Guozhong Cao. Nanostructures & Nanomaterials: Synthesis, Properties & Applications
Подождите немного. Документ загружается.

126
Nanostructures and Nanomaterials
the
[OOOl]
direction (that is, along the c-axis), with diameters of about
266 nm and a length of
-3
pm.
Nanowires can also be grown using the same methods commonly used
for the synthesis of nanocrystals, i.e. by decomposing of organometallic
compounds in the presence of coordinating organics. For example, Urban
and co-w~rkers~~~~~ synthesized single crystal BaTiO, nanowires with diam-
eters ranging from
5
to
70
nm and lengths up to
>
10
km, by solution-phase
decomposition of barium titanium isopropoxide, BaTi[OCH(CH,),],. In a
typical reaction, an excess of
30%
H202
was added at 100°C to a heptade-
cane solution containing a 10
:
1 molar ratio of BaTi[OCH(CH3)2]6 to oleic
acid. The reaction mixture was then heated to 280°C for 6 hrs, resulting in
a white precipitate composed of nanowire aggregates. Well-isolated
nanowires were obtained by sonication and fractionation between water and
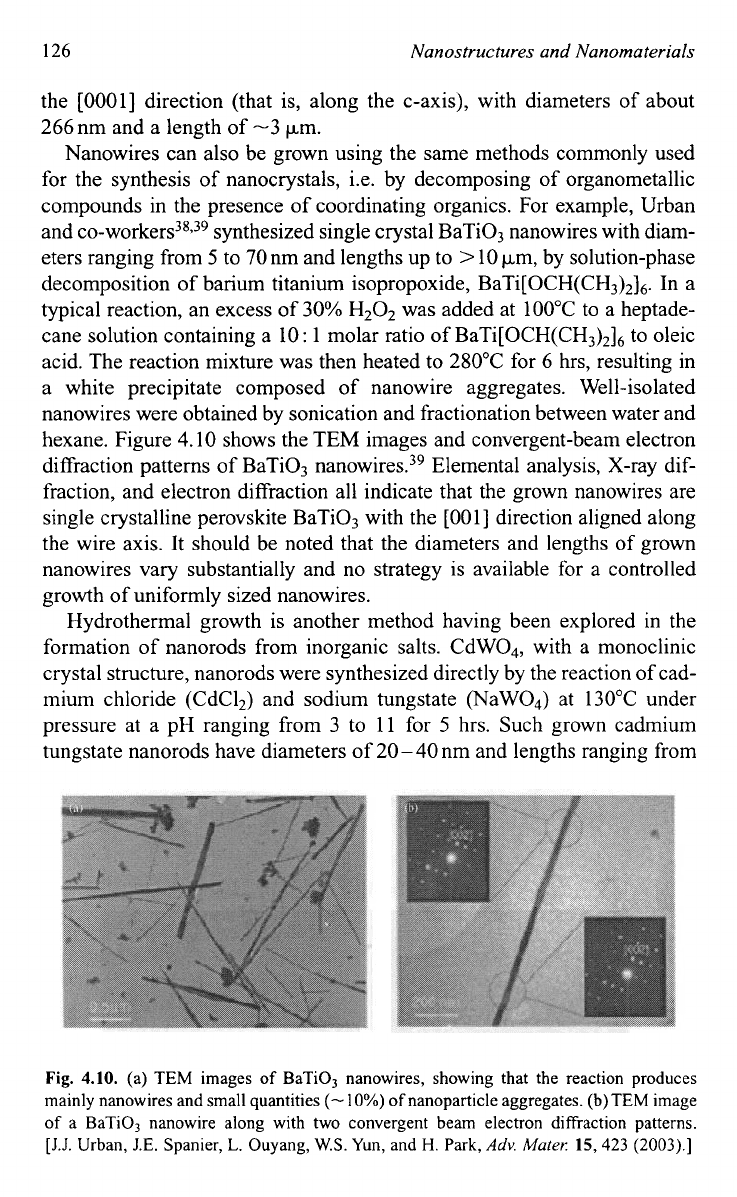
hexane. Figure 4.10 shows the TEM images and convergent-beam electron
diffraction patterns of BaTi03 nan~wires.,~ Elemental analysis, X-ray dif-
fraction, and electron diffraction all indicate that the grown nanowires are
single crystalline perovskite BaTiO, with the
[00
11
direction aligned along
the wire axis. It should be noted that the diameters and lengths of grown
nanowires vary substantially and no strategy is available for a controlled
growth of uniformly sized nanowires.
Hydrothermal growth is another method having been explored in the
formation of nanorods from inorganic salts. CdW04, with
a
monoclinic
crystal structure, nanorods were synthesized directly by the reaction of cad-
mium chloride (CdC12) and sodium tungstate (NaW04) at 130°C under
pressure at a pH ranging from
3
to 11 for
5
hrs. Such grown cadmium
tungstate nanorods have diameters of 20 -40 nm and lengths ranging from
Fig.
4.10. (a) TEM images
of
BaTi03 nanowires, showing that the reaction produces
mainly nanowires and small quantities
(-
10%)
of
nanoparticle aggregates.
(b)
TEM
image
of
a BaTiO, nanowire along with two convergent beam electron diffraction patterns.
[J.J.
Urban,
J.E.
Spanier,
L.
Ouyang,
W.S.
Yun, and
H.
Park,
Adv.
Muter:
15,423 (2003).]

