropenia, anemia,
decreased pigmentation,
neurological, skeletal,
cardiovascular, and
cartilage abnormalities
Gastrointestinal discomfort
(> 5 mg day
1
), liver
damage, weakness,
nausea
900 mg
Fluoride
b
Fluoridated water,
seafood, tea
Precipitates calcium and
phosphorus in bone and
teeth
Increased dental caries Mottled tooth enamel, joint
stiffness UL 10 mg day
1c
3.0 mg
d
Iodine Saltwater fish,
iodized salt,
bakery goods
Thyroid hormones in basal
metabolism
Goiter, stunted growth,
mental retardation,
myxedema, cretinism,
hypothyroidism
Goiter, hypothyroidism 150 mg
Iron Liver, meats,
molasses,
prunes, nuts
Hemoglobin and
myoglobin formation for
oxygen transport,
cellular oxidation
Anemia, poor body
temperature regulation,
impaired psychomotor/
intellectual performance
Liver damage, diabetes,
increased risk of heart
disease due to lipid
oxidation
8mg
e
Manganese Tea, nuts, oatmeal,
bran, pineapple
Cartilage and bone equity,
brain function, lipid and
carbohydrate
metabolism
Rash, nervous disorders,
hypocholesterolemia,
skeletal/mitochondrial
abnormalities
Brain or neurological
disorders resembling
Parkinson’s disease,
schizophrenia
2.3 mg
Silicon Pectin, grains, beer,
cereals
Bone calcification and
cartilage formation,
growth
Depressed growth and
skeletal development
(chick)
Kidney stones; generally
nontoxic
40 mg
Zinc Meats, shellfish,
liver, legumes
Reproduction, growth, skin
integrity, wound healing,
taste acuity, immune
response
Impaired sexual
development/growth,
skin lesions, hair loss,
anorexia, behavioral
disturbances
Poor immune response,
lower high-density
lipoprotein-cholesterol,
copper deficiency
(chronic > 0.50 mg
day
1
)
11 mg
a
Daily recommendations or usual dietary intakes for an adult male from the Institute of Medicine (2001) Dietary Reference Intakes for Vitamin A, Vitamin K,
Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press.
b
May be beneficial rather than essential.
c
Tolerable upper intake level (UL) recommended for adults from the Institute of Medicine (1999) Dietary Reference Intakes for Calcium, Phosphorus,
Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press.
d
Daily recommendation for an adult male from the Institute of Medicine (1999) Dietary Reference Intakes for Calcium, Phosphorus, Magnesium,Vitamin D, and
Fluoride. Washington, DC: National Academy Press.
e
18 mg for adult female.
MINERALS – DIETARY IMPORTANCE 4007
dietary protein increase the absorption of copper. The
milk sugar lactose increases the absorption of calcium
by approximately 30%.
Requirements
000 8 The amount of a mineral required in the diet for
optimal health and prevention of chronic disease is
the basis for new dietary recommendations, dietary
reference intakes (DRIs), that have been formulated in
the USA in conjunction with the reference nutrient
intakes (RNIs) from Canada. These have been set for
major minerals (calcium,magnesium,and phosphorus)
and trace elements (chromium, copper, fluoride,
iodine, iron, manganese, molybdenum, selenium, and
zinc). The DRIs consist of four standards:
1.
0009Estimated average requirements (EAR), based on
the median of a population;
2.
0010Recommended dietary allowances (RDA),
designed to meet nutrient needs of almost all
(97.5%) healthy individuals;
3.
0011Adequate intakes (AI) for minerals with insuffi-
cient data to establish an EAR;
4.
0012Tolerable upper intake levels (UL) based on the max-
imum intake per day that appears to be safe for an
individual over a long period of time (Figure 2).
0013No recommendations are made for other trace and
ultratrace elements that participate in biological pro-
cesses because of a lack of research.
0014In the UK, dietary reference values have been sug-
gested for six minerals (calcium, phosphorus, magne-
sium, sodium, potassium, and chloride), and for
five trace elements (iron, zinc, copper, iodine, and
selenium). ‘Safe’ intakes have been set for molyb-
denum, manganese, chromium, and fluoride. (See
Dietary Reference Values.)
