Crank J. Free and Moving Boundary Problems
Подождите немного. Документ загружается.

102
Analytical solutions
The
problem is formulated by
the
system
o<x<s(t),
x>s(t),
aUt
aU2
ds
-Kt-+K
-=Lp-
ax
2
ax
dt'
x =
set),
Ut=Ut>
x=o,
t:;;:;,:o,
U2=-U
2
, x
~
00,
t:;;:;,:o,
x =
set),
t:;;:;,:o,
(3.1)
(3.2)
(3.3)
(3.4)
(3.5)
(3.6)
where
kt
=
KJ
PG,
i = 1, 2. No initial conditions are specified
at
this stage
of
Neumann's solution. They emerge later. Equations (3.1)
and
(3.4)
are
satisfied by
(3.7)
and (3.2)
and
(3.5) by
(3.8)
where A
and
B
are
constants
to
be determined. Condition (3.6) requires
(3.9)
The
two relationships (3.9) can only
be
satisfied for all t
if
s
=at~,
(3.10)
where a is a constant. By differentiating (3.7)
and
(3.8)
and
using (3.3)
and
(3.9)
the
constant a is seen
to
be
given by
the
root
of
UtKte-ot2/4k,
U
2
K
2e
--ot
2
/4/s
Lpa
(
'7Tkt)~
erf(
a/2kt)
(
'7T~)!
erfc(
a/2Iq)
=
2:
'
(3.11)
where p
is
the
density
of
both
ice
and
water
implying
that
no
volume
change occurs
on
melting.
Table 3.7 gives values of A =
a/(2ki)
computed by Churchill
and
Evans
(1971) for a wide selection of parameters. Some roots for water
and
ice
and different initial
and
surface temperatures
are
given by Carslaw
and
Jaeger (1959,
p.
286). Once a has
been
found from (3.11),
Ut
and
U2 can
Neumann's solution; generalizations; volume changes
103
be
written down from (3.7), (3,8), and (3.9). They are
U
l
X
Ul
= U
1
-
erf(a/2kt) erf 2(k
1
t)!'
(3.12)
U
2
x
~
= - U
2
+ erfc(a/2k!) erfc
2(k2t)~
.
(3.13)
It
is
now possible to see what initial conditions
are
satisfied by (3.10) and
(3.13).
At
t = 0 they give s = 0 and
~
= - U
2
,
i.e. the whole region
x>
0
is
solid
at
uniform
temperatur~
- U
2
•
The
special case in which the solid
is
initially at its melting temperature, so that U
2
= 0, is
the
single-phase
problem introduced in §1.2.1 and (3.11) reduces to
Ae
A2
erf A = U1cl/(L-rr!), (3.14)
where A =
a/(2kl).
Carslaw and Jaeger (1959, p. 287) show a graph of the
left-hand side of (3.14)
as
a function of A from which values of A can be
read for any given value
of
UlCl/(L~).
They also point
out
that for
small values of
A,
and hence of the right-hand side of (3.14), use of the
first
term
in the series expansion for erf A gives approximately
(3.15)
The
solutions (3.12), (3.13) and
the
equation (3.11) are quoted by
Carslaw and Jaeger (1959, p. 288). Their solution of
the
corresponding
solidification of a liquid in
x > 0 initially
at
a uniform temperature above
freezing
is
deducible by suitable changes of nomenclature.
Carslaw
and
Jaeger (1959, Chapter XI) also present Neumann-type
solutions for
other
physically important problems including the region
x>
0 initially liquid and x < 0 solid, the case in which melting occurs over
a temperature range, and three-phase problems. They solve the commonly
neglected problem in which motion of the liquid results from a change of
volume
on
solidification, due
to
a difference between the densities of solid
and liquid. As an example they consider the freezing of a semi-infinite
liquid in the region
x>
0 when the density of the solid,
PI'
exceeds that of
the liquid,
P2,
and both phases are incompressible. Thus
the
problem
is
defined by equation (3.1) in
the
solid phase but in the liquid phase (3.2)
is
replaced by
x>s(t),
(see equations (1.32) and (1.33».
On
the solidification boundary, x = s(t),
they take
U1
=
~
=
UM,
together with (3.3).
For
their solidification prob-
lem, instead
of
(3.4), (3.5), they have
U1
= 0, X = 0,
U2
~
U
as
x
~
00.
