Crank J. Free and Moving Boundary Problems
Подождите немного. Документ загружается.


242
Fixed-domain methods
Crowley and Ockendon (1977) solved a similar problem with body
heating in modelling a thermal switch. They obtained an asymptotic
solution of an integral equation for large times and enthalpy-based
numerical results.
6.2.7. Other conseroation forms and weak solutions
The
essential features of
the
enthalpy method, introduced in §6.2, are
that the problem
is
reformulated in terms of a single differential equation
which
is
solved over a fixed domain
to
obtain values of temperature and
the enthalpy function.
The
position of
the
moving boundary emerges as a
by-product of
the
computation.
In
heat-How problems, enthalpy has a
real physical significance and the reformulated problem
is
in a heat
conservation form. This means
that
a boundary jump condition such as
(6.4)
is
automatically satisfied. Even in problems where the concept of
enthalpy has no direct relevance it
is
sometimes possible
to
secure
the
advantages of computing in a fixed domain by rewriting
the
equations in a
form in which some essential physical quantity is conserved and which
permits a weak solution.
The
following are some examples.
(i)
Cases
of
zero 'specific heat'.
In
the
classical Stefan problem, specific
heat
c and conductivity K are both positive and
the
enthalpy H
is
a
strictly monotone function of temperature, while temperature is a single-
valued function of
H. Crowley (1979) formulated certain problems by
introducing a 'generalized enthalpy' function incorporating a zero 'specific
heat' in one
or
both phases.
An
example of the latter case
is
the
electro-chemical machining problem described by McGeough and Ras-
mussen (1974) (see §2.12.3). Their quasi-steady model
is defined by the
equation
~<f>=o,
(6.66)
where
<f>
is
the electric potential, with the boundary conditions at the
anode
<f>
= 0,
ds
M-=-V<f>·
Vs
dt
and
on
the cathode, r =
a,
on
<f>=-V<o.
s(r, t) = 0,
M>O,
(6.67)
(6.68)
This
is
a one-phase problem governed by an elliptic equation and a Stefan
boundary condition with non-zero latent heat, M.
In
order to formulate
the equations
on
a fixed domain, the anode can
be
regarded as a region
where
<f>
=0,
H=M,
and solutions are required of
(6.69)

Enthalpy method
243
with boundary conditions
c(>
=
-v,
H = 0
on
r =
a,
and initially
cf>=
-v,
H=O,
t=O,
outside s(r, t) = 0,
and
cf>
= 0,
H=M,
t=O,
within s(r, t) =
O.
The
conservative form of (6.69) across a surface of discontinuity s(r, t) =
0, obtained by integrating over a small volume,
is
[H]ds/dt=M
ds/dt =
[Vcf>
• Vs],
(6.70)
where [ ] denotes the jump between the sides where
H~O
and
H;;:.:M
respectively. Since
V2c(>
= 0 inside
the
anode
it
follows
that
(6.67)
is
satisfied by a solution of (6.69). Crowley (1979) obtained an implicit,
finite-difference solution for this problem by expressing (6.69) in cylindri-
cal polar coordinates.
Her
results are compared with those of Christian-
sen and Rasmussen (1976) and Elliott (1980) in Table 8.25.
The
flow of an incompressible fluid in a Hele-Shaw cell (Richardson
1972) (see §2.12.2) can be formulated in the same way (see Figs. 2.19,
20).
In
Crowley'S second problem the 'specific heat'
is
zero only in one
phase in which, therefore, an elliptic differential eqution holds, but we
have a parabolic equation in
the
other
phase. This is the situation for
saturated/unsaturated
flow
in a porous medium (Hornung 1978).
In
one
region the flow
is
unsaturated,
that
is
the medium is only partially filled
with fluid and its fluid content may change.
In
a second region the
medium is saturated and no more fluid can
be
added.
In
terms of a
velocity potential,
C(>,
the
relevant equation
is
(6.71)
where H measures
the
air content of
the
medium. Hornung (1978) adopts
the
'generalized-enthalpy relationships',
c(>
>0,
c(><0,
(6.72)
and
cf>
can
be
regarded as a generalized temperature. A sample problem
is
solved by Crowley (1979).