0015Recommendations for minerals are more difficult
to define than other nutrients as minerals can be
Mn onl
y
0
20
40
Plasma (nmol l
−1
)
60
80
Mn + CaMn + PMn + CuMn + Zn
fig00 02 Figure 2 Plasmauptakeofmanganeseasaffectedbyoral
loadsofmanganese(Mn),calcium(Ca),phosphorus(P),copper
(Cu),andZinc(Zn).Areasunderthecurveforresponseofplasma
manganesewhenthefollowingwasadministered:40mgMn,
40mgMnplus800mgCa,40mgMnplus800mgP,40mgMn
plus2mgCu,and40mgMnplus50mgZn.
tbl0003 Table 3 Foodsources,physiologicalfunctions,deficiencysymptoms,andrequirementofessentialultratraceminerals
MineralFoodsourcesPhysiologicalfunctionsDeficiencysymptomsToxicitysymptomsRequirement
a,b
ArsenicFish,meat,poultryTaurineandpolyamine
metabolism
Depressedgrowth,
reproductive
abnormalities,sudden
death(animals),
nervousdisorders
Dermatosis,low
hematopoiesis,sensory
disturbances,skin
cancer
2–29 mg
BoronMilkanddairy
products,fruits,
nuts,vegetables
Energyutilization,
development/
maintenanceofbone
Elevatedurinarycalcium,
aggravatedarthritis
(humans),poorbone
development(chicks)
Nausea,anorexia,weight
loss,decreasedsexual
activityandspermcount
1mg
MolybdenumLegumes,cereals,
leafygreen
vegetables
Sulfur,pyrimidine,and
purinemetabolism
Lowuricacid,tachycardia,
tachypnea(humans),
kidneystones(sheep)
Gout45 mg
NickelOatmeal,legumes,
peas,nuts,
chocolate
Productionofhormones,
membraneproperties,
oxidation/reduction
Lowbloodglucose,
abnormalbonegrowth,
poorironabsorption,
alteredcalcium
metabolism
Nasalandlungcancers,
asthma,contact
dermatitis,poorstress
andimmuneresponse
<100 mg
SeleniumMeats,fish,grainsAntioxidant,thyroid
hormonemetabolism,
capillaryintegrity
Cardiomyopathy,liver
damage,birthdefects
(wildfowl)
Skinlesions,brittlehair,
nails,andhooves
(animals)UL400 mg
55 mg
VanadiumParsley,black
pepper,
mushroom,dill,
shellfish
IodinemetabolismReducedgrowth,poor
bonegrowth,impaired
reproduction(animals),
poorfeathers(chicks)
Greentongue,intestinal
upset,dehydration,
breathingdifficulty
6–18 mg
a
DailyrecommendationsorusualdietaryintakesforadultsfromtheInstituteofMedicine(2001)DietaryReferenceIntakesforVitaminA,VitaminK,Arsenic,
Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon,Vanadium, and Zinc. Washington, DC: National Academy Press.
b
NutrientinformationfromtheAmericanSocietyofNutritionalSciences:www.nutrition.org(2000).
UL, upper intake level.
4008 MINERALS – DIETARY IMPORTANCE
both essential and toxic. The safe range of intake is
somewhere between levels known to produce a defi-
ciency and those that result in toxicity with continued
exposure (Figure 3). Some minerals, such as zinc and
copper, have a relatively large difference between the
adequate and toxic doses, whereas others, such as
iron and selenium, have a fairly narrow window of
optimal intakes.
0016 It should be emphasized that the recommendations
mentioned above are for healthy persons as they
consume their usual diets. The values may not be
applicable to individuals receiving parenteral solutions,
those with inborn errors that may influence absorption,
transport, utilization, storage, or excretion, and during
conditions of malnutrition, disease, and increased
demand (pregnancy, lactation, growth, stress, injury).
Physiological Importance of Minerals
0017 Minerals serve structural, catalytic, and regulatory
functions in the body. The major minerals serve as
structural components of tissues and function in
cellular and basal metabolism, as well as water and
acid–base balance (Table 1). Three of the major min-
erals – sodium, potassium and chloride – are called
electrolytes. Although the functions of the trace and
ultratrace minerals vary considerably, as shown in
Tables 2 and 3, these minerals are primarily catalytic.
