Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

unsaturated bonds. The resulting lipid hydroper-
oxides, peroxy radicals, and hydroxyl radicals cause
rancidity and are potential DNA-damaging agents. A
variety of epoxides and aldehydes are also formed,
including malondialdehyde, a potential mutagen
(Figure 2).
Food Additives and Contaminants
0036 Food additives and contaminants (including pesticide
residues) are compounds that are added to food at
some point during its production. Exposure to these
substances can be controlled, and the introduction of
new chemicals used in food manufacture falls under
government regulation. Older additives or chemical
contaminants are also reviewed periodically for
exposure and health effects.
0037Use of the preservatives nitrite, butylated hydroxy-
anisole (BHA) and butylated hydroxytoluene (BHT)
as food preservatives has been controversial. Nitrite
enters the diet both through its use as a common food
preservative and through gut microbe-mediated reduc-
tion of nitrate from ingested vegetables. Nitrite cycles
from the gut to the saliva and can react at acidic pH
with amines, amino acids, phenols, and mercaptans,
frequently resulting in the formation of mutagenic
(nitroso) compounds. Mutagens can also be found
in the smoke of nitrite-treated meat during frying.
The formation of 2-chloro-4-methylthiobutanoic
O
O
3-Hydroxyflavone general structure
CH
2
CH
NH C
COOH
O
O
OH
O
CH
3
Ochratoxin A
N
Structural backbone of
pyrrolizidine alkaloids
O
O
O
Psoralen
O
O
O
O
O
OCH
3
Aflatoxin B
1
OH
COOH
NH
NH
2
Hydrazinobenzoic acid
OH
O
O
HO
OH
O
OH
OH
Ptaquiloside
OH O
OH
O
OH
OH
Altertoxin I
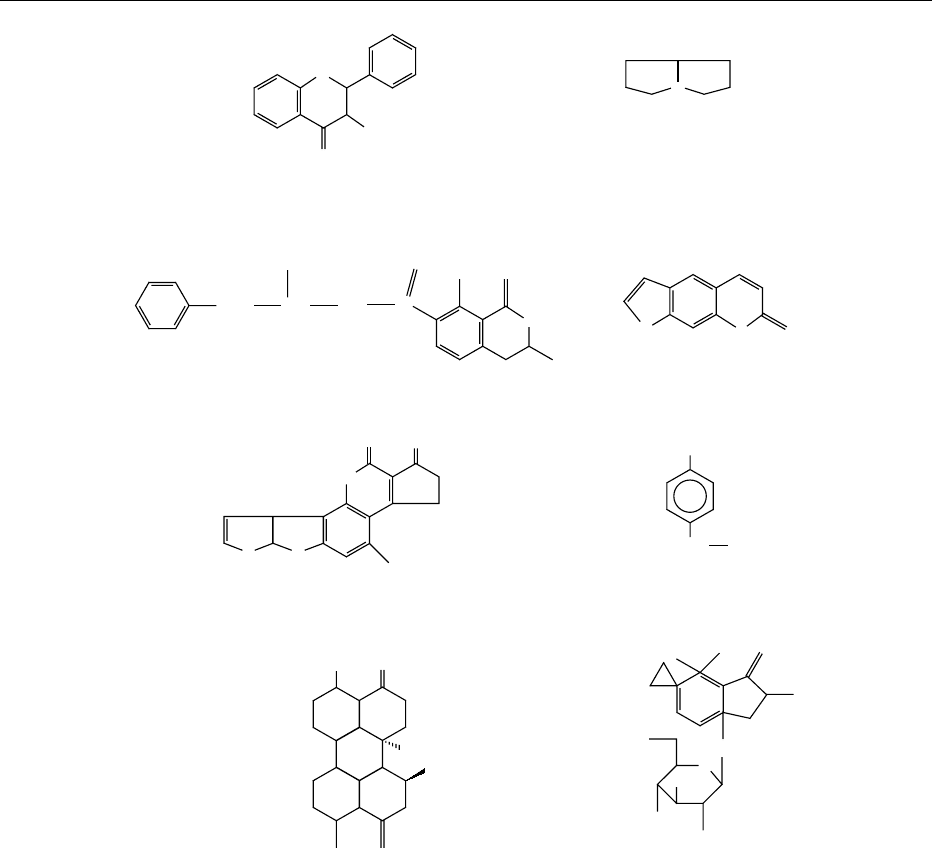
fig0001 Figure 1 Structures of selected naturally occurring mutagens.
4064 MUTAGENS
acid, a mutagen in salted, pickled fish, has also been
linked to nitrite. BHA and BHT have been added to
foods and packaging as synthetic antioxidants. Previ-
ous concerns over BHA’s carcinogenic risk to humans
appear unwarranted and related to the large doses
used in rodent-feeding studies. BHT increases the
proliferation of pretumor liver cells in rodent models
but has little effect on normal cells.
0038 The artificial sweeteners saccharin and cyclamate
have also been shown to have mutagenic properties,
although saccharin-induced bladder carcinogenesis in
rodents also appears to result from the large doses
used. Also, the mutagenicity of some colors has led to
their use being banned, although low levels of extract-
able mutagenic contaminants have been detected in
others. Mutagenic heterocyclic amino acids have
been found in some flavors.
0039 Contaminants may occur in food as residues of
pesticides and fungicides, solvents, or compounds,
such as styrene leaching from packaging material.
The persistent organochlorine pesticide toxaphene
has been found in rural foods consumed by indigen-
ous peoples in the Arctic region, having been carried
and deposited through airborne transport from sites
in the south.
Mutagens in Water
0040Drinking water provides another potential source
of mutagenic compounds. Water disinfection by
chlorination can produce many types of mutagenic
chlorinated organic compounds, of which 3-chloro-4-
(dichloromethyl)-5-hydroxy-2-(5H)-furanone (muco-
chloric acid, MX) is the most potent. Other mutagenic
chlorinated butenoic acids, including EMX (the re-
duced form of MX), brominated trihalomethanes,
and dichloroacetonitrile, are also produced. Dichlor-
oacetonitrile is a direct-acting mutagen and induces
DNA-strand breaks in cultured human lymphoblastic
cells. Other less potent mutagenic disinfection by-
products include dichloroacetic acid, trichloroacetic
acid, and chloral hydrate. Radioactive contamination
can also contribute mutagenic activity in areas where
water is contaminated with
40
K or uranium and
thorium decay products (such as radon).
