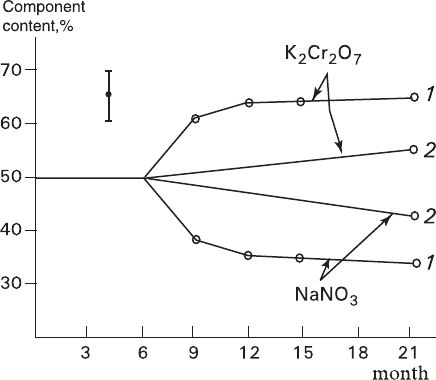
Glikin A.E. Polymineral-Metasomatic Crystallogenesis
Подождите немного. Документ загружается.

202 5 Metasomatic Transformation of Aggregates
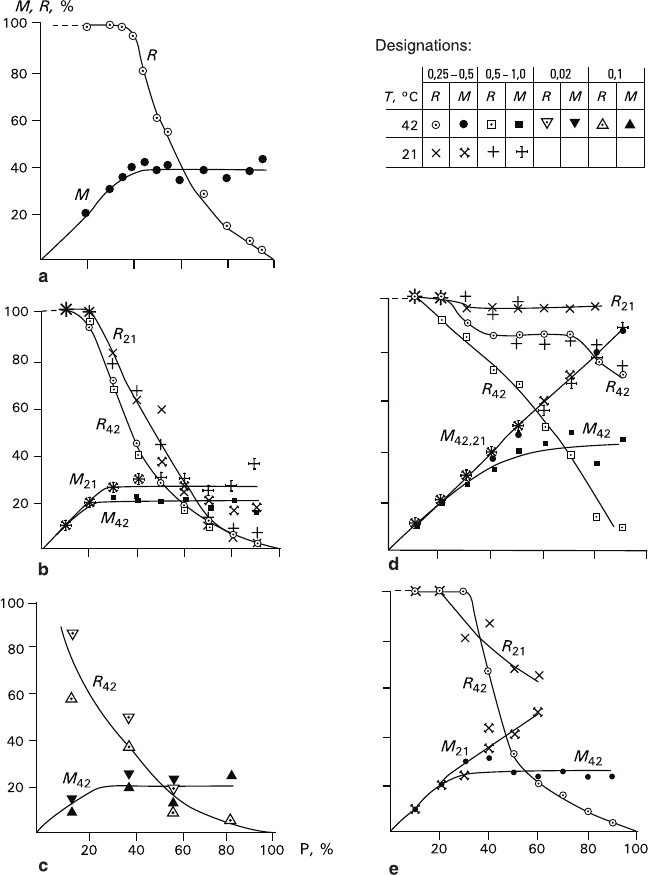
Fig. 5.13
Dependencies of compositional characteristics of recrystallized mixture aggregates on
their initial total composition (Data from Table 5.2 (pages 190–192), explanations are given in the
text): (a–e) – mixtures (separated component is printed in bold): (a) NaNO
3
–K
2
Cr
2
O
7
, (b)
Ba(NO
3
)
2
–BaCl
2
·2H
2
O, (c) KAl(SO
4
)
2
·12H
2
O–NiSO
4
·7H
2
O, (d) NaCl–K
2
Cr
2
O
7
, (e) NH
4
Cl–
K
2
Cr
2
O
7
. Indices 42 and 21 – temperature modes 1 and 2 from Fig. 5.10a; numeric values in the
designation table are fractions of the powders (in millimeters)
It can be seen that if initial content of NaNO
3
was less than 35 wt%, this compo-
nent was contained solely in the aggregate, i.e., the layering did not occur. With
higher initial amounts of NaNO
3
layering resulted in obtaining identical composi-
tion of bimineral aggregate, which consisted of about 61.6 wt% of K
2
Cr
2
O
7
and
38.4 wt% of NaNO
3
(Fig. 5.12). Compositions of aggregates obtained via recrystal-
lization of divided monomineral powders were also close to the above values:
34.0 wt% of NaNO
3
and 66.0 wt% of K
2
Cr
2
O
7
, correspondingly, if K
2
Cr
2
O
7
was
initially placed in the upper part of the container; and 37.5 wt% of NaNO
3
and
62.5 wt% of K
2
Cr
2
O
7
, if NaNO
3
was initially in the upper part of the container.