These function as cofactors for enzymes or essential
components of important biomolecules. For example,
copper, manganese, and zinc are important com-
ponents of enzymes, zinc is a component of some
transcription factors (DNA-binding proteins) that
regulate gene function, iron is an essential component
of the heme portion of hemoglobin, iodine is an inte-
gral part of thyroid hormones, and cobalt is a necessary
element in vitamin B
12
(cobalamin). (See Coenzymes.)
0018 It should be emphasized that the presence of a min-
eral in the body is not proof of essentiality. Metals that
are toxic may enter the body via contaminated foods
and nonfoods, inhalation of fumes, and absorption
through the skin. They remain in the body since there
is no mechanism for excretion. For example, strontium
may be found in bones as a result of drinking contamin-
ated milk; mercury may be deposited in the brain after
inhalation of vapors; and lead may be present in the
brain following ingestion of lead-containing paint.
0019The standard criteria of essentiality are as follows:
(1) a deficiency or conditioned deficiency state is
induced and prevented or cured by a mineral; (2) the
mineral is required as a structural component by body
tissues, regulators, or is a dietary essential; (3) bio-
logical fluids maintain a certain concentration of the
mineral as a component electrolyte; and/or (4) a
molecular process uses the mineral as a cofactor.
For the ultratrace elements, another criteria for essen-
tiality may be that (5) a deficiency state creates a
suboptimal biological function, which can be circum-
vented or revoked by the addition of a usual dietary
level of the mineral. (See Coenzymes.)
Minerals and their Interactions
0020Minerals act as cofactors in biochemical reactions
since they have the ability to form stable chelates with
nitrogen donor ligands in amino acids, peptides, and
proteins. They can stabilize the structure and change
the conformation (which may influence function), bind
substrates or cofactors, activate an enzyme–substrate
complex, and influence the enzyme’s affinity for a
substrate by the specificity of the metallic ion.
0021Binding to proteins can be beneficial as it limits the
amount of free ions available. When some minerals
(such as arsenic, chromium, copper, iron, nickel) are
consumed in excess they may bind to DNA in a cross-
link manner. This type of cross-link binding may
create a dysfunctional or mutated DNA that may
play a role in carcinogenesis (initiating cancer).
Excess free ions of iron, for example, may stimulate
the formation of free radicals which attack lipids or
the free ions may bind to DNA in cells. Mutations in
DNA may be related to the association between
excess iron and colon cancer.
0022Some minerals, such as the cupric ion, bind
strongly to proteins, and are not freed by dialysis. In
contrast, zinc undergoes nonenzymatic chelation.
Zinc ions are known to bind covalently with the
nitrogen from the imidazole moiety of histidine and,
possibly, glutamate. The binding of copper and zinc
to metallothionein, a protein involved in the homeo-
stasis of these minerals, confers resistance against the
enzymatic degradation of this protein. Metallothio-
nein chelates copper and zinc via the sulfhydryl
groups (—SH) of its cysteine moieties. The affinity
of this protein for copper is 105 times greater than for
EAR
Deficiency
Toxicity
RDA
Safe range of intake
Dietary intake
UL
fig0003 Figure 3 Dietary recommendations for minerals are located
within a continuum between deficiency and toxicity. EAR, Esti-
mated average requirements; RDA, recommended dietary allow-
ances; UL, upper intake level.
MINERALS – DIETARY IMPORTANCE 4009

zinc. (See Protein: Interactions and Reactions In-
volved in Food Processing.)
0023 In calcium binding, coordination primarily involves
oxygen atoms. A main-chain carbonyl (—COO—)
is one ligand and each oxygen is the carboxyl group
(—COOH) of glutamyl and aspartyl residues. Iron
in nonheme proteins, such as transferrin, invariably
binds to the sulfhydryl group of a cysteine residue or
inorganic sulfur. In heme proteins, one ligand is the
nitrogen of imidazole in histidine. A sulfhydryl group
is a ligand for copper, zinc, cadmium, mercury, and
other heavy metals. Some proteins have double metal
sites (e.g., aspartate, glutamate) so that different
metallic ions can have a site in common.