Mutagens in Feces
0041Human feces have been found to contain a class of
mutagenic compounds known as fecapentaenes, the
major forms of which are fecapentaene-12 and
fecapentaene-14. These compounds appear to be
synthesized by intestinal bacteria and are lipid-sol-
uble, direct-acting base pair and frameshift mutagens.
Fecapentaene-12 also causes increased sister chroma-
tid exchanges, HGPRT mutagenesis, and oxidative
damage. Bile salts have been shown to augment the
ability of fecapentaenes-12 and -14 to induce DNA
repair in bacteria, underscoring their possible role in
colon carcinogenesis associated with high-fat, low-
fiber diets.
Prevention and Modification of Mutagen
Formation
0042A well-balanced diet also provides many compounds
that can decrease exposure to mutagens. Studies con-
sistently show that a greater consumption of fruits
and vegetables is associated with a lowering of cancer
incidence. These compounds can act at all levels of
mutagenesis by: (1) preventing mutagen formation
and acting as antioxidants, (2) deactivating muta-
genic compounds, and (3) inducing detoxifying
enzymes or inhibiting monoxygenase activity.
0043Several natural antioxidants, such as ascorbic acid,
b-carotene, a-tocopherol, polyphenolic acids, flavo-
noids, and other plant phenolics have a direct muta-
gen-scavenging activity. For example, in addition to
neutralizing oxygen radicals, many of these com-
pounds inhibit the formation of nitrosamines by com-
peting with the amine group. Mutagenic agents may
N
N
N
CH
3
NH
2
IQ
N
N
H
3
C
NH
2
CH
3
N
N
8-MeIQx
N
N
N
NH
2
CH
3
PhIP
N
CH
3
NH
2
CH
3
N
H
Trp-P-1
Benzo[a]pyrene
N
CH
3
N
O
N-Nitrosodimethylamine
H
3
C
H
2
N
C OC
2
H
5
O
Urethane
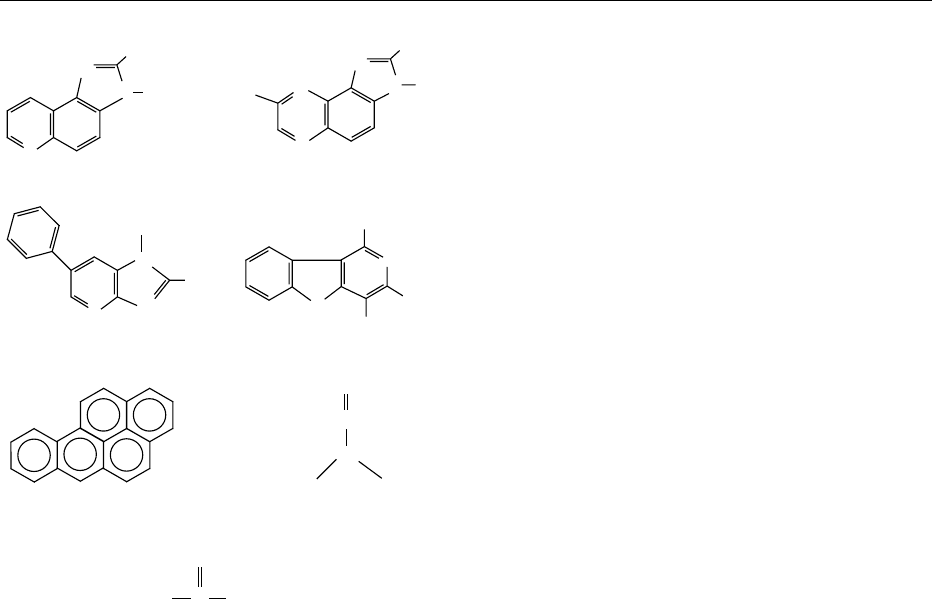
fig0002 Figure 2 Structures of selected mutagens formed during
grilling and processing.
MUTAGENS 4065
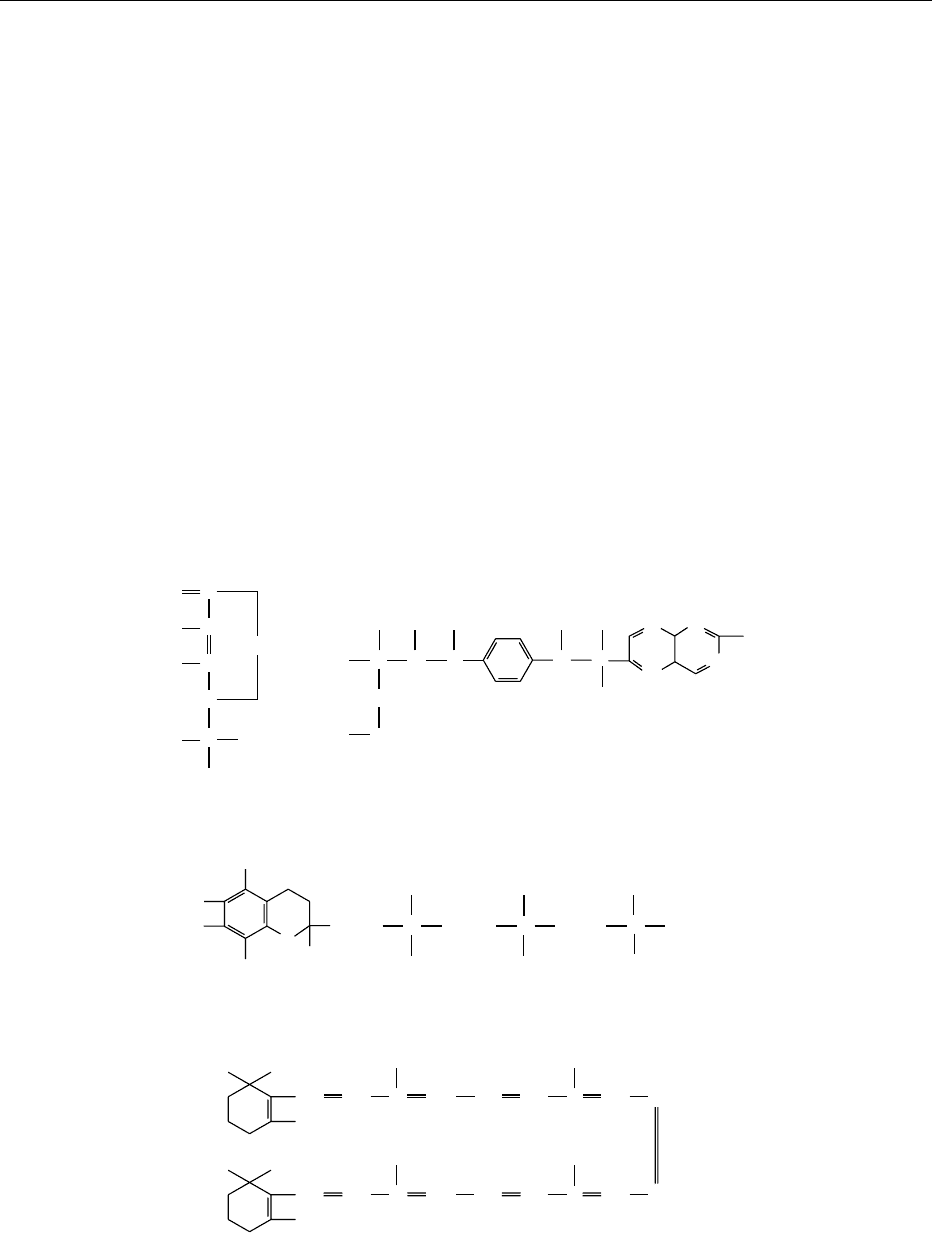
be inactivated through the formation of complexes
with calcium, casein, chlorophyl and carbonyl com-
pounds. Some evidence suggests that calcium can
inhibit familial colon carcinogenesis through inhib-
ition of lipid peroxidation. Both iron deficiency and
excess have been associated with oxidative DNA
damage, and zinc deficiency causes chromosome
breaks. The activity of glutathione-dependent detoxi-
fication systems is increased by selenium and diallyl
disulfide, from garlic, cysteamine, cysteine, as well as
a variety of phenolic compounds. Monooxygenase
activity and the formation of reactive compounds
can be inhibited by phenolic acids, free fatty acids,
flavonoids, and other polyphenols, methylxanthines,
vitamins, and biogenic amines. Conjugated dienoic
isomers of linoleic acid are antimutagenic compounds
in extracts of fried ground beef and dairy products
that appear to act through signal-transduction path-
ways and prostaglandin synthesis. Tannins or poly-