If initial contents of NaNO
3
are high, recrystallization in this system is accom-
panied by formation of intermediate unstable macrostructures resulting from
cumulative recrystallization in the lower part of the container. They exist for about
1.5–2 months and then transform into the bimineral aggregate described above. Big
sectors (10–12 mm) highly enriched with K
2
Cr
2
O
7
are formed, when the initial
NaNO
3
content is about 70–80%, and when this content is about 80–90%, it results
in formation of 3–4 mm thick layers stretching in perpendicular directions and
enriched with K
2
Cr
2
O
7
, located at the distance of 5–6 mm from each other; the lay-
ers are gradually merged to form a bimineral aggregate. Contents of NaNO
3
ranging
from 90% to 95% cause formation of large joint crystals of K
2
Cr
2
O
7
(the aggregate
sizes are about 4–6 mm, while the sizes of individual crystal in the aggregates are
about 2.5 mm; content of K
2
Cr
2
O
7
in this zone is about 2–3%) with their longer
[001] axes located in perpendicular directions to the column vertical axis and also
a thin bottom bimineral layer. When the content of NaNO
3
in flat samples exceeds
80%, accumulations of diffused K
2
Cr
2
O
7
can be visible in randomly located sites of
the column. If the component contents are close to equal, formation of intermediate
net structures gradually transforming in uniformly grained bimineral aggregates is
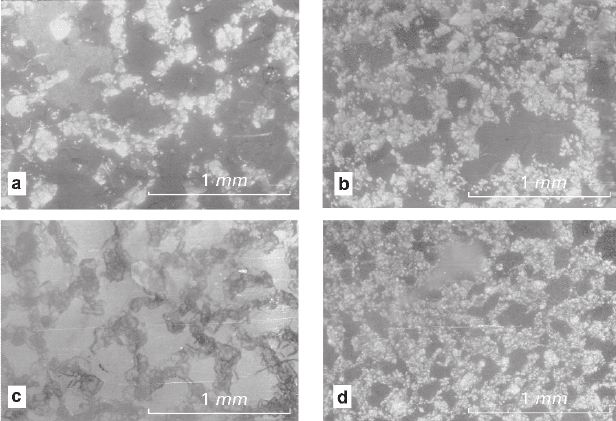
observed (Fig. 5.14a).
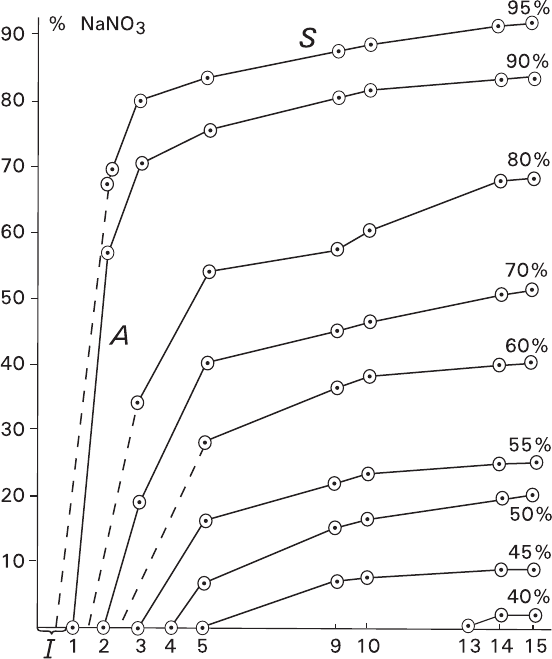
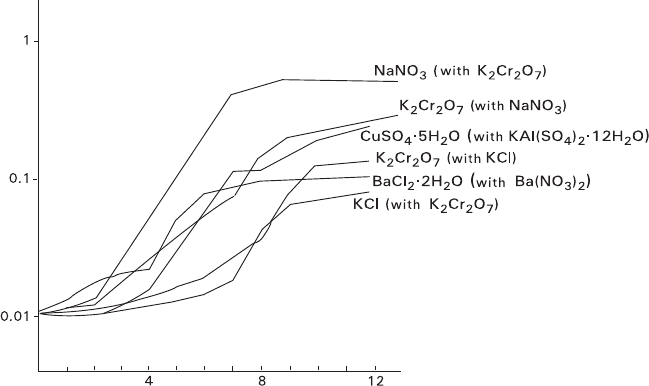
Kinetic curves of layering the mixture containing 40–95% of NaNO
3
are repre-
sented in Fig. 5.15. Each curve can be divided into three definite parts and corre-
sponding to three following stages: incubation interval I (merges with abscissa),
active stage A, and stationary stage S. Increasing the initial contents of NaNO
3
results in shortening incubation interval and active stage as well as accelerating the
active stage.
