Guozhong Cao. Nanostructures & Nanomaterials: Synthesis, Properties & Applications
Подождите немного. Документ загружается.

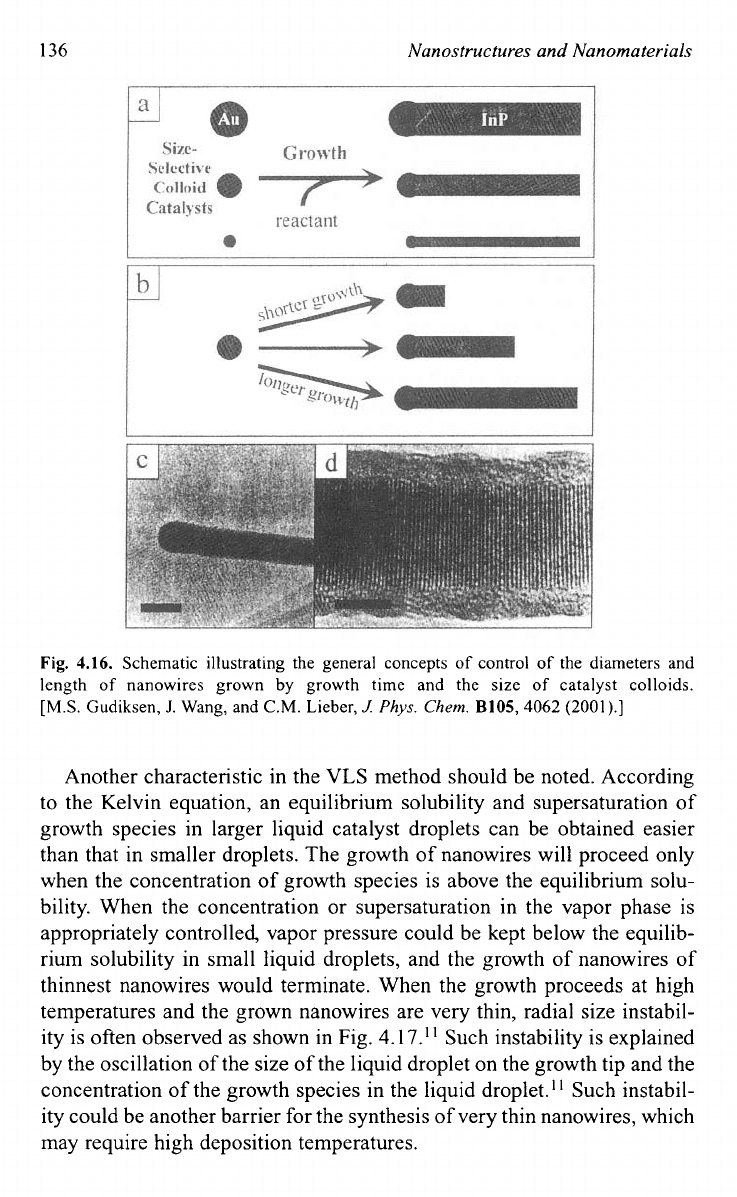
136
Nanostructures and Nanomaterials
Fig.
4.16.
Schematic illustrating the general concepts
of
control
of
the diameters and
length
of
nanowires grown by growth time and the size
of
catalyst colloids.
[M.S.
Gudiksen,
J.
Wang, and
C.M.
Lieber,
J.
Phys.
Chem.
B105,4062
(2001).]