phenols in green tea have been found to be
antimutagenic in some assays, but the effects appear
to depend upon the dose and timing of exposure.
Several other compounds have shown antimutagenic
activity, including b-diketones, catechins, lignans,
and isoflavones. Finally, dietary fiber can complex
with mutagenic compounds and limit their uptake.
0044Mutagenic effects resulting from altered DNA me-
tabolism can also be influenced by diet. Adequate
intake of essential nutrients such as folate, vitamin
B
12
and vitamin B
6
aids nucleotide synthesis and
prevents DNA-strand breakage. Niacin, methyl-
xanthines, and some phenols have been attributed to
promotion of DNA-damage repair (Figure 3).
Further Considerations
0045Much work remains to completely characterize the
role of food-associated mutagens and antimutagens
in human carcinogenesis. Despite the magnitude and
frequency of exposure to these compounds, they
C
C
C
C
CH
2
OH
O
O
HO
HO
HC
HO H
L-Ascorbic acid
(Vitamin C)
(CH
2
)
3
C
C
(CH
2
)
3
(CH
2
)
3
CH
3
C
CH
3
HO
H
3
C
CH
3
O
CH
3
CH
3
CH
3
CH
3
HHH
α-Tocopherol
(Vitamin E)
β-Carotene
(provitamin A)
HOOC
C
NC NC
NH
2
N
OH
N
N
N
HH O H H
H
CH
2
CH
2
HOOC
Folic acid
H
3
CCH
3
CH
3
CH CH C CH CH CH C CH CH
CH
3
CH
3
H
3
CCH
3
CH
3
CH CH CCH
CH
CH CH C CH CH
CH
3
CH
3
fig0003 Figure 3 Structures of common dietary antioxidant antimutagens.
4066 MUTAGENS
remain underrepresented in mutagenesis studies rela-
tive to synthetic chemicals. Additional characteriza-
tion is needed of the human consequences of exposure
to many antioxidants that appear to inhibit mutagen-
esis at low concentrations but have the opposite effect
at high concentrations. Finally, more emphasis should
be placed on the effects of chemical mixtures to study
the effects of combinations of compounds encoun-
tered in the diet.
See also: Aflatoxins; Amines; Carcinogens:
Carcinogenicity Tests; Food Additives: Safety;
Mycotoxins: Classifications; Occurrence and
Determination; Toxicology; Polycyclic Aromatic
Hydrocarbons; Saccharin
Further Reading
Ames BN (1998) Micronutrients prevent cancer and delay
aging. Toxicology Letters 102–103: 5–18.
Clayson DB (2001) Toxicological Carcinogenesis. Boca
Raton, FL: Lewis Publishers.
Franks LM and Teich NM (eds) (1997) Introduction to the
Cellular and Molecular Biology of Cancer, 3rd edn.
Oxford: Oxford University Press.
Friedberg EC, Walker GC and Siede W (1995) DNA Repair
and Mutagenesis. Washington, DC: ASM Press.
Hui YH, Kitts D and Stanfield PS (2001) Foodborne
Disease Handbook, Seafood and Environmental Toxins,
2nd edn., vol. 4. New York: Marcel Dekker.
Hui YH, Smith RA and Spoerke DG, Jr. (eds) (2001) Food-
borne Disease Handbook, Plant Toxicants, 2nd edn, vol.
3, New York: Marcel Dekker.
Kilbey BJ, Legator M, Nichols W and Ramel C (1984)
Handbook of Mutagenicity Test Procedures. Amster-
dam: Elsevier Science.