Mineral–Mineral Interactions
0024 Minerals interact with each other according to their
physicochemical properties, i.e., valence shell elec-
tronic structure, ionic radius, coordination number
and geometric configuration, redox potential, spin
transition state, and ligand exchange. Minerals with
similarities in some of these properties can be predicted
to develop antagonistic relationships. For example,
both divalent iron and trivalent cobalt have a d
6
elec-
tronic configuration (six electrons of the d orbital),
divalent copper (0.81) and divalent iron (0.83) have
similar ionic radii, and vanadate and chromate
have similar 3d-4s configurations. Thus antagonistic
relationships can be predicted between iron and cobalt,
copper and iron, and vanadate and chromate.
0025 A calcium–phosphorus interaction occurs in the
body when levels of serum calcium decline. Parathy-
roid hormone is secreted, which decreases the renal
reabsorption and urinary excretion of phosphorus,
resulting in lower serum phosphorus. This hormone
also induces the active form of vitamin D which
increases intestinal calcium absorption and renal
phosphorus reabsorption, resulting in higher serum
levels of calcium and phosphorus. This feedback
mechanism maintains a homeostatic control of the
serum levels of these minerals. During low dietary
intake of calcium, zinc supplementation has an
inhibitory effect. (See Cholecalciferol: Physiology.)
0026 Magnesium also affects parathyroid hormone
secretion and competes with calcium for intestinal
absorption. At the cellular level, calcium transport by
the sarcoplasmic reticulum is a magnesium-dependent
process. When dietary magnesium is high, intestinal
absorption of phosphorus is inhibited. It has been ob-
served that both calcium and magnesium negatively
influence fluoride uptake at the intestine.
0027 Diets high in zinc have been shown to be detrimen-
tal to the status of both iron and copper. Excessive
zinc results in iron-deficiency anemia via a direct
effect on absorption. The opposite is true, i.e., high
dietary iron reduces zinc absorption. The antagonism
between zinc and copper is being exploited in the
treatment of Wilson’s disease, a genetic disease in
which copper abnormally accumulates in the liver.
The administration of 75 mg zinc day
1
blocks the
intestinal absorption of copper and returns copper
balance to normal quantities.
0028Iron-deficiency anemia is also produced by excessive
quantities of manganese. During periods of low man-
ganese intake, the absorption of iron greatly increases.
(See Anemia (Anaemia): Iron-deficiency Anemia.)
0029The significant interactions that can occur when
excessive amounts of minerals are ingested suggest
that mineral supplements be avoided under usual
circumstances. High-dose supplements of one min-
eral can upset the delicate balance of other minerals
in the body. Consumption of a varied, nutrient-dense
diet is a better choice than supplements for achieving
optimal mineral status.
Influence of Minerals on Food Processing
and Food Quality
0030Minerals have the potential to affect the color,
texture, flavor, pH, and nutritive value of foods and
are often used as food additives.
Color
0031The brilliant colors of fruits and vegetables are due to
a variety of plant pigments. Chlorophyll pigments,
which contribute blue-green, yellow-green, and
gray-green colors, react with both zinc and copper
to produce a bright green color. The color change
occurs when these minerals replace the central
magnesium atom in the chlorophyll molecule. (See
Chlorophyl; Colorants (Colourants): Properties and
Determination of Natural Pigments.)
0032Anthocyanins are red and blue pigments which
turn red with acid, blue with an alkali, and are some-
times colorless with prolonged heating. When foods
containing anthocyanins are processed in tin (actually
tin-coated) cans, the tin must be lacquered to prevent
the anthocyanins from forming greenish-blue pig-
ments. A greenish-blue color is also created when
rusted tin pans are used for fruit pies. Contact of a
fruit filling, such as blueberry or raspberry, with the
tin and iron salts may cause it to discolor.
0033Anthoxanthins (flavones) are pigments that are
clear and white in acid, yellow in alkali, and pink
with prolonged heating. Tin and aluminum react
with these pigments and change the color to a bright
yellow. This is illustrated by the bright-yellow
cooking water produced when onions are cooked in
an aluminum pan. When anthoxanthins react with
4010 MINERALS – DIETARY IMPORTANCE

iron and copper, blue-black and reddish-brown colors
appear, as seen in onions fried in such pans.