The
104
Analytical solutions
similarity solution based
on
s =
2A(klt)~
leads
to
the
equation for A
e-
x2
(U
-
U~Kzkte-x2""k,l""k2
AL7r~
erf'\' -
UMK
l
k!
erfc(APl
kif
1>214
Cl
U
M
'
which reduces
to
(3.11)
if
Pl =
Pz,
remembering
that
a = 2.\.kt.
Rubenstein (1971) in considering
the
crystallization of a supercooled
melt in contact with its solid phase (see Chambre 1956) also
took
density
changes into account
but
did
not
impose
the
condition of incompressibil-
ity in
the
liquid phase.
Thus
the
condition (1.32) still holds
on
the
phase-change boundary with v = v(s(t), t),
but
an equation
of
motion
av
av
aZv
-+v-=v-
s(t)<x<oo,
t>O
at ax ax
z
'
is
to
be
satisfied by v(x, t). Rubinstein takes
the
solid phase
to
occupy
the
space
-oo<x<s(t)
for
t>O
and
the
liquid phase
s(t)<x<oo,
coupled
with initial and boundary conditions
v=o,
v~o,
The
heat
flow equations are
auz=
kz
a
Z
U2
at ax
z
'
t=o,
x
~
00,
x
~
-00.
-oo<x<s(t),
t>o,
s(t)<x<oo,
t>o,
and conditions (3.3)
and
Ul
= U
z
= U
M
still hold
on
the
phase-change
boundary. Rubinstein derives a similarity solution based
on
s(t)=2~(vt)!,
v =
2(v/mN(1)),
Uj =
Uj(1)),
i = 1; 2
1)
=
x/{2(vt)~},
where
~,
f(1)), and
U;(1))
are
to
be
determined from
/(1)) - (4/7r!)/(1))f(1)) + 2{f(1)) +
TJ!(1))}
=
0,
ul(1))+{2ul1)
- (4/7r!)Ut!(1))}Ul(1)) = 0,
uz(1))
+
2uz1)u
z
(1))
= 0,
Uj(m =
UM,
Uj(1))
~
0;,
11)1
~
00,
i=1,2,
f(m
=
-17r~e~,
where e =
(Pz
- Pl)/Pt.
/(1))
~
f(1))
~
0,
1)
~
00;
-IL~
={Ul(1))-'Y
U
z{1))},
1)
=~,
with
Neumann's ;;olution; generalizations; volume changes
105
It
follows easily
that
where
with
and
finally
e~
exp{-(T/2-~2)}
!(T/) = -
2/7T~+
e~(erf
T/
-erf
~)exp(~2)
,
Ul(T/) = U
1
+
(UM-
U
1
)R(T/,
m,
1
+erf{T/.JO":J
U2(T/)
= U
2
+(UM-
U~
l+erf{~.JO":J·
These relationships yield
an
equation for
~.
Rubinstein pursues the
simplest case
of
0"1 = 1, which
he
considers relevant for a gas, and obtains
(2/.J7T)erfc
T/
Ul
= U
1
+
(UM-
U
1
)
{2/.J7T+
e~(erf
T/
-erf
mexp(~2)}erfc
~
and
{
_ ( _ )}
_~
{(
_ U )
exp(-~2)
'}'O"!(UM-
u~exp(-0"2~2)}
IJ.
eUM
Ul~-.J7T
UM
1
erfc~
+
l+erf(~.JO"~
.
Considerable simplification occurs
if
U
2
=
UM'
i.e.
the
solid phase
is
initially
at
the
crystallization temperature throughout.
In
a problem with several phases of different densities, Wilson (1982)
denoted
by
Sb
i = 1, 2,
...
, n
-1,
the
free boundaries separating phases of
densities Pi, i = 1, 2,
...
,
n,
together with
So
= 0 and
s"
is
the
right-hand
boundary,
not
necessarily
at
infinity.
If
the
whole slab is
of
mass M,
conservation
of
mass
is
expressed by
n-l
s,,(t) = M/p.. + L Sj(t)<Pi+l-fJj)/p..
j=1
as long as
Sn-l(t)
does
not
exceed Sn(t). When
Sn-l(t)
determined by
the
free-boundary problem becomes equal
to
s,,(t),
the
n·th phase has disap-
peared
and
a new free-boundary problem with (n
-1)
phases
is
to
be
considered. Similarly, a growing number of phases could
be
considered.