Kitchin KT (ed.) (1999) Carcinogenicity: Testing, Predict-
ing, and Interpreting Chemical Effects. New York:
Marcel Dekker.
Klug WS and Cummings MR (2000) Concepts of Genetics,
6th edn. Upper Saddle River, NJ: Prentice Hall.
McKinnell RG, Parchment RE, Perantoni AO and Pierce
GB (1998) The Biological Basis of Cancer. Cambridge:
Cambridge University Press.
National Research Council Monograph (1996) Carcino-
gens and Anticarcinogens in the Human Diet. Washing-
ton, DC: National Academy Press.
Pariza MW, Aeschbacher H-U, Felton JS and Sato S (eds)
(1990) Mutagens and carcinogens in the diet. In: Pro-
gress in Clinical and Biological Research, vol. 347. New
York: Wiley-Liss.
Pfeifer GP (ed.) (1996) Technologies for Detection of DNA
Damage and Mutations. New York: Plenum Press.
Twyman RM (1998) Advanced Molecular Biology: A
Concise Reference. Oxford: Bios Scientific.
Mutton See Sheep: Meat; Milk
MYCOBACTERIA
C H Collins and J M Grange, National Heart and Lung
Institute, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The genus Mycobacterium contains over 60 species;
these are divided into rapid-growers, slow-growers,
and the human leprosy bacillus which has not been
convincingly cultivated in vitro. A few of the species
are obligate parasites, but most of them are environ-
mental saprophytes. The latter are widely distributed
in nature and have been isolated from natural waters,
wet soil, mud, compost, grasses, vegetables, unpas-
teurized milk, and butter. They have also been isol-
ated from domestic water pipes from which they
readily enter drinking water. Mycobacteria are char-
acterized by the possession of very thick, waxy, lipid-
rich hydrophobic cell walls. Being hydrophobic, they
tend to grow as fungus-like pellicles on liquid culture
media: hence the name Mycobacterium –‘fungus
bacterium.’
0002Even the rapidly growing mycobacteria grow
slowly in comparison with most other bacteria.
Mycobacteria may play a role in the decomposition
of organic material, particularly in sphagnum
MYCOBACTERIA
4067

marshes, but there are no reports of mycobacteria
causing spoilage in foods. They do not produce
appreciable amounts of toxic substances and do not
cause food poisoning. Pathogenic strains owe their
virulence to their ability to resist immune defense
mechanisms; they cause chronic infections.
0003 The obligate parasites are Mycobacterium tubercu-
losis (the human tubercle bacillus), M. bovis (the
bovine tubercle bacillus), and M. africanum (a species
first described in equatorial Africa and having rather
variable properties that are intermediate between the
other two). Variants of these species are occasionally
encountered and include M. microti (the vole tubercle
bacillus) and biochemically and genetically distinct
isolates from goats termed the caprine genotype or
M. tuberculosis subsp. caprae. All these species, often
grouped as the tuberculosis complex, are very closely
related and should be regarded as variants of a single
species. Other pathogens thought to be obligate para-
sites are M. leprae (the causative organism of lep-
rosy), M. paratuberculosis (the cause of Johne’s
disease or hypertrophic enteritis in cattle and other
ruminants) and M. lepraemurium (the cause of rat
leprosy). As M. leprae is uncultivable and other two
are very difficult to cultivate in vitro, the possibility
that they can replicate in the inanimate environment
cannot be excluded.
0004 Some of the saprophytic species occasionally cause
opportunistic infections of animals and humans. The
principal opportunist species are M. avium,
M. intracellulare, M. scrofulaceum, M. kansasii,
M. xenopi, M. malmoense, and the rapidly growing
species M. chelonae and M. fortuitum. The first two
species are closely related and are often grouped to-
gether as the M. avium complex (MAC). Although
essentially saprophytic, MAC are the most patho-
genic of the opportunist mycobacteria. Members of
this complex are a common cause of tuberculosis in
birds and they also cause limited lesions, particularly
cervical lymphadenopathy, in mammals, including
pigs, cattle, and deer. Closely related to the MAC
are M. lepraemurium, M. paratuberculosis and the
wood pigeon bacillus (M. avium subsp. sylvaticum)
which resembles M. paratuberculosis in requiring
mycobactin, an iron-binding lipid extracted from
mycobacterial cell walls, for its in vitro cultivation.
A few other nutritionally very fastidious mycobac-
teria, requiring enriched media for their cultivation,
have recently been described. These include
M. genavense, which was originally detected in ac-
quired immunodeficiency syndrome (AIDS) patients
by means of DNA amplification techniques. This
species has also been isolated from pet birds and a
dog and infected pets may thus pose a health hazard
to severely immunosuppressed persons.
Mycobacterial Disease
0005Although the human mycobacterial diseases differ
enormously in their clinical features they have certain
characteristics in common. They all commence with a
local lesion, which may or may not be clinically
evident, at the site of implantation of the causative
organism. They may be in the lung in the case of
inhaled bacilli, in the skin following trauma, or in
the pharynx or intestinal tract following ingestion of
the bacilli in contaminated food or water. In some
cases, notably in tuberculosis, the local lymph nodes
are also involved in the primary infection and the
lymphatic lesion may be much more extensive than
that at the site of implantation. Thus, ingestion of
milk contaminated with M. bovis often leads to a
cryptic tonsillar or pharyngeal lesion and gross en-
largement of one or more of the lymph nodes in the
neck – a condition known as scrofula. Bacilli may
enter the blood stream from the primary lesion and
then cause serious forms of primary tuberculosis,
notably tuberculous meningitis and disease of the
kidneys, bones, and joints. Thus, in countries where
there is (or was) a high incidence of human tubercu-
losis of bovine origin, scrofula and associated non-
pulmonary manifestations of the disease are (or were)
commonly seen in children.