In
the
same paper, the uniqueness
of
the
weak solutions for these cases
in which
the
'specific heat' may vanish was proved by necessary exten-
sions
of
Oleinik's (1960) methods for the classical Stefan problem.
It
is
because zero specific heat means that temperature
is
no
longer a singie-
valued function of enthalpy
that
Oleinik's proof needs modification.
Crowley (1979) introduced a function strictly monotone in both
H and
C(>,
defined by
F(
cf>,
H)
= H +
'Ycf>
where
'Y
is
any positive constant, into the
244
Fixed-domain methods
uniqueness proof. This has the additional consequence that the finite-
difference scheme used to solve (6.69), for example, cannot be explicit
but
must be implicit. Thus, an implicit scheme of the form
H(x,
t + 8t) -
H(x,
t) =
8t{1-'-
V
2
cf>(x,
t + 8t) +
(I-I-'-)
V
2
cf>(x,
t)}
for ° <
I-'-
.:;;;
1 can evaluate a linear combination of
Hand
cf>
of the form
H
+
"Icf>
used in the uniqueness proof. This follows immediately by writing
the implicit scheme for a one-dimensional problem, for example, as
(H::.+
1
+
21-'-
8tcf>::'+1)
= H::' +
I-'-
8t(cf>::.-t:!-1
+
cf>::'~\)
+
(I-I-'-)
8t(cf>::'-1
+
cf>::'+1
-
2cf>::'),
and solving by successive over-relaxation with
the
right-hand side treated
as known from
the
previous iteration (see solution of (6.51)). From
numerical experiments Crowley (1979) showed
that
the finite-difference
scheme converges but no analytical proof was available because of the
vanishing specific heat. NiezgOdka (1980) and Visintin (1983) estab-
lished the existence of weak solutions for problems with zero specific heat
and examined their stability.
We
note in passing
that
Fasano and
Primicerio
(1979d) proved existence, uniqueness, and continuous depen-
dence theorems for classical solutions for saturated/unsaturated flow in
one space dimension.
Crowley's (1979) approach can
be
adopted in solving the oxygen
diffusion with absorption problem which
is
formulated classically in
equations (1.57-60). By introducing a generalized enthalpy function H
related to oxygen concentration
u by
H={U,
0,
and an absorption term
Q defined by
Q={-I,
0,
the problem can be reformulated as
u>o,
u.:;;;O,
u>o,
u.:;;;O,
O<x<l,
au/ax
=0,
x=O;
u=o,
x=l,
H=u=!(1-x?,
t>o,
t=O.