0034 Sulfur prevents the darkening of foods which
occurs when melanin is formed. This brown-black
pigment is created via enzymatic browning in the
presence of oxygen. Dried fruits, such as apricots
and golden raisins, which might turn an unappetizing
brown when drying, can be dipped in a sulfur solution
or exposed to sulfur fumes to prevent discoloration.
Pineapple juice is used as a dipping solution for cut
fresh fruits, such as bananas, because its high sulfur
content retards color changes. Cut lettuce for salad
bars was once sprayed or dipped in a sulfite solution
to retard browning, but this practice has been banned
since over 100 people had allergic reactions (includ-
ing one death) in response to the sulfur.
0035 Chloride salts, such as chlorine dioxide and nitro-
syl chloride, and chlorine are used to accelerate the
natural aging and bleaching of flour.
Texture
0036 When canned vegetables and fruits are cooked during
processing, the tissue softens as cementing pectic sub-
stances in the cell walls of plant tissue degrade. This
softening can be counteracted by the addition of cal-
cium ions in the form of calcium hydroxide and cal-
cium pectate. The calcium salts react with the pectic
substances to form a firm material. Calcium salts are
often added to canned tomatoes as a firming agent.
The presence of phytates in vegetables, such as peas,
decreases the firming effect of the calcium ions due to
formation of a calcium–phytate complex.
0037 In commercial baking, the texture and baking qual-
ity of bread dough are improved by the use of bro-
mates and iodates which act as oxidizing agents. If the
dough must wait for the oven, gas that is evolving
may be lost before cooking and the baked goods
will lose their characteristic light texture. To prevent
the loss of gas, baking powders have been specially
formulated to produce two reactions. In sodium
aluminum sulfate–phosphate powder, monocalcium
phosphate reacts first when moistened at room tem-
perature to create a smooth, light batter; then sodium
aluminum sulfate reacts when it is solubilized by hot
water. In sodium acid pyrophosphate (SAPP)-baking
powder, a pyrophosphate replaces the monocalcium
phosphate because it has a slower reaction rate. An
even slower reaction rate is seen with sodium acid
aluminum phosphate (SALP) powder that contains
sodium aluminum phosphate. SALP powder is used
in cakes because it retains carbon dioxide until the
gluten strands coagulate, thus preventing formation
of tunnels. (See Bread: Chemistry of Baking.)
0038 Mineral salts are also used as anticaking agents and
flow conditioners for powdered foods that have a
tendency to cake or form lumps, such as salt, confec-
tioner’s sugar, and baking powder. Some compounds
used are tricalcium phosphate, silicon dioxide, cal-
cium silicate, aluminum stearate, ferric ammonium
citrate, and monocalcium phosphate.
Flavor and pH
0039The intensity of flavor and tartness in sherbets, car-
bonated beverages, and fruit drinks is enhanced by
the addition of potassium citrate and phosphoric
acid. The correct proportion of acidity and alkalinity
is critical in controlling the correct flavor, texture,
and keeping quality of several dairy products. Buffer-
ing agents such as sodium bicarbonate, calcium car-
bonate, hydrogen chloride, sodium citrate, sodium
hydroxide, and calcium oxide may be used to control
pH. (See pH – Principles and Measurement.)
Safety and Quality of Foods
0040Sulfur dioxide and sulfites are added to foods because
of their ability to act as antioxidants, and to fermenting
alcoholic beverages because it is more toxic to bacteria
and molds than to yeast. Wine can have an exception-
ally high sulfur concentration, as much as 200 mg per
0.5 l. This level far exceeds the acceptable daily intake
of 0.7 mg kg
1
. In the USA, all foods containing detect-
able levels of 10 mgg
1
sulfite or more are labeled. (See
Antioxidants: Synthetic Antioxidants.)
0041Mold inhibitors, such as calcium and sodium
propionate, monocalcium phosphate and sodium dia-
cetate, are added to baked goods. These prevent ropi-
ness in bread and increase the shelf-life. (See Spoilage:
Molds in Spoilage.)
0042Sequestrants or chelating agents are added to foods
to bind metals, such as calcium, iron, and copper.
When the metals bound to the chelator are no longer
in an ionized form, oxidative changes such as stale-
ness, rancidity, and off-flavors are prevented from
developing. These compounds are important in fruit
juices, canned seafood, milk, and salad dressings.