It is important to note that recrystallization kinetics depend essentially upon the
structure of an initial aggregate. The differences in the process of transformation of
mixtures and various monomineral combinations (Figs. 5.11 and 5.12) are dis-
cussed above. Also, influence of primary granulometric heterogeneity of the aggre-
gates should be taken into consideration. It can be seen in Fig. 5.16 that the active
stage of bimineral aggregate formation in a mixture containing equal amounts of
NaNO
3
and K
2
Cr
2
O
7
abruptly slows down if the mixture is seeded with individual
large crystals (5–8 mm, 4–7 wt%).
At the later stages of process, morphology of individuals is characterized by
a high degree of idiomorphism. Crystals of K
2
Cr
2
O
7
are faceted as monohedrons
{100}, {010}, {001}, {110}, {11
¯
0}, and {111
¯
} and, as a rule, flattened in parallel
to {010} (crystal class 1, indexing according to Groth, 1906) that corresponds to
5.3 Recrystallization of Polymineral Aggregates 203
204 5 Metasomatic Transformation of Aggregates
their most abundant form. Crystals of NaNO
3
(crystal class 3
¯
m2) are faceted with
{101
¯
1}, and surprisingly have faces {0001} (up to 60% of crystals) and {112
_
1}.
The crystal idiomorphism distinctly increases in transition from monomineral sec-
tors to bimineral ones, idiomorphism of K
2
Cr
2
O
7
being always higher than that of
NaNO
3
. At the same time, K
2
Cr
2
O
7
crystals initially appearing in NaNO
3
mass have
needle-like shape elongated along [001].
Observations conducted in thin sections (see Fig. 1.2a) used to minimize gravi-
tation influence upon the mass exchange showed that large crystals of both NaNO
3
and K
2
Cr
2
O
7
dissolved when placed in monomineral fine-grained mass of the other
substance, which formed druses in cavities appearing due to dissolution. Dissolution
of NaNO
3
crystals was approximately twice as fast in comparison with that of
K
2
Cr
2
O
7
. At the same time, big crystals located in fine-grained mass of the same
substance did not change significantly at least for 1 month. Development of “comb
structures” was observed in vertical section-type samples. The structures appeared
on large (5–8 mm) flat crystals of K
2
Cr
2
O
7
surrounded by NaNO
3
mass; the “comb
cogs” (usually about 10) had almost the same length as the crystal and were its
continuation; they were surrounded with fine-grained NaNO
3
mass (Fig. 5.17).
Recrystallization results in enlargement of NaNO
3
and K
2
Cr
2
O
7
grains, which
can reach their maximal sizes observed under stationary condition, i.e., 2–2.5 mm
and 1.5–2 mm, respectively. The size distribution of the crystal grains is multimo-
Fig. 5.14 Intermediate net structures formed in thin sections via recrystallization of finely frac-
tionated mixtures NaNO
3
–K
2
Cr
2
O
7
(a), Ba(NO
3
)
2
–BaCl
2
·2H
2
O (b), KAl(SO
4
)
2
·12H
2
O–NiSO
4
·7H
2
O
(c) and KAl(SO
4
)
2
·12H
2
O–CuSO
4
·5H
2
O (d)
dal. Reaching the stationary stage leads to disappearance of all differences in the
multimodal distributions of the grain sizes obtained in different series of experi-
ments (Petrov et al. 1988). Coarsening the fine-grained (0.005–0.008 mm) mixtures
of NaNO
3
and K
2
Cr
2
O
7
(as well as other mixture discussed below) in the course of
recrystallization in thin sections proceeds with a high rate and includes an incuba-
tion interval, and the active and stationary stages (Fig. 5.18).