(6.73)
(6.74)
(6.75)
(6.76)
(6.77)
Integration of (6.75) over a small volume element around the moving
front,
x = s(t), for a time interval 8t
~
0 shows
that
condition (1.59) is
satisfied. Furzeland's (1980) results obtained in this way are included in
Enthalpy method
245
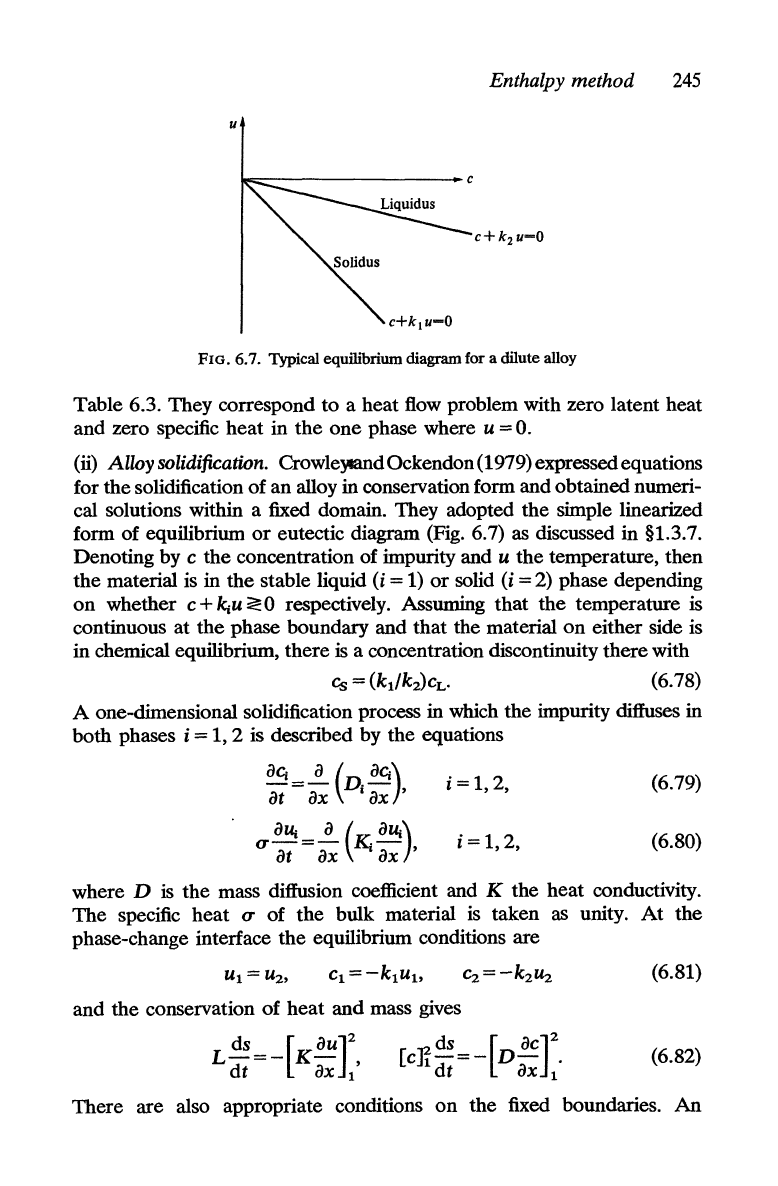
u
FIG. 6.7. Typical equilibrium diagram for a dilute alloy
Table 6.3. They correspond
to
a heat flow problem with zero latent heat
and zero specific
heat
in
the
one
phase where u =
O.
(ii)
Alloy
solidification. Crowleymnd Ockendon (1979) expressed equations
for
the
solidification
of
an alloy in conservation form and obtained numeri-
cal solutions within a fixed domain. They adopted
the
simple linearized
form of equilibrium
or
eutectic diagram (Fig. 6.7) as discussed in §1.3.7.
Denoting by c
the
concentration of impurity and u
the
temperature, then
the
material is in
the
stable liquid
(i
= 1)
or
solid
(i
= 2) phase depending
on
whether c +
k;u
~O
respectively. Assuming
that
the
temperature is
continuous
at
the
phase boundary and that the material
on
either side is
in chemical equilibrium,
there
is
a concentration discontinuity
there
with
Cs
= (kl/k2)CV (6.78)
A one-dimensional solidification process in which the impurity diffuses in
both
phases i = 1, 2 is described by the equations
aG=~(n.
a
G
)
at
ax
'ax'
i = 1, 2,
(6.79)
u
aUt
=
~
(Kt
aUt),
at
ax ax
i =
1,2,
(6.80)
where
D
is
the
mass diffusion coefficient and K the heat conductivity.
The
specific
heat
u of
the
bulk material is taken as unity.
At
the
phase-change interface
the
equilibrium conditions are
Ul
=
U2,
Cl
=
-k1Ul>
C2 =
-k
2
Uz
(6.81)
and
the
conservation
of
heat
and
mass gives
L
dS=_[Kau]2,
[c]ids=_[Dac]2.
dt
ax
1
dt
ax
1
(6.82)
There
are also appropriate conditions
on
the fixed boundaries.
An
246
Fixed-domain methods
enthalpy form which matches the first of (6.82) is
H-{U,
u+L,
u+c/k
1
<0,
u+c/k
2
>0.
(6.83)
Making the usual assumption for dilute solutions,
that
the concentration
of impurity is proportional
to
its chemical potential
or
activity
v,
we have
i=1,2,
(6.84)
where
b is some constant.
The
interface condition (6.78) becomes
Vi
=
V2
and so V, as well as u,
is
continuous across
the
phase boundary u + bv =
O.