Another characteristic in the
VLS
method should be noted. According
to the Kelvin equation, an equilibrium solubility and supersaturation
of
growth species in larger liquid catalyst droplets can be obtained easier
than that in smaller droplets. The growth
of
nanowires will proceed only
when the concentration of growth species is above the equilibrium solu-
bility. When the concentration or supersaturation in the vapor phase is
appropriately controlled, vapor pressure could be kept below the equilib-
rium solubility in small liquid droplets, and the growth of nanowires of
thinnest nanowires would terminate. When the growth proceeds at high
temperatures and the grown nanowires are very thin, radial size instabil-
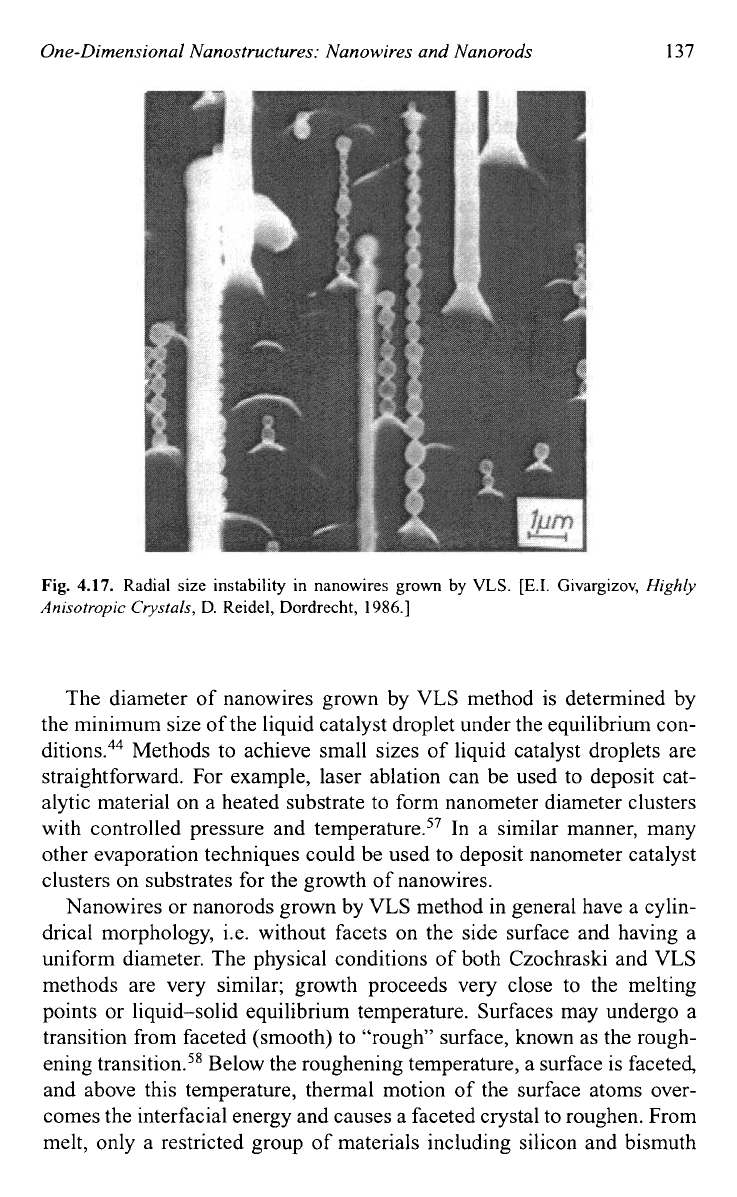
ity is often observed as shown in Fig.
4.17."
Such instability is explained
by the oscillation of the size of the liquid droplet on the growth tip and the
concentration of the growth species in the liquid droplet." Such instabil-
ity could be another barrier for the synthesis of very thin nanowires, which
may require high deposition temperatures.
One-Dimensional Nanostructures: Nanowires and Nanorods
137
Fig.
4.17.
Radial size instability in nanowires grown by
VLS.
[E.I.
Givargizov,
High/y
Anisotropic
Crystals,
D. Reidel, Dordrecht,
1986.1
The diameter of nanowires grown by
VLS
method is determined by
the minimum size of the liquid catalyst droplet under the equilibrium con-
dition~.~~ Methods to achieve small sizes of liquid catalyst droplets are
straightforward. For example, laser ablation can be used to deposit cat-
alytic material on
a
heated substrate to form nanometer diameter clusters
with controlled pressure and temperat~re.~~ In a similar manner, many
other evaporation techniques could be used to deposit nanometer catalyst
clusters on substrates for the growth of nanowires.