The
first local coordinate gl(X, t) is simply gl(X, t) = x,
O~x
~SI(t),
and
the
position
of
the
first moving boundary
is
denoted by f
1
(t) in
the
local
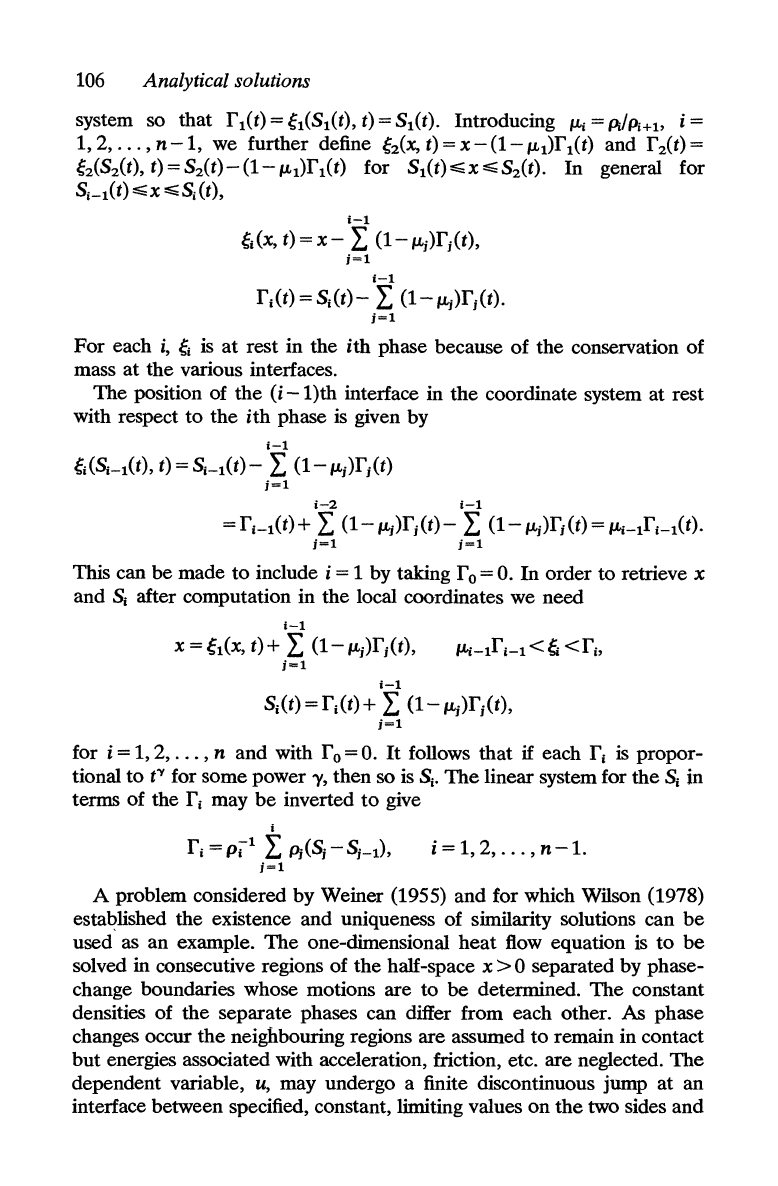
106
Analytical solutions
system so that r
1
(t) = gl(SI(t), t) = SI(t). Introducing
fLi
= pdPi+l> i =
1,2,
...
,n-1,
we further define
gz{x,t)=X-(l-ILI)rI(t)
and
rz<t)=
g2(S2(t),
t)=S2(t)-(1-ILI)rI(t)
for
SI(t)~X~S2(t).
In
general for
SI_l(t)~X~SI(t),
1-1
gl
(x, t) = x - L
(1-
ILj)rj(t),
j=1
1-1
rl(t)
=
SI(t)-
L
(1-
ILj)rj(t).
j=1
For
each
i,
gi
is
at
rest in the
ith
phase because
of
the
conservation of
mass
at
the
various interfaces.
The
position of
the
(i
-l)th
interface in
the
coordinate system
at
rest
with respect
to
the
ith
phase is given by
1-1
gi(SI-l(t), t) =
S;-I(t)-
L
(1-
ILj)rj(t)
j=1
i-2
1-1
= r
i
-
1
(t) + L
(1-
ILj)rj(t)- L
(1-
ILj)rj(t) =
fLi-l
r
i-l(t).
j=1
j=1
This can
be
made
to
include i = 1 by taking r 0 =
O.