Since
~
= constant in this formulation, V can
be
defined everywhere by
(6.84), though in a more general case this relation only holds
on
the phase
boundary.
Thus, the chemical activity and concentration in
the
mass transfer
problem are
the
direct analogues
of
temperature and enthalpy in the
heat
transfer and (6.79) may
be
written in aliform analogous
to
(6.80), i.e.
with
aG
=
~
(D~
aVi)
at
ax
•
ax
'
D;=Dib~,
[v]i=O,
[
c
12 ds =
-[D'
av]2
Jl
dt
ax
l'
i=1,2,
u+bv=O
at x = s(t). Finally, therefore, we can write
the
conservation forms
ac
=~
(D~
av)
at
ax
•
ax
'
aH=~
(K;
au)
at
ax ax
coupled with
C
=bk
1
v,
bk
1
v:S;;c:s;;bk
2
v,
c
=bk
2
v,
H=u,
u+bv<O,
u:s;;H:s;;u+L,
u+bv=O,
H=u+L,
u+bv>O.
(6.85)
(6.86)
Crowley and Ockendon (1979) were unable to prove either existence
or
uniqueness of a weak solution of (6.85) in general. Following Oleinik
(1960) proofs can
be
established for
the
particular case of
Ki
= Ki(u) and
D;=D;(v)
for a
pure
metal where
c=v=O.
Accordingly, Crowley and
Ockendon (1979) proposed a numerical discretization
of
the general case
(6.85), (6.86) with
Ki
and
Di
constant in each phase for simplicity, and
tested their solution by comparison with Rubinstein's (1971) explicit
similarity solutions.
For
the numerical scheme, (6.86) must
be
rewritten
to
give
u,
V in
Enthalpy method
247
terms
of
H,
c,
i.e.
u = H, v =
c/bkt.
H +C/k1
<0,
u=H-L,
v==c/bk
2
,
H+c/k2>L.
(6.87)
The
interval between H
+c/k
1
>0
and H
+C/k2<L
needs special consid-
eration since
u is
not
defined there. Since the material cannot exist in
equilibrium in this range, Chalmers (1964) took
the
values
of
H and c to
represent
an
element of material of which a fraction f is solid
and
1 - f is
liquid. This is reminiscent of the procedure adopted by Voller and Cross
(1981a) (see
eqn
(6.55)).
The
vaues of
u,
CS,
CL
for
the
element are
compatible with local equilibrium. Thus we have
H
==
u +
(1-
f)L,
where
Cs
=
-k
1
u,
CL
=
-k
2
u.
Elimination
of
cs,
CL,
f yields a quadratic
equation for
u with
the
solutiQn corresponding to taking
the
positive
square
root
given by
u
==u*
-{k
1
-
H(k
1
- k
2
)/L}+[{k
1
-
H(k
1
-kz)/L}2_4c(k
1
- k
2
)/Ly.
2(k
1
-k
2
)/L
(6.88)
so
that
u = H
on
the
solidus and u = H - L
on
the
liquidus boundary.
The
inverted form
of
(6.86) is therefore (6.87) together with
u=u*,
v
=-u*/b,
H+c/k
2
-L<0<H+c/kt.
(6.89)
where
u*
comes from (6.88).
A simple explicit, finite-difference scheme for (6.85)
if
the
mesh points
(n
-1)
8x, n 8x, (n + 1)
8x
are
in
the
same phase is
H
m+1_Hm+Km
8t
(m
2 m+ m )
n - n n (8X)2 U
n
+1
-
Un
u
n
-1 ,
(6.90)
m+1
m+D,m
8t
(m
2 m+ m )
(691)
Cn = C
n
n
(8X)2
Vn+1
-
Vn
V
n
-1,
.
where H~ =
H(n
8x, m 8t), m = 0,
...
,M,
n = 1,
...
,N,
etc., and
K~,
D~m
take
the
values appropriate
to
that
phase.