Nanowires or nanorods grown by
VLS
method in general have a cylin-
drical morphology, i.e. without facets on the side surface and having a
uniform diameter. The physical conditions of both Czochraski and
VLS
methods are very similar; growth proceeds very close to the melting
points or liquid-solid equilibrium temperature. Surfaces may undergo a
transition from faceted (smooth) to “rough” surface, known as the rough-
ening tran~ition.~~ Below the roughening temperature, a surface is faceted,
and above this temperature, thermal motion of the surface atoms over-
comes the interfacial energy and causes a faceted crystal to roughen. From
melt, only a restricted group of materials including silicon and bismuth
138
Nunostructures and Nanomateriuls
can grow faceted single crystals.59 However, facets may develop if there is
vapor-solid
(VS)
deposition on the side surface. Although the
VS
deposi-
tion rate is much smaller than the
VLS
growth rate for a given tempera-
ture, it is still effective in controlling the morphology. Since the difference
in the two deposition rates decreases with increasing temperature, the
VS
deposit will greatly influence the morphology in the high temperature
range. It is noted that the diameter of the nanowire may change if the
growth conditions vary or the catalyst evaporates or is incorporated into
the nanowires.
4.2.2.4.
Precursors
and
catalysts
A variety of precursors have been used for the
VLS
growth as that for
evaporation-condensation methods. Gaseous precursors such as SiC14 for
silicon nan~wires~~ are convenient sources. Evaporation of solids by heat-
ing to elevated temperatures
is
another common practice.60 Laser ablation
of solid targets is yet another method used in generating vapor precur-
sor~.~~,~~
To
promote the evaporation of solid precursors, formation of
intermediate compounds may be an appropriate approach. For example,
Wu
et
al.54
used a mixture of Ge and GeI, as precursors for the growth
of
Ge nanowires. The precursors evaporated through the formation of
volatile compound via the following chemical reaction:
The Ge12 vapor was transported to the growth chamber, condensed into the
liquid catalyst (here is AdSi) droplets, and disproportionated according
to
Other precursors have also been explored in the
VLS
growth of nanowires
including ammonia and gallium acetylacetonate for GaN nanorods,62
closo- 172-dicarbadodecaborane (C2BI0Hl2) for
B4C
nan~rods,~~ and
methyltrichlorosilane for
ZnO nanowires have been grown on Au-coated (thickness ranging from
2 to 50nm) silicon substrates by heating a 1
:
1 mixture of ZnO and
graphite powder to 900-925°C under a constant flow of argon for
5-30
min.65 The grown ZnO nanowires vary with the thickness of the ini-
tial Au coatings. For a
50
nm Au coating, the diameters of the nanowires
normally range from
80
to 120nm and their lengths are 10-20pm.
Thinner nanowires of
40-70
nm with lengths of 5-10 pm were grown on
3
nm Au-coated substrates. The grown ZnO nanowires are single crystals
One-Dimensional Nanostructures: Nanowires
and
Nanorods
139
with a preferential growth direction of
(001).
The growth process of ZnO
is believed to be different from that of elementary nanowires. The process
involves the reduction of ZnO by graphite to form Zn and
CO
vapor at
high temperatures (above
900°C).
The Zn vapor is transported to and
reacted with the Au catalyst, which would have already reacted with sili-
con to form a eutectic Au-Si liquid on silicon substrates, located down-
stream at a lower temperature to form Zu-Au-Si alloy droplets.
As
the
droplets become supersaturated with Zn, crystalline ZnO nanowires are
formed, possibly through the reaction between Zn and CO at a lower
temperature. The above process could be easily understood by the fact that
the reaction:
ZnO
+
C
f~
Zn
+
CO
is a reversible at temperatures around
900°C.66
Although the presence
of a small amount of CO is not expected to change the phase diagram
significantly, no ZnO nanowires were grown on substrates in the absence
of
graphite.