0006Unless one of the serious sequelae of primary
tuberculosis develops, the primary complex usually
resolves, but the disease reactivates in about 5% of
infected persons to cause postprimary tuberculosis,
years or even decades later. (The risk of reactivation
is much higher in immunosuppressed persons and
human immunodeficiency virus (HIV) infection has
emerged as the most prevalent predisposing factor
for the development of tuberculosis worldwide. A
person dually infected by HIV and M. tuberculosis
has a 8–10% chance of developing active tuberculosis
annually.) Cases of tuberculosis due to late reactiva-
tion of M. bovis infection still therefore occur in
countries that have virtually eradicated tuberculosis
from cattle. In Europe and Australia, M. bovis
accounts for around 1% of cases of tuberculosis.
0007The way in which residual mycobacteria persist in
tissues for long periods is unknown. The reactivation
may occur at the site of the primary lesion (such as
cervical lymph nodes in the case of tuberculosis of
bovine origin), but more often it reactivates, for un-
known reasons, in the upper lobes of the lung. This
leads to open or infectious tuberculosis and there
have been reports of cattle being infected by such
patients. Also, and for unknown reasons, some cases
(about 25%) of postprimary human tuberculosis due
to M. bovis in Europe involve the genitourinary tract.
This rather insidious form of tuberculosis often passes
4068
MYCOBACTERIA

undiagnosed for long periods and farm workers with
this condition are known to have transmitted tuber-
culosis to cattle by urinating in cowsheds.
0008 In humans, some environmental mycobacteria are
able to cause lymphadenopathy, particularly in young
children; skin lesions following injections of contam-
inated material, superficial or penetrating injuries,
and surgery involving implantation of contaminated
prostheses such as porcine heart valves; pulmonary
disease, particularly in patients with pneumoconiosis
or other predisposing lung diseases; and disseminated
disease. The last, once rare, is now a common AIDS-
related condition and, for unknown reasons, is usu-
ally caused by the MAC, although some cases are due
to the more recently described M. genavense, referred
to above.
0009 In recent years there has also been speculation that
ingested mycobacteria, possibly from milk, might be
responsible for Crohn’s disease, a granulomatous
disease of the human intestine. Normal forms and
spheroplasts of mycobacteria have been isolated
from tissue excised from a few patients with Crohn’s
disease and DNA supposedly specific for M. para-
tuberculosis, the cause of Johne’s disease in cattle,
has been detected by the polymerase chain reaction
(PCR) in biopsies from patients with Crohn’s disease.
In some studies such DNA was detected in the major-
ity of specimens but in other studies no such DNA
was detected. These conflicting findings may be at-
tributable to methodological problems and techno-
logical advances may resolve the issue but at present
no firm conclusions can be drawn. Likewise, attempts
to detect antibodies to M. paratuberculosis in serum
and antigen in tissues, as well as trials of antimyco-
bacterial drugs, have yielded unconvincing results.
Sources of Infection
0010 The human tubercle bacillus, M. tuberculosis,isusu-
ally spread from person to person via expectorated
aerosols and causes pulmonary tuberculosis. Individ-
uals with open tuberculosis who work in the food
industry may therefore transmit the organisms to
other people and could possibly, in theory, contamin-
ate food. The bovine bacillus, M. bovis, causes pul-
monary tuberculosis in farm workers who inhale the
bacilli in aerosols expectorated by diseased cattle
while town dwellers usually become infected by
drinking contaminated milk and predominantly
develop nonpulmonary tuberculosis.
0011 Although persons working with other animals with
disease due to M. bovis, including seals, elk, and a
rhinoceros with tuberculous rhinitis, have become
tuberculin-positive, overt disease has seldom been
reported. A few strains of the caprine genotype
have, however, been isolated from humans with
tuberculosis, including one isolate from a veterinary
surgeon with a recent history of working with tuber-
culous goats, and a seal trainer contracted tubercu-
losis due to an M. bovis-like strain from a captive seal
in Australia.
0012Mycobacterial disease may result from the inocu-
lation of the bacilli into the skin. In areas where
tuberculosis of cattle is common, food handlers
are at risk of developing tuberculous lesions of the
hands as a result of contamination of minor cuts and
abrasions, the resulting condition being known as
butcher’s wart (it is also known as prosector’s wart,
as anatomists and pathologists were likewise exposed
to this occupational hazard).
0013Food and drink may serve as vectors for the trans-
mission of pathogenic mycobacteria. By far the most
likely vehicle, leading to disease of the alimentary
tract, is unpasteurized milk. Meat and water are
also possible sources, but are much less important.
(See Milk: Processing of Liquid Milk.)
Unpasteurized Milk
0014There is a long history of human infection resulting
from the consumption of milk that contained
M. bovis. In developed countries such as the UK
these infections are now rare for two reasons: the
eradication of tuberculosis among cattle by tubercu-
lin testing and slaughter; and the pasteurization of
milk for human consumption. Both have been the
subject of legislation. Milk-borne tuberculosis is still
a hazard, however, in developing countries.
0015The history of milk-borne tuberculosis in the UK
illustrates this. The disease in cattle (and other
animals) had been recognized as an economic prob-
lem for centuries. By the turn of the century it was
known that tuberculosis in cattle was caused by
M. bovis, but Robert Koch, who had discovered the
tubercle bacillus in 1882 and was regarded as a world
authority on the subject, told the British Congress on
Tuberculosis in 1901 that the bovine organism did
not cause the disease in humans and that measures
to counteract infection with that organism were
unnecessary. This was disputed by certain British
bacteriologists and veterinarians and it was not
until after the publications of the findings of a Royal
Commission in 1911 that the bovine bacillus was
accepted as the causative organism of many cases of
nonpulmonary tuberculosis, notably cervical lymph-
adenopathy, abdominal and skeletal tuberculosis,
particularly in children.
0016Official action was slow, however, and in the 1930s
it was known that about 40% of all cattle slaughtered
in public abattoirs had tuberculous lesions and that
about 0.5% of all dairy cows produced milk that
MYCOBACTERIA
4069

contained the bovine tubercle bacillus. The eradica-
tion scheme that followed was initially voluntary and
did not become compulsory until after World War II.
By 1960 all dairy herds in the UK had been tested and
by 1979 the reactor rate had fallen dramatically and
only 0.18% of herds were infected. This may well
represent an irreducible minimum, because reinfec-
tion, from humans and other animals (e.g., badgers)
may still occur and therefore there is still a risk of
infection from unpasteurized milk although, at pre-
sent, as a result of more legislation, very little of this is
sold for immediate consumption in this country.