Other systems are characterized by various tendencies though scarcity of the
data prevents development of an unequivocal picture.
During recrystallization, mixtures of Ba(NO
3
)
2
–BaCl
2
·2H
2
O and KAl(SO
4
)
2
·
12H
2
O–NiSO
4
·7H
2
O undergo separation into layers in accordance with the above
scheme (Figs. 5.13b, c; Table 5.2), but a fraction of substance transferred to the
Fig. 5.15 Time variation of separated amount of monomineral NaNO
3
in the course of recrystal-
lization of mixtures containing NaNO
3
(40–95%) and K
2
Cr
2
O
7
5.3 Recrystallization of Polymineral Aggregates 205
206 5 Metasomatic Transformation of Aggregates
monomineral part is higher in comparison with that of NaNO
3
–K
2
Cr
2
O
7
mixture. The
amount of separated substance insignificantly depends upon the temperature con-
ditions of recrystallization; it is indicated by a slight divergence of plots showing
separation of Ba(NO
3
)
2
–BaCl
2
·2H
2
O mixture into layers in different temperature
modes. The fraction size does not affect or only slightly affects the separation; the
plots drawn for different fractions of this mixture coincide almost completely. Also,
there is not any significant difference in the curves drawn for KAl(SO
4
)
2
·12H
2
O–
NiSO
4
·7H
2
O mixture, and their absolute values do not much differ from the charac-
teristics of the two previous compounds, despite the fact that the difference in the
grain sizes is about an order of magnitude. A mixture of KAl(SO
4
)
2
·12H
2
O and
CuSO
4
·5H
2
O also undergoes layering with formation of a monomineral alum aggre-
gate in the upper part of the container and a bimineral aggregate in its lower part.
Unstable intermediate structures, analogous to those of NaNO
3
–K
2
Cr
2
O
7
, were
observed during recrystallization of Ba(NO
3
)
2
–BaCl
2
·2H
2
O, KAl(SO
4
)
2
·12H
2
O–
NiSO
4
·7H
2
O, and KAl(SO
4
)
2
·12H
2
O–CuSO
4
·5H
2
O mixtures. The net structures
formed by fine-grained aggregates of one substance grain surrounding the individuals
of the other were observed in flat samples in the fine-grained (0.01 mm) aggregates
of all three pairs of compounds (Figs. 5.14b–d); the individuals were represented by
a separated component, and the nets were formed by the other one. In the cylindrical
columns the mixture of KAl(SO
4
)
2
·12H
2
O and NiSO
4
·7H
2
O formed isometric spotty
segregations enriched with NiSO
4
·7H
2
O, which subsequently transformed into a lay-
ered structure (in perpendicular direction to the gravitation vector) containing 3–5 mm
thick alternating layers. Contacting of monomineral KAl(SO
4
)
2
·12H
2
O and
CuSO
4
·5H
2
O columns resulted in formation of the “upper” substance aggregates
Fig. 5.16 Formation of bimineral aggregate in a 50%:50% mixture of NaNO
3
–K
2
Cr
2
O
7
of homo-
geneous structure (1) and that containing big crystals of both substances (2)
(K-Al-alum) in the mass of the “lower” substance. When KAl(SO
4
)
2
·12H
2
O was
arranged in the lower zone, large (up to 2–4 mm) joints of CuSO
4
·5H
2
O crystals were
formed in its bulk; and if the arrangement was inversed, the lower CuSO
4
·5H
2
O mass
became broken into blocks divided by veins composed of KAl(SO
4
)
2
·12H
2
O.