A variety
of
materials can be used as catalysts for the
VLS
growth
of
nanowires. For example, silicon nanowires were grown using iron as a cat-
aly~t.~~ Any materials or mixtures can be used as catalyst as far as they
meet the requirements described by Wagner.44 For example, a mixture
of
Au
and Si was used for the growth
of
germanium nan~wires.~~
Single crystal monoclinic gallium oxide (P-Ga203) nanowires were
synthesized with a conventional
DC
arc-discharge method.67 GaN powder
mixed with
5
wt% of transition metal powders (Ni/Co
=
1
:
1
and
Ni/Co/Y
=
4.5
:
4.5
:
1)
was pressed into a small hole of the graphite
anode.
A
total pressure of
500
torr of argon and oxygen gases in a ratio
of
4:
1
was maintained during the growth. The typical diameter of the
nanowires is about
33
nm with a growth direction of
[OOl],
and no amor-
phous layer was founded on the surface. Possible chemical reaction for the
formation of Ga,O, is proposed to be:
(4.9)
(4.10)
Single crystal Ge02 nanowires were grown by evaporation of a mixture of
Ge powder and
8
wt% Fe at
820°C
under a flow
(130
sccm) of argon gas
under a pressure
of
200 torr.68 The nanowires have diameters ranging from
15
to
80
nm. Although Fe was added as a catalyst to direct the growth of
nanowires, no globules were found on the tips
of
grown nanowires. The
authors argued that the Ge02 nanowires were grown by mechanisms other
than
VLS
method. It is also noticed that during the experiment, no oxygen
140
Nanostructures and Nunomaterials
was intentionally introduced into the system. Oxygen may leak into the
reaction chamber and react with germanium to form germanium oxide.
The catalyst can be introduced
in
situ
as well. In this case, the growth
precursor is mixed with the catalyst and evaporated simultaneously at a
higher temperature. Both the growth precursor or species and the catalyst
condense at the substrate surface when
a
supersaturation
is
reached at a
temperature lower than the evaporation temperature. The mixture of the
growth species and catalyst react either in the vapor phase or on the sub-
strate surface to form a liquid droplet. The subsequent nanowire growth
would proceed as discussed before.
Yu
et
uZ.~~
reported the synthesis of amorphous silica nanowires by
VLS
method. A mixture of silicon with 20 wt% silica and
8
wt% Fe was
ablated using an excimer laser of 246 nm wavelength under flowing argon
at
100
torr. Fe was used as
a
catalyst and the growth temperature was
1200°C. The nanowires have a chemical composition of Si
:
0
=
1
:
2, and
a uniform size distribution with a diameter of 15 nm and a length
up
to
hundreds micrometers.
GaN nanowires were prepared using elemental indium
as
a catalyst
in the reaction of gallium and ammonia.70 Nanowires have diameters
ranging from 20 to 50nm and lengths up to several micrometers, and
they are high-purity crystalline with
a
preferred
(100)
growth direction. It
should
also
be noted that GaN nanowires has to be grown with Fe as the
catalyst.71 However, no GaN nanowires were grown when gold was used
as a catalyst.70
NiO and FeO have
also
been reported to act
as
catalysts for the growth
of GaN nan~wires.~~ Solid gallium was reacted with ammonia at temper-
atures
of
920-940°C. The single crystal GaN nanowires have diameters
of
10-40
nm and
a
maximum length of
-500
p,m, with a preferential growth
direction
of
[OOl].
It is assumed that under the growth conditions,
NiO
and FeO were first reduced to metals and the metals reacted with gallium
to form liquid droplets permitting the growth of
GaN
nanowires via
VLS
method.
4.2.2.5.
SLS
growth
In general,
a
high temperature and a vacuum are required in the growth
of
nanowires by
VLS
method. An alternative method called solution-
liquid-solid
(SLS)
growth method was developed by Buhro’s research
and first applied for the synthesis
of
InP, InAs and GaAs
One-Dimensional Nanostructures: Nunowires
and
Nunorods
141
nanowires with solution-phase reactions at relative lower temperatures
(5203°C).