In
order
to
retrieve x
and
SI
after computation in
the
local coordinates
we
need
1-1
X=gl(X,t)+
L
(l-ILj)r
j
(t),
j=1
i-I
Si(t) =
rj(t)
+ L
(1-
ILj)rj(t),
j=1
for i =
1,2,
...
,n
and
with r 0 =
O.
It
follows that
if
each r
j
is
propor-
tional
to
f( for some power
'Y,
then so is
Sj.
The
linear system for
the
Sj
in
terms of the r
i
may
be
inverted
to
give
j
rj =
pi
1
L
Pi(Sj
- Sj-l),
j=1
i = 1,
2,
...
, n
-1.
A problem considered by Weiner (1955)
and
for which Wilson (1978)
established
the
existence and uniqueness of similarity solutions can
be
used as an example.
The
one-dimensional
heat
flow equation
is
to
be
solved in consecutive regions of
the
half-space
x>
0 separated by phase-
change boundaries whose motions are
to
be determined.
The
constant
densities of
the
separate phases can differ from each other. As phase
changes occur
the
neighbouring regions are assumed
to
remain in contact
but
energies associated with acceleration, friction, etc. are neglected.
The
dependent variable,
u,
may undergo a finite discontinuous
jump
at
an
interface between specified, constant, limiting values
on
the
two sides
and

Neumann's solution; generalizations; volume changes
107
is also constant
at
the
fixed boundary, x =
O.
Initially all free boundaries
coincide
at
x = 0, and u =
Un
= constant for
x>
0, for t = 0 and u
~
Un
as
x
~
00,
t~O.
Here
So
= 0 and
Sn
=
00.
By using
the
nomenclature defined above
and
assuming
the
usual
Stefan condition
to
hold
on
each interface, with
~
denoting latent heat
per
unit mass of
the
ith
phase for the change from
the
ith
to
the (i +
l)th
phase,
the
problem can
be
stated as follows:
U;t
=
k;Uq;g,
U;-lfi-l(t)
<
~
<fi(t),
t > 0, i =
1,
...
,
n,
u;(t, lJ-i-lI7-l(t)) = V';-h t
>0,
i =
1,
...
,
n,
u;(t,ri(t))=U
i
+.:1j,
t>O,
i=
1,
...
,
n-1,
t>O,
i = 1,
...
,
n-1,
t~O,
fi(O)
=0,
i = 1,
...
,
n-1,
~>O,
t=O.
Here
K; denotes
heat
conductivity and
k;
heat diffusivity. Weiner's (1955)
similarity solution is expressed
by
Wilson (1982) as
U;(t,
~)
=
(Vi-l
+ a;)[erfcWv'(4k;t)}/erfc{a;-llJ-i-
l
v'(k;-l/k;)}]-a;,
t>O,
IJ-i-lfi-l(t)<~<fi(t),
i = 1,
...
,
n-1,
fi
(t) = arv'( 4k;t), i = 1,
...
, n
-1,
and for
~>rn-l(t),
t>O
In
these equations, for i = 1,
...
, n
-1,
if
if
k,.
=0,
k,.>0.
al
=
-Vi-l
+
(Vi-l
-
V';
-
.:1
i
)erfc{a;-llLi-lv'(k;-l/k;)}/<l>;,
<l>i
= erf(a;) -erf{a;-llJ-i-lv'(k;-l/k;)},
and
the
a;
(i
= 1,
...
, n
-1)
are
the
unique solution of
the
non-linear
equations
-(J;~k;a;v'
7T
= - K; (V';-l -
Vi
-
.:1;)exp(
-ai)/<I>lv'k;)
+ K
i
+1(Vi
-
Vi
+1
-.:1
i
+
l
)exp(-arlLrk;/k;+l)/(<I>i+lv'k;+l),
i
=1,
...
,
n-2,

108
Analytical
solutions
and
Pn-lL..-lk,,-Ia..-I-17T + K;..-I(U
n
-
2
-
U
n
-
1
-
A.
n
-
1
)exp(
-a~-I)/(<Pn-l-1k,,-l)
{
o
if
k,,=0
=
Kn
(U
n
-
1
-
Un)exp(-a~-I#L~-Ik,,-I/k,,)/(<Pn
-Ik,,)
'if
k"
1
O.