Behavior of NaCl–K
2
Cr
2
O
7
and NH
4
Cl–K
2
Cr
2
O
7
mixtures (Figs. 5.13d, e; Table
5.2, pages 190–192) and mixtures of KCl and K
2
Cr
2
O
7
reveals other regularities.
During recrystallization of a fine-grained fraction under low-temperature condi-
tions, mixtures of NaCl and K
2
Cr
2
O
7
do not practically undergo any separation into
layers, while elevation of temperature (and increase in the amplitude of temperature
fluctuations; see Fig. 5.10a) or increasing the amount of the fraction increases the
tendency of the separation. At elevated temperature and increasing the amount of the
fraction, the plot shapes become similar to those of the layered systems. Under
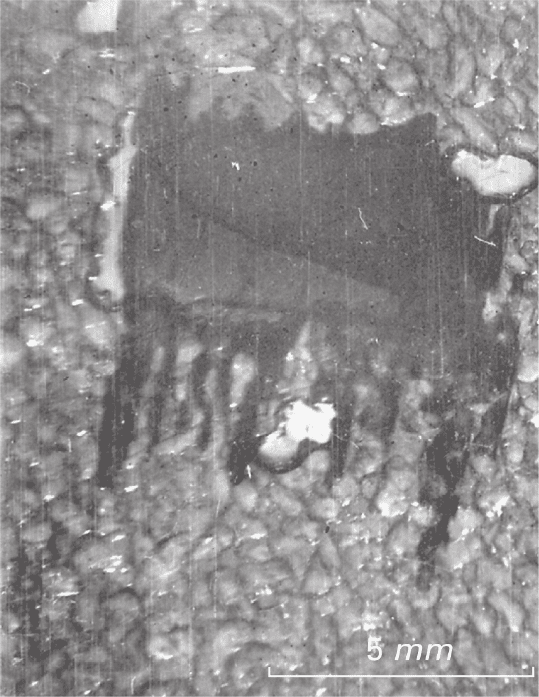
Fig. 5.17 A “comb-like” structure on a big crystal of K
2
Cr
2
O
7
surrounded by NaNO
3
5.3 Recrystallization of Polymineral Aggregates 207
208 5 Metasomatic Transformation of Aggregates
low-temperature conditions the mixture NH
4
Cl–K
2
Cr
2
O
7
undergoes insignificant
layering; elevated temperatures produce alterations similar to those occurring in the
layering systems. Mixture of equal contents of KCl and K
2
Cr
2
O
7
(78 experiments)
does not undergo layering, but predominance of KCl (80–90%) results in accumula-
tion of potassium dichromate in the form of big crystal joints chaotically distrib-
uted within the container and undergoing no changes for a long time in contrast to
the morphologically similar but unstable joint crystals of NaNO
3
(95%)–K
2
Cr
2
O
7
mixture. When a monomineral mass of K
2
Cr
2
O
7
is placed over a mass of KCl, big
stable joint crystals of K
2
Cr
2
O
7
are also formed in the mass of KCl; the crystals grow
bigger in the narrow bordering zones of the both substances. It is to be noted that recrys-
tallization of the above mixture was accompanied by mutual epitaxial growth of
components, including formation of KCl incrustations all over big K
2
Cr
2
O
7
crystals.
Coarsening the grains, which was determined in the series of mixtures in the
fine-grained preparations (Fig. 5.17), includes three stages (similar to the coarsening
in a NaNO
3
–K
2
Cr
2
O
7
mixture). As a result, the aggregate acquires a stationary state.
Coarsening of crystals in KCl–K
2
Cr
2
O
7
mixture proceeds with the least extent and
the rate in comparison with coarsening processes taking place in NaNO
3
–K
2
Cr
2
O
7
,
Ba(NO
3
)
2
–BaCl
2
·2H
2
O, and KAl(SO
4
)
2
·12H
2
O–CuSO
4
·5H
2
O mixtures.