SLS
method is very similar to
VLS
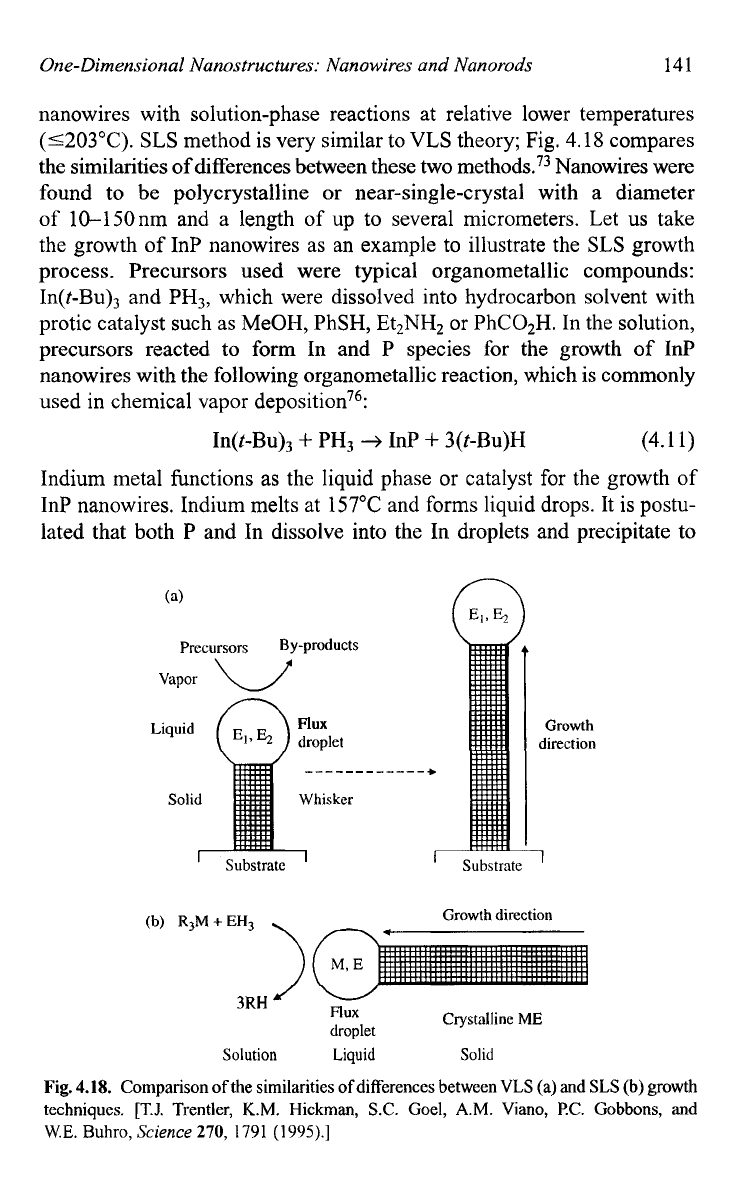
theory; Fig.
4.18
compares
the similarities
of
differences between these two methods.73 Nanowires were
found to
be
polycrystalline or near-single-crystal with a diameter
of 10-150nm and a length of up to several micrometers. Let us take
the growth
of
InP nanowires as an example to illustrate the
SLS
growth
process. Precursors used were typical organometallic compounds:
1n(t-B~)~ and PH3, which were dissolved into hydrocarbon solvent with
protic catalyst such as MeOH, PhSH, Et,NH, or PhC02H. In the solution,
precursors reacted to form In and P species for the growth
of
InP
nanowires with the following organometallic reaction, which is commonly
used in chemical vapor deposition7?
1n(t-B~)~
+
PH3
+
InP
+
3(t-Bu)H (4.1 1)
Indium metal functions as the liquid phase or catalyst for the growth
of
InP nanowires. Indium melts at
157°C
and forms liquid drops. It is postu-
lated that both
P
and In dissolve into the In droplets and precipitate to
Liquid
a
droplet
I
I
Substrate
'
Substrate
'
(b)
R3M+EH,
Growth
direction
JW
Flux
drop
1
e
t
Crystalline
ME
3RH
Solution Liquid
Solid
Fig.
4.18.
Comparison ofthe similarities of differences betwecn VLS
(a)
and
SLS
(b) growth
techniques. [T.J.