In
order
to
use (6.90) and
(6.91) successfully over the whole
of
a fixed domain including
both
phases, we require
them
to
conserve
heat
and mass across
the
phase
boundary. Integration
of
(6.90) shows
that
the
change in heat content in
an
interval
8t
is given by
N-1 N-1
8x
L
(H~+l-
H~)
= (8t/8x) L
{K~(u~+1
-
u~)-
K~(u~-
U~-l)}
1 1
N-1
= (8t/8x) L
(K~-
~+1)(U~+1
-
U~)
+
K;:':(u;:':-
U;:':-l)
-
Kl'(ul'-
u;;').
1
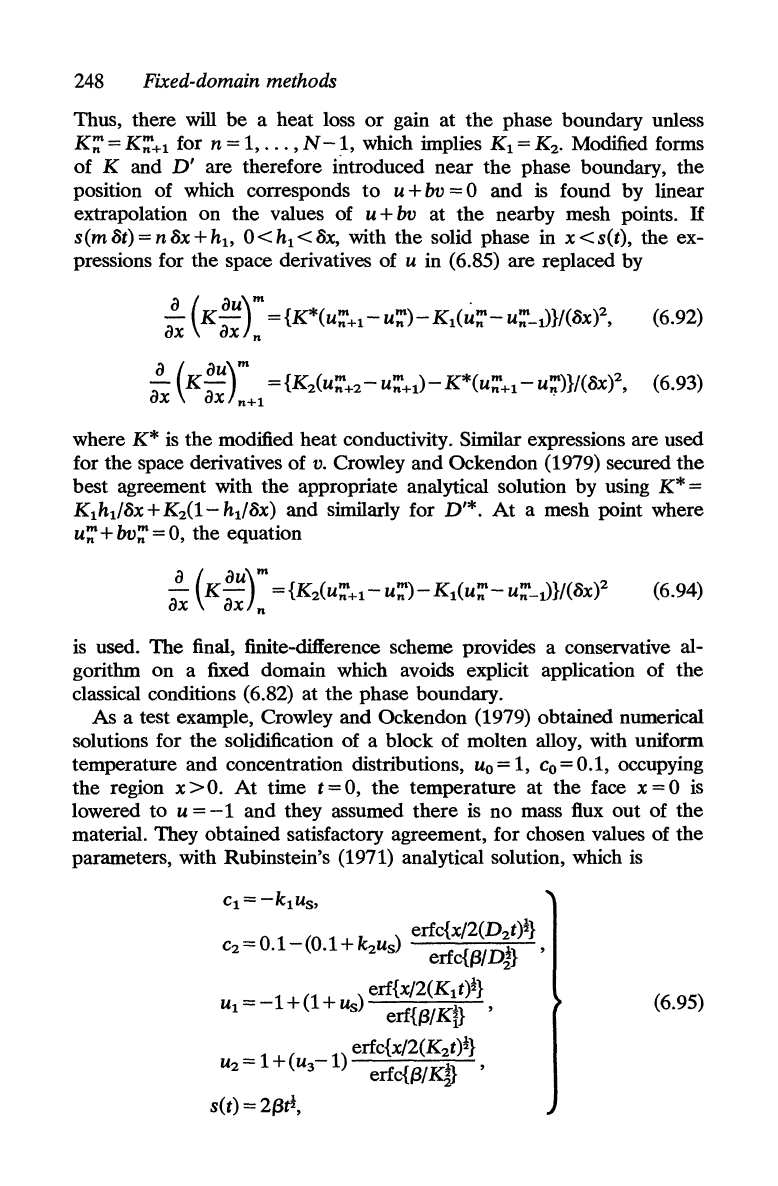
248
Fixed-domain methods
Thus, there will
be
a heat loss
or
gain
at
the
phase boundary unless
K;:'
=
K;:'+l
for n = 1,
...
, N
-1,
which implies
Kl
= K
2
•
Modified forms
of
K and
D'
are therefore introduced
near
the
phase boundary, the
position
of
which corresponds
to
u +
bv
= 0
and
is found by linear
extrapolation
on
the
values of u +
bv
at
the
nearby mesh points.
If
s(mcSt)=ncSx+hh
0<h1<cSx, with
the
solid phase in
x<s(t),
the ex-
pressions for the space derivatives of
u in (6.85) are replaced by
where
K*
is
the
modified heat conductivity. Similar expressions are used
for
the
space derivatives
of
v.