0017 Goats may also be infected, and tubercle bacilli
have been recovered from their milk. The consump-
tion of raw goats’ milk is therefore not without risk.
0018 Little is known about the role of milk in other
mycobacterial infections. The possible link between
milk-borne M. paratuberculosis and Crohn’s disease
has been referred to above. Members of the MAC and
other saprophytic mycobacteria have been isolated
from milk, but no direct link has been established
between the presence of these organisms in milk and
human disease, although contaminated milk is a
possible source of M. avium causing disease in AIDS
patients. Although DNA specific for M. avium, in-
cluding the sylvaticum subspecies, has been detected
in a few biopsies from patients with Crohn’s disease,
their role as primary pathogens remains, as in the case
of M. paratuberculosis, highly speculative.
Meat
0019 In most parts of the world meat is cooked before it is
eaten, but there is nevertheless the possibility that
there is insufficient heat penetration and that tubercle
bacilli may remain viable in parts of the meat. Cross-
contamination from infected meat to food ready for
consumption is also a possibility. As indicated above,
some infected cattle still reach slaughterhouses and
the meat of some may be tuberculous. Five cases of
tuberculosis due to M. bovis occurred over a 2-year
period in abattoir workers in South Australia: four of
the five cases involved the lung, suggesting that infec-
tion followed inhalation of aerosols. In addition, a
DNA fingerprinting study of strains of M. bovis
isolated from patients in Australia between 1970
and 1994 revealed that the majority of Australian-
born patients had worked in the meat and livestock
industries and had disease due to strains similar to
those found in Australian cattle.
0020 The incidence of tuberculosis among sheep is low,
as it is among pigs, although members of the MAC
have been responsible for outbreaks and single cases
of tuberculous disease in piggeries. Bovine tubercle
bacilli have been isolated from rabbits, wild and
farmed, and along with the avian bacillus, from a
variety of poultry. In the UK and New Zealand
farmed, but not wild, deer are now known to suffer
from tuberculosis. In the Middle East and India there
is evidence that some of the camels that are slaugh-
tered for their meat are tuberculous.
0021Fortunately, in developed countries, the systems of
meat inspection, required by law, prevent the sale for
human consumption of bovine, ovine, and porcine
carcasses, or parts of them, that may be infected.
There is still a trade, however, in carcasses, e.g., of
goats, pigs, rabbits, and poultry, that escapes an
official inspection and as yet in the UK carcasses of
farmed deer are exempt from official inspection. All
these animals offer a degree of hazard to the food
handler and the consumer.
0022Fish are not normally a source of mycobacterial
disease, although there have been a few cases of
disease due to M. marinum infection, manifesting as
warty skin lesions, in fishermen and fishmongers,
presumably as a result of infection of dermal abra-
sions. Multiple regression analysis has shown that
regular consumption of raw or partly cooked fish
and shellfish is associated with an increased risk of
disseminated disease due to the MAC in AIDS
patients.
Water
0023Several species of mycobacteria, some of which are
opportunist pathogens, have been isolated from piped,
bottled, and natural waters. Tubercle bacilli, however,
have never been recovered from water supplies.
0024There is adequate evidence that infections due to
some opportunist mycobacteria result from the inhal-
ation of aerosols containing the organisms. In the
great majority of cases ingestion of these species
does no harm (overt abdominal infection associated
with them is extremely rare), but they may temporar-
ily colonize the alimentary and upper respiratory
tracts. There is evidence that they may cross the epi-
thelial barrier (translocation) and persist for some
time in the draining lymph nodes: thus they have
been isolated from excised tonsils and abdominal
lymph nodes. Water has been postulated as a source
of infection in AIDS patients with disseminated
mycobacterial disease due to the M. avium, although
epidemiological evidence is conflicting, but M. gene-
vense was detected in tap water at a hospital with an
unusually high incidence of HIV-related disseminated
disease due to this species.
Isolation of Mycobacteria from Milk, Meat,
and Water
0025Tubercle bacilli and other pathogenic mycobacteria
are in Hazard Group 3 of the Advisory Committee on
4070
MYCOBACTERIA

Dangerous Pathogens and other similar authorities.
Such organisms may be dispersed as aerosols during
laboratory manipulations, and individuals who inhale
them may become infected. Any bacteriological pro-
cedure involving them or material that may contain
them must be carried out under Containment Level 3
conditions in microbiological safety cabinets.
0026 In some parts of the world the conventional tech-
niques for isolating mycobacteria on solid media are
being replaced by more rapid radiometric methods
and more recent nonradiometric automated systems
and also by the PCR and its derivatives. Originally
evaluated in the field of human disease, they are
increasingly being applied to veterinary microbiology,
food hygiene, and environmental work. There
have, for example, been reports of the use of PCR
to detect and identify waterborne mycobacteria,
M. paratuberculosis in pasteurized milk, and M. bovis
in tissues obtained postmortem from various animals.
Likewise, DNA fingerprinting techniques suitable for
epidemiological studies on M. bovis from animal and
human sources are now available.
0027 The traditional methods for the isolation of myco-
bacteria are described below, but the final identifica-
tion of tubercle bacilli and other mycobacteria is too
detailed to be included. Alternatively, cultures of
suspected mycobacteria may be sent to a reference
laboratory, a regional tuberculosis laboratory, or a
veterinary laboratory. For information on automated
systems and nucleic acid-based technology, the rap-
idly changing primary literature should be consulted.
Milk
0028 Direct microscopic examination of milk for acid-fast
bacilli is unrewarding as saprophytic mycobacteria
are often present, as are artifacts that retain the
fuchsin stain.
0029 A suitable method for recovering tubercle bacilli
(and other mycobacteria) from the milk of individual
cows or from small groups of cows is given below.
0030 Centrifuge at least 50 ml of the milk at 3000 rpm
for 20 min. Remove the cream to a small tube, add
4 ml of 4% sodium hydroxide and stand for 15 min;
add 6 ml of 14% monopotassium phosphate contain-
ing phenol red indicator to indicate neutralization;
centrifuge at 3000 rpm for 15 min; discard super-
natant fluid and culture the deposit.