Trentler,
K.M.
Hickman,
S.C.
Goel,
A.M.
Viano, PC. Gobbons, and
W.E.
Buhro,
Science
270,
1791
(1995).]

142
Nunostructures
and
Nanomaterials
form nanowires of InP. The growth direction of InP nanowires was found
to be predominated by
(1 1
l), similar to that with
VLS
method.
Holmes
et
uZ.~~
used colloidal catalysts to control the diameters of sili-
con nanowires grown by solution-liquid-solid growth. Bulk quantities
of defect-free silicon nanowires with nearly uniform diameter ranging from
4
to
5
nm were grown to a length of several micrometers. Alkanethiol-cated
gold nanoclusters of
2.5
nm in diameter were used to direct the growth of
silicon nanowires in a solution heated and pressurized above its critical
point. The solution consisted of hexane and diphenysilane, as silicon
precursor, and were heated to
500°C
and pressurized at
200
or 270 bar.
Under the above growth conditions, the diphenysilane decomposes to
silicon atoms. Silicon atoms difise to and react with gold nanoclusters to
a silicon-gold alloy droplet. When silicon concentration reaches a super-
saturation, silicon precipitates out from the alloy droplets, resulting in the
formation of silicon nanowires. The supercritical conditions are required to
form the alloy droplets and to promote silicon crystallization. The growth
directions of silicon nanowires were found to be pressure-dependent. The
wires formed at
200
bar exhibited a preponderance of
[
1001 orientation,
whereas the samples synthesized at 270 bar oriented almost exclusively
along the [l
1
I] direction.
A
thin coating of oxide or hydrocarbon was
found on all the nanowires, though it was not possible to tell if the coatings
were formed during or after the growth. The diameter and length of
nanowires grown by
SLS
methods can be controlled by controlling the size
of liquid catalyst and the growth time, in the same way as that in
VLS
meth-
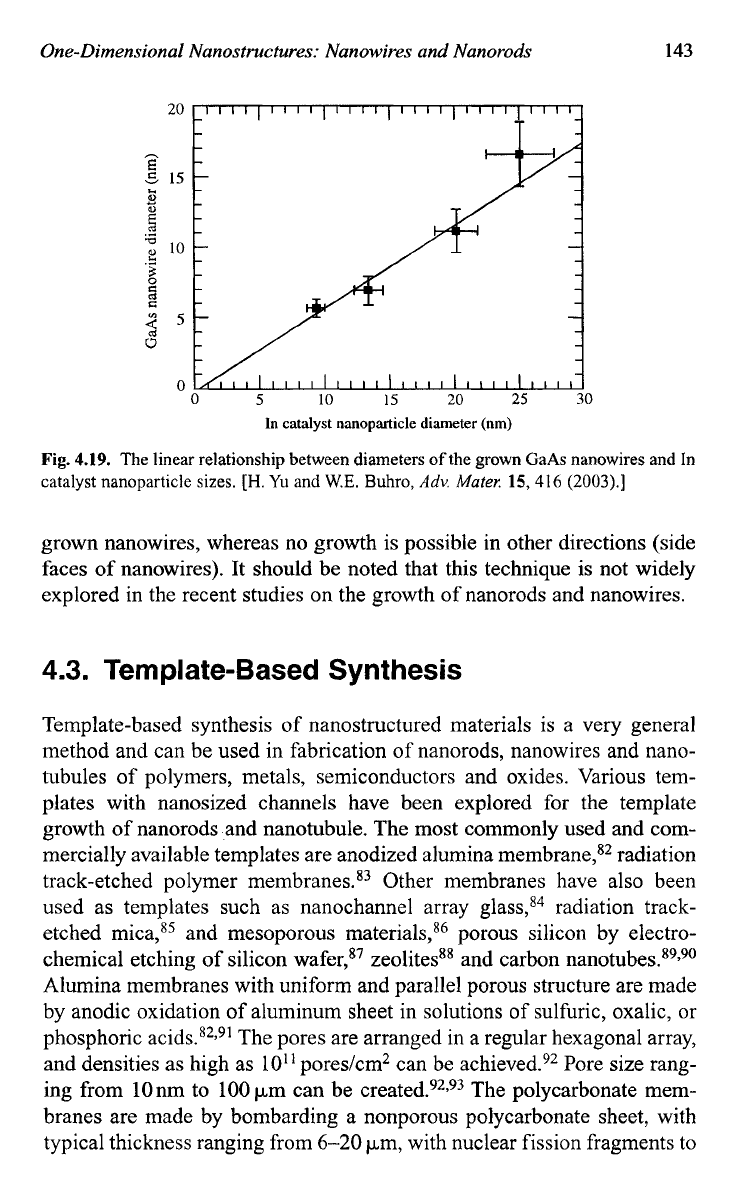
ods. Figure 4.19 shows the linear relationship between diameters of the
grown GaAs nanowires and In catalyst nanoparticle sizes.75
4.2.3.