Crowley and Ockendon (1979) secured
the
best agreement with
the
appropriate analytical solution by using
K*
=
Kl
h1/cSx
+
Kz(l
-
h1/cSx)
and
similarly for D'*.
At
a mesh point where
u;:'+
bv;:'
= 0,
the
equation
(6.94)
is used.
The
final, finite-difference scheme provides a conservative al-
gorithm
on
a fixed domain which avoids explicit application of
the
classical conditions (6.82) at the phase boundary.
As
a test example, Crowley
and
Ockendon (1979) obtained numerical
solutions for
the
solidification of a block
of
molten alloy, with uniform
temperature and concentration distributions,
Uo=
1,
co=O.l,
occupying
the
region
x>
O.
At
time t = 0,
the
temperature at
the
face x = 0 is
lowered
to
u =
-1
and they assumed
there
is no mass flux
out
of
the
material. They obtained satisfactory agreement, for chosen values of
the
parameters, with Rubinstein's (1971) analytical solution, which is
Cl
=
-k1Us,
erfc{x/2(D2t)~}
c2=0.1-(0.1+k
2
u
s)
erfc{{3ID1} ,
1
(1
) erf{x/2(Klt)!}
Ul
= - + +
Us
erf{{3/Ki}
,
- 1 (
-1)
erfc{x/2(K2t)!}
U2
- + U
3
erfc{f3/
Iq} ,
s(t) =
2{3t!,
(6.95)

Enthalpy method
249
where
(3
is
the
root of
L(37T!
erf«(3/Kl)erfc«(3/Iq)exp«(32/K
1
+
(32/K~
-
K~rfc«(3/Iq)exp«(32/K2)
+ Iqerfc«(3/KVexp«(32/K1)
= {Ki
erfc«(3/Iq)exp«(32/K~
+
K~
erfc«(3/Kf)exp«(32/K
1
)}us, (6.96)
and where
Us,
the
constant phase-change temperature, is given by
O.lD!
(6.97)
The
error
in
the
position
of
the
phase boundary obtained from the
finite-difference scheme compared with
the
analytical solution (6.95)
is
less
than 5
per
cent. Slight oscillations in
the
numerical solution are less
noticeable
the
greater
the
number
of
mesh points.
A more general enthalpy-type formulation by Wilson
et
al.
(1982)
removes
the
restriction that the heat capacities in the liquid
and
solid
phases must
be
equal
and
allows more general boundary conditions
to
be
considered. They give a number of numerical finite-difference solutions
and some comparisons with Rubinstein's solution (1971).
The
possible
appearance of a mushy region just in front of the interface is mentioned.
(iii) Flame-front problems. Sometimes
the
equations in
one
part
of
a fixed
domain are special cases
of
the
equations in the remainder of
the
domain.
In these cases,
the
latter equations are valid for
the
whole domain
but
when written in conservation form they may not satisfy
the
jump
condi-
tion
on
the
moving boundary, which forms
part
of the classical formula-
tion. Crowley (1981) handles this situation by adding a term
to
the
differential equation which vanishes everywhere except
on
the moving
boundary where it has
the
required jump.
The
context is an enthalpy
formulation of Buckmaster's (1979) model of flame fronts, in particular a
steady-state flame tip for laminar, pre-mixed flames with large activation
energy.
The
reaction region is considered
to
be
confined
to
a flame sheet
y = h(x), which divides two regions where the simple diffusion
and
heat
conduction equations are satisfied. Jump conditions hold
at
the
flame
sheet.
The
governing equations considered are
(6.98)
(6.99)
T=l+T"",
y
>h(x).
(6.100)
250
Fixed-domain methods
The
conditions
on
the flame sheet, y = h(x), are
T=1+Too,
[<1>]
= 0 =
[T],
[
a<l>]
[all
{<I>Y=h}
ay
=-A
ayJ=Aex
p
2(1+T.Y
,
(6.101)
(6.102)
(6.103)
where [ ] denotes
the
jump from
one
side of
the
front
to
the
other
as
usual.