0031 Discard the remaining supernatant fluid from the
original centrifugation and add 2 ml of 4% sodium
hydroxide to the deposit and stand for 15 min; add
3 ml of 14% monopotassium phosphate (3 ml) and
proceed as above.
0032 Divide the deposits between several tubes of
Lo
¨
wenstein–Jensen medium or Middlebrook medium
containing antibiotics. Incubate at 37
C for 6–8
weeks. Check the identity of any organisms that
grow by Gram and Ziehl–Neelsen stains.
0033Identify acid-fast bacilli by the methods described
in standard textbooks or send cultures to a specialist
laboratory.
Meat
0034For full details, the World Health Organization
guidelines for speciation within the Mycobacterium
tuberculosis complex should be consulted. The
recommended methods are briefly summarized here.
0035The material should be selected by a veterinarian or
meat inspector familiar with the macroscopic appear-
ance of tuberculous lesions. The usual specimens are
lymph nodes from the respiratory and gastrointest-
inal tracts, lung, and liver tissue and these should be
placed in wide-mouthed, hermetically sealed plastic
or glass pots.
0036Cut about 10 g of the sample into small pieces with
sterile instruments and further homogenize the pieces
in a Griffith tube or mechanical blender.
0037Remove about 3 ml of homogenized tissue with a
wide-bore pipette and add to an equal quantity of
autoclaved 4% sodium hydroxide in a screw-capped
tube, mix well and stand for 15–20 min, with occa-
sional shaking.
0038Neutralize with a 14% w/v solution of monopotas-
sium phosphate containing phenol red indicator
(about 40 mg l
1
) and allow to stand until the indica-
tor turns red, indicating that neutralization is com-
plete. Centrifuge fluid at 3000 rpm for 20 min, decant
the supernatant fluid, and inoculate the deposit on
two slopes of Lo
¨
wenstein–Jensen media and two
slopes of a related medium, such as Stonebrink’s
medium, that contains sodium pyruvate instead of
glycerol and which favors the growth of M. bovis.
0039Incubate slopes at 35–37
C for up to 8 weeks. Read
weekly, examine colonies for acid-fastness, and
arrange for identification by a specialist laboratory.
Water
0040The sample should consist of at least 1 l of water and
be delivered to the laboratory as soon as possible.
0041Pass separate 100-ml volumes of the whole sample
through membrane filters. Place the membrane filters
in 4% sodium hydroxide solution for 5 min (e.g., in a
Petri dish), then in 14% monopotassium phosphate
solution containing phenol red for several minutes.
0042Cut the membranes into strips 5 mm wide and
place each strip on the surface of Lo
¨
wenstein–Jensen
or Middlebrook antibiotic medium in screw-capped
tubes. Incubate at 35–37
C. Examine every few days
as some mycobacteria grow rapidly.
0043Examine colonies for acid-fastness and arrange for
identification, if necessary.
MYCOBACTERIA
4071

0044 In developed countries, foodborne mycobacteria
do not offer a serious health hazard and routine
laboratory examinations are not justified. In special
circumstances attempts may be made to culture
the organisms by the methods described above.
However, the essential and expensive equipment
requirements for Containment Level 3 preclude iden-
tification in most laboratories. The advice and ser-
vices of reference and other specialized laboratories
should be sought.
See also: Milk: Processing of Liquid Milk
Further Reading
Collins CH and Grange JM (1983) A review: the bovine
tubercle bacillus. Journal of Applied Bacteriology 55:
13–29.
Collins CH and Grange JM (1987) Zoonotic implications
of Mycobacterium bovis infections. Irish Veterinary
Journal 41: 363–366.
Collins CH, Grange JM and Yates MD (1984) A review:
mycobacteria in water. Journal of Applied Bacteriology
57: 193–211.
Collins CH, Lyne PM and Grange JM (eds) (1995) Collins
and Lyne’s Microbiological Methods, 7th edn. Oxford:
Butterworth Heinemann.
Collins CH, Grange JM and Yates MD (1997) Tuberculosis
Bacteriology Organization and Practice, 2nd edn.
Oxford: Butterworth Heinemann.
Cosivi O, Grange JM, Daborn CJ et al. (1998) Zoonotic
tuberculosis due to Mycobacterium bovis in developing
countries. Emerging Infectious Diseases 4: 59–70.
Cousins DV and Dawson DJ (1999) Tuberculosis due to
Mycobacterium bovis in the Australian population:
cases recorded during 1970–1994. International Journal
of Tuberculosis and Lung Disease 3: 715–721.
Cousins DV, Williams SN and Dawson DJ (1999) Tubercu-
losis due to Mycobacterium bovis in the Australian
population: DNA typing of isolates, 1970–1994. Inter-
national Journal of Tuberculosis and Lung Disease 3:
722–731.
Covert TC, Rodgers MR, Reyes AL and Stelma GN (1999)
Occurrence of nontuberculous mycobacteria in environ-
mental samples. Applied and Environmental Microbiol-
ogy 65: 2492–2496.
Fordham von Reyn C, Arbeit RD, Tosteson AN et al. (1996)
The international epidemiology of disseminated Myco-
bacterium avium complex infection in AIDS. Inter-
national MAC Study Group. AIDS 10: 1025–1032.
Grange JM and Collins CH (1987) Bovine tubercle bacilli
and disease in animals and man. Epidemiology and
Infection 92: 221–234.
Grange JM, Yates MD and de Kantor IN (1996) Guidelines
for Speciation within the Mycobacterium tuberculosis
complex, 2nd edn. WHO/EMC/ZOO/96.4. Geneva:
World Health Organization.
Hubbard J and Surawicz CM (1999) Etiological role of
Mycobacterium in Crohn’s disease: an assessment of
the literature. Digestive Diseases 17: 6–13.
Millar D, Ford J, Sanderson J et al. (1996) IS900 PCR to detect
Mycobacterium paratuberculosis in retail supplies of whole
pasteurized cows’ milk in England and Wales. Applied and
Environmental Microbiology 62: 3446–3452.
Moda G, Daborn CJ, Grange JM and Cosivi O (1996) The
zoonotic importance of Mycobacterium bovis. Tubercle
and Lung Disease 77: 103–108.