Stress-induced recrystallization
It is worth noting that nanowires can be synthesized by stress-induced
recrystallization, though
it
has attracted little attention in the nanotech-
nology community. Application of pressure on solids at elevated tempera-
tures is known to result in the growth of whiskers or nanowires with
diameters as small as
50
nm.78 It was demonstrated that the growth rate
of
tin whiskers increased proportionally with the applied pressure78 and
could be four orders of magnitude when a pressure of 7,500psi was
applied.79 The growth of such nanowires or whiskers is based on a dislo-
cation at the base of the whisker80 and the growth proceeds from the base
and not from the tip.81 The formation of metallic nanorods is likely due to
the confined growth at the surface between the metallic film and the
One-Dimensional Nanostructures: Nanowires and Nanorods
143
/
1
0
5
10
15
20
25
30
In
catalyst nanoparticle diameter (nm)
Fig.
4.19.
The
linear relationship between diameters
of
the grown GaAs nanowires and In
catalyst nanoparticle
sizes.
[H.
Yu
and
W.E.
Buhro,
Adv.
Muter.
15,416
(2003).]
grown nanowires, whereas no growth is possible in other directions (side
faces of nanowires). It should be noted that this technique is not widely
explored in the recent studies on the growth
of
nanorods and nanowires.
4.3.
Tern plate-Based Synthesis
Template-based synthesis
of
nanostructured materials is a very general
method and can be used in fabrication of nanorods, nanowires and nano-
tubules of polymers, metals, semiconductors and oxides. Various tem-
plates with nanosized channels have been explored for the template
growth of nanorods and nanotubule. The most commonly used and com-
mercially available templates are anodized alumina membrane,82 radiation
track-etched polymer mernbrane~.~~ Other membranes have also been
used as templates such as nanochannel array glass,s4 radiation track-
etched mica,85 and mesoporous materials,86 porous silicon by electro-
chemical etching of silicon wafer,87 zeolites88 and carbon nanot~bes.
Alumina membranes with uniform and parallel porous structure are made
by anodic oxidation of aluminum sheet in solutions of sulfuric, oxalic, or
phosphoric
acid^.^^>^'
The pores are arranged in a regular hexagonal array,
and densities as high as
10'
pores/cm2 can be achieved.92 Pore size rang-
ing from lOnm to 100 pm can be ~reated.~~,~~ The polycarbonate mem-
branes are made by bombarding a nonporous polycarbonate sheet, with
typical thickness ranging from
6-20
pm, with nuclear fission fragments to

144
Nunostructures
and
Nanomaterials
create damage tracks, and then chemically etching these tracks into
pores.83 In radiation track etched membranes, pores have a uniform size
as small as
10
nm, though randomly distributed. Pore densities can be as
high as
lo9
pores/cm2.