In
this last expression consistency with the previous definition
is
achieved
by taking erf(a..)
==
1 and
<Pn
= 1-erf{a..-l#Ln-l-1(k,,-I/k,,)}.
In
the defini-
tion of
<PI
the dummy parameters ao,
ko
are taken to
be
zero.
Wilson
(1978) established
the
existence and uniqueness of
the
similar-
ity solution subject
to
the following conditions:
Ie;
>0,
K;
>0,
i =
1,
...
,
n-l,
and either
k"
>0,
Kn
>0,
or
k"
=
Kn
=0;
and either
Vi-I>
Vi
+.1;,
1..;
~O,
i = 1,
...
,
n-l
and U
n
-
1
~
Un;
or
Vi-I <
Vi
+.1;,
1..;
~O,
i = 1,
...
,
n-l
and U
n
-
1
~
Un;
provided
that
if
U
n
-
1
=
Un
then L..-l
10;
and
if
k"
=Kn
=0
then U
n
-
1
=
Un
Reverting to the original frame of reference we see that
i=2,
...
,n,
and SI(t)
=fl(t)
=
2a
l-1(k
1
t).
Although a similarity solution has
been
obtained for this example,
Wilson
(1982) suggests
the
use of
the
same local coordinates in combina-
tion with finite elements
or
finite differences in
more
complicated prob-
lems.
A problem relating to
the
manufacture of glass in which volume
changes are substantial enough to
be
of
practical importance
is
reported
by Gelder and
Guy
(1975).
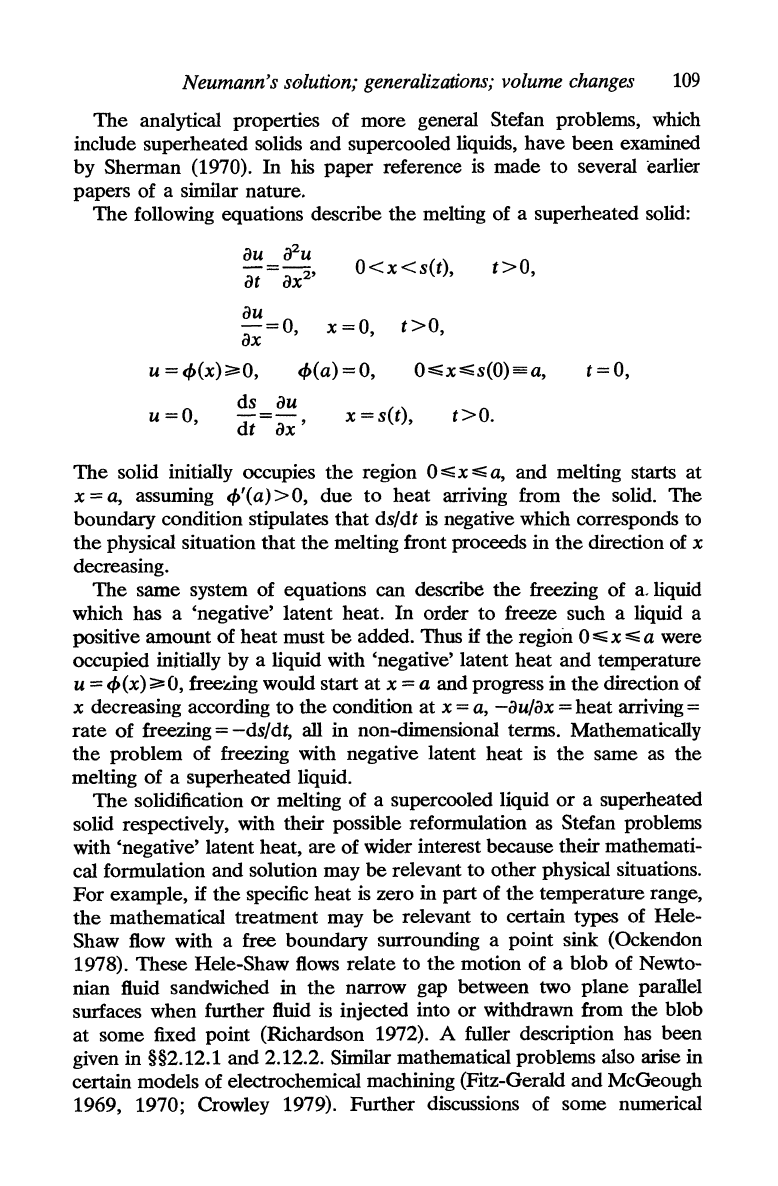
Carslaw and Jaeger (1959, p. 287) derive a similarity solution for
the
solidification of a supercooled liquid initially in x > 0, i.e.