They are also used to clarify wine and other beverages
of minerals.
0043The presence of minerals in foods may influence
the cooking or processing time. Calcium ions, for
example, have a firming effect, which prolongs the
length of the cooking time. This may occur when hard
water is used since it naturally contains calcium salts.
If a long cooking period is desired for flavor develop-
ment, as in preparing baked beans, calcium, as well as
acids, can be added. In home cooking, molasses is
used since it contains high concentrations of calcium
as well as aconitic acid.
0044The use of soft water in food processing, however,
may not always be desirable. In the formulation of
MINERALS – DIETARY IMPORTANCE 4011

low-sodium foods, soft water derived from a water
softener should be avoided because it may contain as
much as 50 mg of sodium dl
1
. Sodium is also added
when foods are brined, as in preparing pickles or
preventing the discoloration of vegetables. When
meats are processed according to kosher laws, large
amounts of sodium are added as the blood is removed
by salting.
See also: Anemia (Anaemia): Iron-deficiency Anemia;
Antioxidants: Synthetic Antioxidants; Bioavailability of
Nutrients; Cholecalciferol: Physiology; Coenzymes;
Colorants (Colourants): Properties and Determination of
Natural Pigments; Dietary Fiber: Effects of Fiber on
Absorption; Dietary Reference Values; Food
Fortification; Functional Foods; Heavy Metal
Toxicology; Protein: Interactions and Reactions
Involved in Food Processing; Spoilage: Molds in
Spoilage; Trace Elements; Vegetarian Diets
Further Reading
Department of Health (1991) Dietary Reference Values for
Food Energy and Nutrients for the United Kingdom.
Report of the Panel on Dietary Reference Values of the
Committee on Medical Aspects of Food Policy. London:
Her Majesty’s Stationery Office.
Freeland-Graves J (1985) Mineral adequacy of vegetarian
diets. American Journal of Clinical Nutrition 48: 859–
862.
Freeland-Graves J (1999) Manganese: development of a
dietary recommendation for an essential yet toxic trace
element. In: Abdulla M, Bost M, Gamon S, Arnaud P
and Chazot G (eds) New Aspects of Trace Element
Research, pp. 22–27. London: Smith-Gordon.
Freeland-Graves J and Peckham G (1996) Foundations of
Food Preparation, 6th edn. Englewood Cliffs, NJ: Pren-
tice Hall.
Institute of Medicine (1999) Dietary Reference Intakes for
Calcium, Phosphorus, Magnesium, Vitamin D, and
Fluoride. Washington, DC: National Academy Press.
Institute of Medicine (2001) Dietary Reference Intakes
for Vitamin A, Vitamin K, Arsenic, Boron, Chromium,
Copper, Iodine, Iron, Manganese, Molybdenum, Nickel,
Silicon, Vanadium, and Zinc. Washington, DC: National
Academy Press.
National Research Council (1989) Recommended Dietary
Allowances. Washington, DC: National Academy Press.
O’Dell BL and Sunde RA (eds) (1997) Handbook of Nutri-
tionally Essential Mineral Elements. New York: Marcel
Dekker.
Shils ME, Olson JA, Shike M and Ross AC (1999) Modern
Nutrition in Health and Disease, 9th edn. Baltimore:
Williams & Wilkins.
MINERAL WATER
Contents
Types of Mineral Water
Sources and Analysis
Bottling and Storage
Types of Mineral Water
M Abdulla, M Bost and S Gamon, Trace Element –
Institute for UNESCO, Lyon, Cedex, France
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The term ‘mineral water’ is currently applied to sev-
eral types of drinking water originating from under-
ground sources that is packaged and marketed for
human consumption. Mineral water may be defined
as drinking water containing naturally or artifically
supplied minerals, trace elements, and gases. Natural
mineral water is microbiologically wholesome water,
originating from an underground formation and
emerging from a spring tapped at one or more natural
or bore exits. Natural mineral water can be distin-
guished from ordinary drinking water by its natural
and original state.
Formation of Mineral Water
0002It takes a significant number of years for mineral
water to accumulate in an underground formation.