In addition to the desired pore or channel size, morphology, size distri-
bution and density of pores, template materials must meet certain require-
ments. First, the template materials must be compatible with the processing
conditions. For example, an electrical insulator is required for a template to
be used in electrochemical deposition. Except for the template directed
synthesis, template materials should be chemically and thermally inert
during the synthesis. Secondly, depositing materials or solution must wet
the internal pore walls. Thirdly, for the synthesis of nanorods or nanowires,
the deposition should start from the bottom or one end of the template
channels and proceed from one side to another. However, for the growth of
nanotubules, the deposition should start from the pore wall and proceed
inwardly. Inward growth may result in the pore blockage,
so
that should be
avoided in the growth of “solid” nanorods or nanowires. Kinetically,
enough surface relaxation permits maximal packing density,
so
a diffusion-
limited process is preferred. Other considerations include the easiness of
release of nanowires or nanorods from the templates and of handling
during the experiments.
4.3.1.
Electrochemical deposition
Electrochemical deposition, also known as electrodeposition, can be under-
stood as a special electrolysis resulting in the deposition
of
solid material
on an electrode. This process involves (i) oriented diffusion
of
charged
growth species (typically positively charged cations) through a solution
when an external electric field is applied, and (ii) reduction of the charged
growth species at the growth or deposition surface which
also
serves as an
electrode. In general, electrochemical deposition
is
only applicable to elec-
trical conductive materials such as metals, alloys, semiconductors and elec-
trical conductive polymers, since after the initial deposition, the electrode
is separated from the depositing solution by the deposit and the electrical
current must go through the deposit to allow the deposition process to con-
tinue. Electrochemical deposition is widely used in making metallic coat-
ings; the process
is
also known
as
ele~troplating.~~ When deposition
is
confined inside the pores of template membranes, nanocomposites are pro-
duced. If the template membrane is removed, nanorods or nanowires are
prepared. Let us briefly review the hndamentals of electrochemistry,
One-Dimensional Nanostructures: Nanowires and Nanorods
145
before we get into detailed discussion of the growth of nanorods by electro-
chemical deposition.
In Chapter
2,
we have discussed the electrical properties of a solid sur-
face. When a solid immerses in a polar solvent or an electrolyte solution,
surface charge will be developed. At an interface between an electrode and
an electrolyte solution, a surface oxidation or reduction reaction occurs,
accompanied with charge transfer across the interface, until equilibrium is
reached. For a given system, the electrode potential or surface charge den-
sity,
E,
is described by the Nernst equation:
RT
n,F
E
=
Eo
+
-1n(ai)
(4.12)
Where
Eo
is the standard electrode potential, or the potential difference
between the electrode and the solution, when the activity,
ai,
of the ions is
unity,
F,
the Faraday’s constant,
R,,
the gas constant and
T,
temperature.
When the electrode potential
is
more negative (higher) than the energy
level of vacant molecular orbital in the electrolyte solution, electrons will
transfer from the electrode to the solution, accompanied with dissolution
or reduction of electrode as shown in Fig. 4.20(a).95 If the electrode poten-
tial is more positive (lower) than the energy level of the occupied molec-
ular orbital, the electrons will transfer from the electrolyte solution to
the electrode, and the deposition or oxidation of electrolyte ions on the
electrode will proceed simultaneously as illustrated in Fig.
4.20(b).95
The
reactions stop when equilibrium is achieved.
When
two
electrodes of different materials immerse into one electrolyte
solution, each electrode will establish equilibrium with the electrolyte solu-
tion. Such equilibrium will be destroyed, if
two
electrodes are connected with
an external circuit. Since different electrodes have different electrode poten-
tials, this difference in electrode potential would drive electrons migration
from the electrode with a higher electrode potential to the lower one. Let us
take the Cu and Zn electrodes immersed in
an
aqueous solution as
an
exam-
ple to illustrate the electrochemical process.96 Assuming both activities
of
copper and zinc ions in the aqueous solution are unity in the beginning, the
copper electrode has a more positive electrode potential (0.34
V)
than that of
the zinc electrode
(-0.76V).
In the external circuit, electrons flow from the
more negative electrode (Zn) to the more positive electrode (Cu). At the zinc-
solution interface, the following electrochemical reactions take place:
Zn
+
Zn2+
+
2e-
(4.13)
This reaction generates electrons at the interface, which then flow through
the external circuit to another electrode
(Cu).
At the same time, Zn continues