The
other
boundary conditions are
aTlay = 0 =
a<l>lay,
y=O,
(6.104)
and
on
x
=0
we have
T(O,
y) = T .. +exp(y - h
o
),}
<I>
= - A (y - ho)exp(y - h
o
),
O<y<h
o
,
x=O
(6.105)
T=1+T
.. ,
<I>
= 0,
y>ho,
x=O.
(6.106)
In
these expressions T denotes temperature,
<I>
measures the sum
of
temperature
and
concentration
of
fuel related
to
its value far behind the
flame, and A
=
(1-1/L)6,
where 6
is
the activation energy and L
the
Lewis number.
It
is
obvious
on
inspection
that
equations (6.100) are simply the
constant-temperature forms of (6.98)
and
(6.99), which are therefore
valid over the whole domain except perhaps
on
y = h(x).
The
conserva-
tion form of (6.99)
on
the
flame front can
be
obtained by integrating
(6.99) with respect to y across the front, having replaced
a<l>lax
by
(a<l>lay)(dhldx).
It
is
[<1>]
dhldx =
-[a<l>lay
+ A aTlay], which shows that
the
first of the jump conditions in each of (6.102) and (6.103) are satisfied.
On
the other hand,
the
conservative form
of
(6.98) gives [T] dhldx =
[aT lay],
which does not agree with the second of each
of
(6.102) and
(6.103). Crowley (1981) therefore modifies equation (6.98) by the addi-
tion of a term which vanishes everywhere except
on
y = h(x), where it has
the jump required in
the
second
of
(6.103). When (6.98)
is
rewritten as
aT
a {H(1 ) G
<l>y=h
)}
a
2
T
-+-
+T
..
-T
exp
(1
T.)2
=-2'
ax
ay
+..
ay
where
H(u)
= 0, u
~O
and
H(u)
=
1,
u
>0,
the
conservation form at the
discontinuity gives
[T]:~
-
[H(1+T
.. -
T)eXPG(:~~
..
f)]
=-[:~,
which at T = 1 + T .. implies the desired jump conditions in (6.102) and
(6.103).
Enthalpy method
251
For
numerical Sblution, the conditions T = 1 +
Too,
e(>
==
0 on y
==.e
for
some sufficiently large
.e>
ho
are added and then the governing equations
in conservation form, to
be
solved over the whole of the fixed domain,
O~y
~.e,
are
aT
a { (
C(>Y=h
)}
a
2
T
ax
+ ay
H(l
+ Too-T)eXPU(l +
Too?
=
ay2'
ae(>
a
2
T a
2
e(>
-=>..-+-
ax ay2
ay2'
together with
the
conditions (6.104-106).
(6.107)
(6.108)
A convenient numerical procedure, given
e(>,
T at all mesh points j
8y,
i8x,
O<y<.e,
is
(i)
find
h(i
8x) where T = 1 +
Too
by quadratic extrapolation on T from
the region
y<h(i8x),
i.e.
T<l+Too;
(ii) evaluate
e(>y=h
==
e(>~
by quadratic extrapolation from both sides and
taking
the
mean;
(iii) solve (6.107) explicitly using
T}<l+Too,
(iv)
solve (6.108) as
-I..~+1
=
-I..~+~
{(-I..!+1_
2-1..~+1-
-I..~+1)
'l"J 'l"J
2(8y)2
'l"J+1
'l"J
'l"J-1
+
>"(T}!t
-2T;+1+
T}~D+(e(>}+1
-2e(>}+e(>}-1)
+>"(T}+1-
2T
}+T}-1)},
using successive over-relaxation.
Typical profiles of the flame sheet are shown in Fig. 6.8. Crowley
(1981) was not able
to
quote existence and uniqueness proofs of weak
solutions for the coupled systems of equations nor
to
prove the con-
vergence of the numerical scheme just outlined.
In
another paper Crowley
(1980) suggests enthalpy treatments of flame fronts in solid fuel and a
quite different problem relating
to
the growth of a crystal as it
is
slowly
withdrawn from a bath of its own melt. The movement of an air-liquid
interface has
to
be
considered as well as that of the solidification front,
with the additional complication that a corner exists all the time in the
interface with air.