Fig. 5.18 Temporal evolution of grain size in the course of recrystallization of mixtures contain-
ing equal amounts of finely fractionated components
R, mm
day

Recrystallization of coarse-grained (1–2 mm) ordered NaCl–KCl (1:1) aggre-
gates was studied in nine experiments in flat samples.
4
Amplitudes of the tempera-
ture fluctuations ranged from ambient conditions to 50–55°C and the period varied
from 20 to 60 min. The main peculiarity of the process appeared to be formation of
fine-grained fraction consisting mainly of KCl surrounding individual initial crys-
tals and the whole aggregate (Fig. 5.19). Newly formed crystals precipitated at the
beginning of the process; subsequently they become coarser and sometimes intergrown.
The process terminates when an aggregate consisting of coarse and fine grains
acquires a stationary state (or extremely sluggishly altering state).
Thermogradient recrystallization of aggregates was observed in four binary powder
mixtures: KAl(SO
4
)
2
·12H
2
O–NiSO
4
·7H
2
O, KAl(SO
4
)
2
·12H
2
O–CuSO
4
·5H
2
O, KCl–
K
2
Cr
2
O
7
, (NH
4
)
2
Ni(SO
4
)
2
·6H
2
O–CuSO
4
·5H
2
O (three of them were tested under thermo-
oscillatory conditions) and one ternary mixture NaNO
3
–K
2
Cr
2
O
7
–(NH
4
)
2
Ni(SO
4
)
2
·6H
2
O.
The columns contained equal amounts of components, and the experiments were
carried out using the temperature mode shown in Fig. 5.10b.
Fig. 5.19 Exemplary recrystallized aggregate of KCl–NaCl surrounded by fine-grained precipi-
tated crystals, which also fill up the intercrystalline cavities:
(a–d) various parts of the sample (45 periods of temperature oscillations)
4
Mr. A. V. Talyzin performed the experimental investigations.
5.3 Recrystallization of Polymineral Aggregates 209
210 5 Metasomatic Transformation of Aggregates
The main result was common for all the mixtures and consisted in separating the
mixtures into a series of zones located in perpendicular direction to direction of
coinciding vectors of the temperature and gravity gradients. Zonal structures of all
investigated systems included a central cavity having a thickness of about 5–10 mm,
sharp contacts between the selvage zones, and asymmetric configurations of the
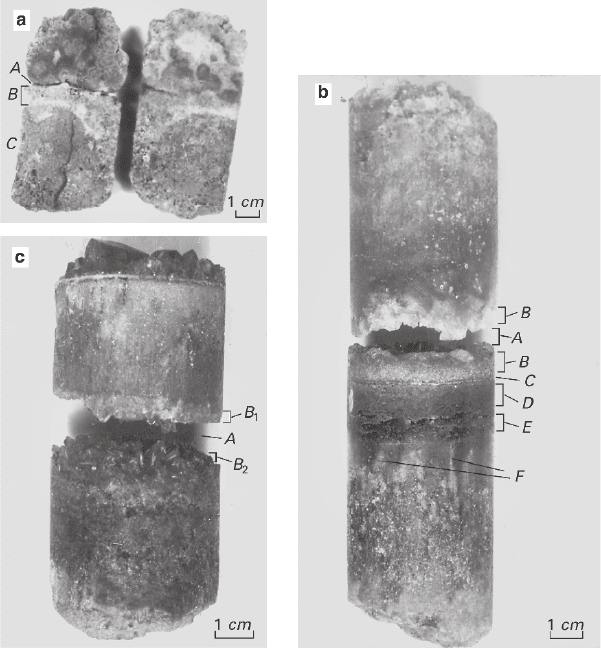
upper and lower selvages in respect to the cavity (Fig. 5.20). The process of separa-
tion into layers takes about 2–3 months, and after that the column is not percep-
tively changing for at least the same period.
In KAl(SO
4
)
2
·12H
2
O–NiSO
4
·7H
2
O mixture (Fig. 5.20a) the cavity A is delimited
by the crystal druses (3–6 mm) of both substances. Zone B having the thickness up
to 50 mm and containing large (up to 10–12 mm) zonal idiomorphic crystals of
Fig. 5.20 Columns obtained after recrystallization of mixtures in a thermogradient mode.