O’Reilly LM and Daborn CJ (1995) The epidemiology of
Mycobacterium bovis infections in animals and man: a
review. Tubercle and Lung Disease 76: 1–46.
Roberts TA (1986) A retrospective assessment of human
health protection benefits from removal of tuberculous
beef. Journal of Food Protection 49: 293–298.
Robinson P, Morris D and Antic R (1988) Mycobacterium
bovis as an ocupational hazard in abattoir workers. Aus-
tralia and New Zealand Journal of Medicine 18: 701–703.
Thoen CO and Steele JH (eds) (1995) Mycobacterium bovis
Infection in Humans and Animals. Ames, Iowa: Iowa
State University Press.
Wards BJ, Collins DM and de Lisle GW (1995) Detection of
Mycobacterium bovis in tissues by polymerase chain
reaction. Veterinary Microbiology 43: 227–240.
Van Kruiningen HJ (1999) Lack of support for a common
etiology in Johne’s disease of animals and Crohn’sdisease
in humans. Inflammatory Bowel Disease 5: 183–191.
MYCOPROTEIN
M J Sadler, MJSR Associates, Ashford, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Myco-protein is produced from microfungi, aerobic
organisms that live in the soil and convert
carbohydrate to protein. Research to produce food
from microfungi began in the early 1960s. This was at
a time when many projects were being set up to
develop single-cell protein sources suitable for animal
and human consumption in response to the predicted
protein gap in developing countries. There are reports
of three products produced form microfungi, but by
far the most successful myco-protein product is sold
under the trade name ‘Quorn
TM
.’
4072 MYCOPROTEIN

0002 Myco-protein is the generic name of the major
raw material used in the manufacture of Quorn
TM
products. It comprises the RNA-reduced biomass
composed of the hyphae (cells) of the organism
Fusarium venenatum A3/5 (deposited with the
ATCC as PTA-2684) grown under axenic conditions
in a continuous fermentation process. Quorn
TM
is
the brand name of a range of meat-alternative
products made from myco-protein. Quorn
TM
products
include pieces and mince for use in home cooking
in addition to a range of convenience products
such as burgers, fillets, goujons, nuggets, and ready
meals.
0003 Quorn
TM
myco-protein is the first truly ‘new’ food
product developed for humans. Introduced to the UK
food market in 1984, Quorn
TM
products are the result
of a development program, designed to produce
single-cell protein, that was started in the 1960s. In
addition to availability in all major UK and Irish food
retailers, Quorn
TM
products are now available in an
increasing number of retailers within Continental
Europe, including Belgium, Luxembourg, Holland,
Sweden, Switzerland and France, and in the USA.
0004 This article covers the cultivation and harvesting
of myco-protein, and its properties including accept-
ability, safety, and nutritional value. Known and
other potential functional health effects of consuming
myco-protein are also discussed.
0005 It was predicted in the late 1950s that within three
decades, there would be a shortage of protein across
the world – the so-called ‘protein gap.’ In response to
this, many projects were set up in the early 1960s to
develop feeds or foods from single-cell protein
sources that would be suitable for animal or human
consumption.
0006 Ranks Hovis McDougall (RHM) Research Centre
in the UK undertook one such project, which started
in 1964. The original aims were to convert starch
into a protein-rich food that was highly nutritious,
delicious, and safe to eat. The project focused on the
potential of microfungi to produce a high-protein
food (myco-protein). This resulted two decades later
in the successful development of the Quorn
TM
(a regis-
tered trade mark of Marlow Foods Ltd, UK) range of
products, which have been marketed as a human food
for the past two decades.
0007 It should be noted that the commercial deve-
lopment of myco-protein was a joint venture
between RHM and ICI (Marlow Foods). In 1990,
ICI assumed full control of the business. However,
in 1993, ICI de-merged into ICI and Zeneca, and
Marlow Foods became part of Zeneca. In 1998,
Zeneca merged with Astra (Sweden) to become
AstraZeneca.
Production
Cultivation
0008Cultivation of fungal mycelia has many factors in
common with the production of yeast and bacteria,
although because fungi are multicellular or coenzo-
cytic, the term ‘single-cell protein’ cannot strictly be
used to describe fungi produced by fermentation. The
advantages of microfungi are that they can utilize a
wide range of substrates, they have straightforward
nutritional requirements, and they are relatively easy
to harvest from culture broths on account of their
particle size.
0009In addition, the whole microfungi can be consumed
without any reported problems of toxicity and with
only a very low level of adverse reactions. Fungal
mycelia have a high nutritional value, desirable tex-
ture, and mild smell and flavour. Acceptability is less
of a problem than for food from other lower organ-
isms, since fungi already play a role in the human diet.
0010The microfungi Fusarium venenatum A3/5 (de-
posited with the ATCC as PTA-2684) was chosen by
RHM for the production of the new myco-protein
product, on account of its suitable organoleptic and
nutritional properties.
0011Growth requirements and conditions Myco-protein
is grown in an airlift or pressure-cycle fermenter in a
liquid medium providing all the nutrients required for
growth. In preference to a batch culture, a continu-
ous-flow aerobic culture system is used in which the
culture medium is continuously fed to the fermenter
and the broth continuously drawn off for filtering
such that the volume of the fermenter is displaced
approximately every 5–6 h. This ensures that steady-
state conditions are maintained and that optimal
productivity is achieved. Optimizing the process con-
ditions improves the yield, quality, and uniformity of
the end product, and the efficiency of the process. The
cell mass doubles every 4–5h.
0012The carbon substrate used is glucose syrup, which
is obtained from hydrolyzed corn. Ammonium is the
source of nitrogen, and is also used to regulate the
pH, which is maintained between 4.5 and 7.0. Salts of
potassium, manganese, cobalt, calcium, magnesium,
iron, copper, and biotin are supplied as a liquid feed-
stock from tanks adjacent to the fermenter. Com-
pressed air is the source of oxygen, and its injection
into the fermenter keeps the broth in continuous
motion. All the ingredients of the culture medium
are sterile and of food- or reagent quality. The tem-
perature of the broth is maintained at 30
C, and it is
therefore necessary to cool the fermenter.
MYCOPROTEIN 4073