the
region x > 0
contains material
at
temperature V <
Tl
which
is
in
the liquid state even
though its temperature
V
is
below
the
solidification temperature T
1
•
Solidification starts at
the
plane x = 0 and moves
to
the
right; no
heat
is
removed from
the
solidified material
and
so
it
will have the constant
temperature
Tl
throughout. In
the
supercooled liquid there will
be
a
temperature profile
of
the form
x
v = V + A erfc 2(k2t)!
and
the
usual Stefan conditions expressing
heat
conservation
on
the
boundary,
s(1)
= 2A(k
2
t)!, between
the
solid and liquid phases, leads to
the
relations V + A erfc A =
Tl
and
Ae-
A2
=
Alm!/c2'
It
follows
that
A
is
the
root of
Ae
A2
erfc A =
(T
1
-
V)c
2
/L7T!
and
Carslaw and Jaeger give a
graph of
Ae
A2
erfc
A.
Neumann's solution; generalizations; volume changes
109
The
analytical properties of more general Stefan problems, which
include superheated solids and supercooled liquids, have
been
examined
by Sherman (1970).
In
his
paper
reference is made
to
several earlier
papers of a similar nature.
The
following equations describe
the
melting of a superheated solid:
u=O,
au
a
2
u
at=
ax
2
'
O<x<s(t),
t>O,
au
=0
0 0
ax
' x
=,
t>,
<f>(a)
=0,
O:s;;x:S;;s(O)==a,
x = s(t),
t>O.
t=O,
The
solid initially occupies
the
region
0,;;;
x
:s;;
a,
and melting starts
at
x
==
a,
assuming
<f>'(a)
>0,
due
to
heat arriving from
the
solid.
The
boundary condition stipulates
that
ds/dt
is
negative which corresponds to
the
physical situation
that
the
melting front proceeds in
the
direction of x
decreasing.
The
same system of equations can describe
the
freezing of
a.
liquid
which has a 'negative' latent heat.
In
order
to
freeze such a liquid a
positive amount
of
heat must
be
added. Thus
if
the
region
O:S;;
x
:s;;
a were
occupied initially by a liquid with 'negative' latent heat
and
temperature
u
==
<f>(x)~O,
freeL.ing
would start
at
x
==
a and progress in
the
direction of
x decreasing according
to
the
condition
at
x =
a,
-au/ax
= heat arriving
==
rate
of
freezing
==
-ds/dt,
all in non-dimensional terms. Mathematically
the
problem of freezing with negative latent heat is
the
same as the
melting
of
a superheated liquid.
The
solidification
Or
melting of a supercooled liquid
or
a superheated
solid respectively, with their possible reformulation as Stefan problems
with 'negative' latent heat, are of wider interest because their mathemati-
cal formulation and solution may
be
relevant
to
other
physical situations.
For
example,
if
the
specific heat
is
zero in part of
the
temperature range,
the
mathematical treatment may
be
relevant
to
certain types of Hele-
Shaw flow with a free boundary surrounding a point sink (Ockendon
1978). These Hele-Shaw flows relate
to
the
motion of a blob of Newto-
nian fluid sandwiched in
the
narrow gap between two plane parallel
surfaces when further fluid is injected into
or
withdrawn from the blob
at
some fixed point (Richardson 1972). A fuller description has been
given in §§2.12.1 and 2.12.2. Similar mathematical problems also arise in
certain models of electrochemical machining (Fitz-Gerald and McGeough
1969, 1970; Crowley 1979). Further discussions of some numerical
110
Analytical solutions
treatments of problems of this kind are
to
be found in §§2.12.2, 2.12.3,
and 6.2.7. Crank (1975a, Chapter 13) gives Neumann-type solutions for
several diffusion problems including
the
general approach
by
Danckwerts
(1950) which also allows for movement of
the
medium on
one
side of
the
boundary relative
to
the
other. Mikhailov (1975) obtains similarity solu-
tions of coupled equations for temperature and moisture distributions
together with
the
position of a moving evaporation front during
the
drying of a porous region x
>0,
and
he
also studied (1976)
the
corres-
ponding freezing problem. Tayler (1975) refers
to
similarity solutions
describing
the
solidification of
an
alloy as formulated in §1.3.7 for some
particular initial
and
boundary conditions.