When rain and snow fall on the land, over a period
of many decades, it percolates through layers of
soil, sand, and clay. Successive layers filter out the
4012 MINERAL WATER/Types of Mineral Water

impurities while adding dissolved minerals and trace
elements. Some trace elements such as arsenic can be
found in significant quantities in certain geographical
areas of our planet, which may contaminate the
water. Finally, perhaps 100 years or more after it
first touched the land, this mineral water settles on
bedrock deep below the surface.
Classification of Mineral Waters
0003 The packaged (bottled/canned/in carton or foil) water
market comprises two main segments: still and spark-
ling water. The main difference between the two cat-
egories is in the carbon dioxide content. Still waters
have no carbon dioxide, are often sold as an alterna-
tive to tap water, and are used for cooking and mixing
with coffee, powdered mixes, concentrated juices,
soft drinks, etc. Sparkling waters contain carbon di-
oxide and are generally used as an alternative to soft
drinks or alcoholic beverages. There are several types
of packaging (bottled/canned/in cartons and poly-
ethylene bags), depending upon the source of water.
The various types of water that are commercially
available at present are shown in Table 1.
Regulations
0004 The definition of mineral waters may differ in differ-
ent countries. The rules and regulations for marketing
the mineral water in the country of origin and outside
that country may also differ. These differences in the
rules and regulations may hinder the free movement
of packaged mineral water outside the boundaries of
the countries producing special brands. Efforts are
being made, especially in the European Community,
to eliminate these differences in order to promote the
free movement of the packaged natural mineral
waters. The producers of the natural mineral water,
however, must fulfill the basic laws and regulations
concerning foodstuffs and water intended for human
consumption. From a global point of view, there is
a standard set by the Food and Agricultural Organ-
ization and the World Health Organization of the
United Nations (FAO/WHO codex standard). In
the countries of the European Communities, in re-
sponse to the opinion of the European Parliament
delivered in October 1995 on the approximation of
the laws of the member states relating to the exploit-
ation and marketing of natural mineral waters, cer-
tain amendments have been made. These
amendments, however, do not differ from those of
the FAO/WHO codex standard. The following con-
ditions apply to natural mineral waters packaged for
human consumption, particularly in affluent industri-
alized countries.
1.
0005Natural mineral water, in its state at source, may
not be the subject of any treatment or addition
other than the addition or elimination of carbon
dioxide. It must contain a certain amount of dis-
solved mineral salts. In many industrialized coun-
tries, this amount is around 500 mg l
1
.
2.
0006Natural mineral water, in its state of source, must
meet the specified microbiological requirements.
It must be free from all pathological organisms.
After packaging, mineral water must not have
more than a specified total colony count. In many
countries, especially in industrialized countries, the
figure may not exceed 100 per ml at 20–22
Cin
72 h on agar or agar–gelatine mixture and 20 per
ml at 37
C in 24 h on agar. The total colony count
shall be measured within 12 h following pack-
aging, the water being maintained at 4+1
C
tbl0001 Table 1 Types of water that are commonly available at present
Type of water Characteristics
Natural mineral water Originates from a geologically and physically protected and approved underground source. It must have a
high quality and constant composition. It must naturally contain a certain amount of dissolved mineral salts
and trace elements, and should not undergo any treatment. The amount of dissolved mineral salts is at least
500 mg l
1
. Nothing should be added or removed other than carbon dioxide. It must be tapped from the
original source and pumped to the surface through stainless steel pipes for packaging in bottles, cans, or
other approved packaging materials.
Spring water Originates from an underground formation and flows naturally to the surface of the earth. It must have a high
quality and constant composition.
Well water Originates from a hole bored, drilled, or otherwise constructed in the ground that taps the water of an aquifer.
Mineral water Originates from a protected underground formation that has a high quality and constant composition. It should
not be modified other than by adding mineral salts, aroma-producing compounds and carbon dioxide.
Other packaged water This applies to all other types of bottled, canned, or packaged table waters. Names such as table water,
carbonated table water, soda water or other accepted names belong to this category. They may be treated
and prepared in the same way as commercial drinking water according to the local public health
regulations. Mineral salts, essential trace elements, aroma-producing substances, and carbon dioxide may
be added.
MINERAL WATER/Types of Mineral Water 4013