Explanations are given in the text
NiSO
4
·7H
2
O bound with KAl(SO
4
)
2
·12H
2
O crystals having the sizes up to 4 mm is
located lower. A fine-grained zone C is located below the zone B; it is enriched with
NiSO
4
·7H
2
O and is gradually transformed into a bound primary aggregate.
The cavity A in KAl(SO
4
)
2
·12H
2
O–CuSO
4
·5H
2
O mixture (Fig. 5.20b) is delim-
ited by crystal druses (8–10 mm) of both substances growing upward from the zone
B and also containing crystals of Cu
3
SO
4
(OH)
4
. Below that cavity are the following
zones: C, which is a monomineral aggregate of CuSO
4
·5H
2
O having the thickness
up to 3–4 mm; D, which is a monomineral aggregate of Cu
3
SO
4
(OH)
4
having the
thickness of about 2–12 mm; E, which is practically a monomineral aggregate con-
taining phenocrysts of CuSO
4
·5H
2
O; F, which is a series of vertical tapered out
monomineral veins having the length up to 20–35 mm. These veins look like a
continuation of the zone E and are surrounded by a fine-grained bound aggregate
of KAl(SO
4
)
2
·12H
2
O and CuSO
4
·5H
2
O (the initial ratio 1:1). The upper zones rep-
resent gradual transition from a zone similar to B to the bound primary aggregate.
The cavity in KCl–K
2
Cr
2
O
7
mixture is delimited by large (4–6 mm) crystals of
K
2
Cr
2
O
7
and by relatively symmetric upper and lower zones enriched with the same
substance and having sharp contact zones with a primary aggregate.
The cavity A in CuSO
4
·5H
2
O–(NH
4
)
2
Ni(SO
4
)
2
·6H
2
O mixture (Fig. 5.20c) is
delimited by crystal druses (up to 8–10 mm) made up of the substances composing
the system and (NH
4
)
2
Cu(SO
4
)
2
·6H
2
O. The borders between the selvages and the
main volume are sharp and their compositions are distinctly asymmetric. The upper
selvage is enriched with (NH
4
)
2
Ni(SO
4
)
2
·6H
2
O (B
1
), whereas the lower one with
(NH
4
)
2
Cu(SO
4
)
2
·6H
2
O and CuSO
4
·5H
2
O (B
2
). The upper and lower parts of the
main mass are represented by a bound primary aggregate enriched with correspond-
ing substances. The upper part contains a druse grown above the massif in a free
part of the solution and consisting of crystals of (NH
4
)
2
Ni(SO
4
)
2
·6H
2
O, CuSO
4
·5H
2
O,
and (NH
4
)
2
H(SO
4
)
2
.
In ternary NaNO
3
–K
2
Cr
2
O
7
–(NH
4
)
2
Ni(SO
4
)
2
·6H
2
O mixture, the cavity is deline-
ated from above and below by crystal druses composed of (NH
4
)
2
Ni(SO
4
)
2
·6H
2
O
(up to 8–10 mm) with insignificant amounts of NaNO
3
and K
2
Cr
2
O
7
crystals. On
either side of the druse boundaries there are located a strong porous macrocrystal-
line aggregate made up of (NH
4
)
2
Ni(SO
4
)
2
·6H
2
O. After the aggregate there are
zones of dense macrocrystalline aggregates of K
2
Cr
2
O
7
containing up to 20% of
NaNO
3
, which form sharp contact zones with the main bound mass. These borders
exist even at the upper zone, but at the lower zone they have wedges intruding into
the main bound mass and gradually disappearing in it.
The results discussed in this section show a variety of transformations of indi-
viduals and phenomena of matter redistribution occurring during recrystallizations
in solutions. However, they have a number of regularities that can be traced despite
the incoherence of the experimental data.
5.3 Recrystallization of Polymineral Aggregates 211