He
also remarks
on
the
limit-
ing cases of instantaneous diffusion of
heat
or
material
and
of
no diffusion
of material. Kamin (1976) and Peletier and Gilding (1976) obtain similar-
ity solutions for a non-linear filtration problem obeying
au/at
=
if'(u
m
)/ax
2
,
m > 1.
3.3.
SimDarity solutions
in
cylindrical
and
spherical
coordinates
Similarity solutions of problems with radial symmetry,
if
they exist, can
be
expected
to
be
functions of
r/t!
only by analogy with
the
linear cases
discussed in §3.2.
Frank
(1950) presents solutions contained in a disserta-
tion by Rieck in 1924
and
quoted by
Huber
(1939),
and
applies them
to
spherical and cylindrical phase growth. Retaining Frank's nomenclature
for ease of reference, concentration (or temperature) is denoted
by
cf>
=
cf>(r,
t) =
cf>(s)
where s = r/(Dt)!, D is
the
diffusivity,
and
r
the
radial
coordinate.
Then
the
usual diffusion equation for spherical symmetry
becomes
and successive integration yields
cf>
-
cf>co
= A {s
-1
exp( -
~S2)
-
!'1T!
erfc@s)}
=
AFis)
,
(3.16)
(3.17)
where
cf>co
is
the
value
of
cf>
at
infinity.
The
constant A is related
by
Frank
(1950)
to
q,
the
amount of diflusant expelled
per
unit volume of new
phase (analagous
to
latent heat) formed as growing proceeds, and
he
deduces from
the
conservation condition on r = R
(3.18)
where S
=
R/(Dt)!
and
R is
the
outer
radius of
the
sphere.
On
the
surface
Similarity solutions and moving heat sources
111
r = R,
cf>
has
the
constant value
cf>s,
where
cf>s
= !qS3 exp(iS
2
)FiS)
+
cf>co
E qf3(S) +
cf>co.
(3.19)
For
the
corresponding radial cylindrical problem Frank (1950) obtains
the
solutions
(3.20)
where
Ei
is
the
exponential integral
and
on
the
surface of
the
growing
cylinder
the
constant
cf>s
is
cf>s
= - iqS2 exp(iS2)Ei( -
iS~
+
cf>co
==
qfZ{S) +
cf>coo
(3.21)
In
the
linear case for comparison
t1(S)
=!S
exp(iS2)F
1
(S) (3.22)
and
F
1
(s) =
7T~
erfc@s). (3.23)
Frank
(1950) tabulates
the
functions
F3(S),
tiS),
F
2
(s),
and
tz{S) and
quotes series
and
asymptotic expansions for F(s)
and
f(S)
for
both
the
spherical
and
cylindrical cases.
Carslaw
and
Jaeger (1959, p. 295) give similarity solutions in cylindri-
cal
and
spherical coordinates where
the
region r < R consists of solid
at
its melting point and r > R of supercooled liquid whose temperature
tends
to
V <
the
melting temperature
as
r
-+
00.
They also solve a cylindri-
cal problem of freezing
by
an
axial line source.
3.4.
SimDarity solutions and moving heat sources
Lightfoot (1929) developed a method suitable
if
the
thermal properties
of
the
solid
and
liquid
are
assumed
to
be
the same.
If
the
solidification
front in a one-dimensional problem
is
at
x =
s(t),
and
moves with a
velocity
s(t),
the
latent
heat
of solidification
is
liberated
on
the
front at a
rate
Lps(t). (3.24)
This liberation of heat may
be
represented by a moving source of heat at
s(t)
of
strength (3.24).
The
temperature at any point will
be
due
to
the
superposed contributions from this moving source, having
the
initial and
boundary conditions in mind.
An
integral equation for
s(t)
can be
developed which expresses
the
fact
that
the
temperature
on
the
boundary
at
x =
s(t)
is
always
the
melting temperature. In this way, Lightfoot