One-Dimensional Nanostructures: Nanowires and Nanorods
127
80
to 280nm.40 The growth of nanorods is attributed to anisotropic growth,
though no specific growth direction was identified. Nanotubes of H2Ti307
have been hydrothermally synthesized from Ti02 powder dissolved in a
NaOH aqueous solution at
130°C
by Chen
et
aL41
The synthesized prod-
ucts were hollow tubes about 9nm in diameter, with lengths from
100
to
several hundred nanometers.
Nanowires or nanorods by the evaporation (dissolution)-condensation
deposition most likely have faceted morphology and are generally short in
length with relatively small aspect ratios, particularly when grown in liquid
medium. However, anisotropic growth induced by axial imperfections, such
as screw dislocation, microtwins and stacking faults, or by impurity poi-
soning, can result in the growth of nanowires with very large aspect ratios.
4.2.2.
Vapor (or
solution)-liquid-solid
(VLS or
SLS)
growth
4.2.2.1,
Fundamental
aspects
of
VLS
and
SLS growth
In the
VLS
growth, a second phase material, commonly referred to as
either impurity or catalyst, is purposely introduced to direct and confine
the crystal growth on to a specific orientation and within a confined area.
A
catalyst forms
a
liquid droplet by itself or by alloying with growth mate-
rial during the growth, which acts as a trap of growth species. Enriched
growth species in the catalyst droplets subsequently precipitates at the
growth surface resulting in the one-directional growth. Wagner
et
al.42,43
first proposed the
VLS
theory over
40
years ago to explain the experi-
mental results and observations in the growth of silicon nanowires or
whiskers that could not be explained by the evaporation-condensation
theory. These phenomena include:
(1)
There are no screw dislocations or other imperfections along the
(2)
The growth direction
(1
1
1)
is the slowest as compared with other low
(3)
Impurities are always required and
(4)
A
liquid-like globule is always found in the tip of nanowires.
Wagner44 summarized the experimental details, results, and the
VLS
the-
ory in a truly elegant way in a classical paper, and Givargizov' further elab-
orated the experimental observations and models and theories developed
regarding the
VLS
process. The readers who want to learn more about this
growth direction.
index directions such as
(1
10)
in silicon.

128
Nanostructures and Nunomaterials
subject are strongly recommended to read those literatures. Although an
extensive research in this field has been carried out in the recent years,
fundamentals of the
VLS
method have not been changed significantly.
Wagner44 summarized the requirements for the
VLS
growth
30
years ago,
which are still valid in today’s understanding:
(1)
The catalyst or impurity must form a liquid solution with the crys-
talline material to be grown at the deposition temperature,
(2)
The distribution coefficient
of
the catalyst or impurity must be less
than unity at the deposition temperature.
(3)
The equilibrium vapor pressure of the catalyst or impurity over the
liquid droplet must be very small. Although the evaporation of the cat-
alyst does not change the composition of the saturated liquid compo-
sition,
it
does reduce the total volume of the liquid droplet. Unless
more catalyst is supplied, the volume of the liquid droplet reduces.
Consequently, the diameter of the nanowire will reduce and the
growth will eventually stop, when all the catalyst
is
evaporated.
(4)
The catalyst or impurity must be inert chemically. It must not react
with the chemical species such as by-products presented in the growth
chamber.
(5)
The interfacial energy plays a very important role. The wetting char-
acteristics influence the diameter of the grown nanowire. For a given
volume of liquid droplet, a small wetting angle results in a large
growth area, leading to a large diameter of nanowires.
(6)
For a compound nanowire growth, one
of
the constituents can serve
as
the catalyst.
(7)
For controlled unidirectional growth, the solid-liquid interface must
be well defined crystallographically. One of the simplest methods is
to choose a single crystal substrate with desired crystal orientation.
In a
VLS
growth, the process can be simply described as following as
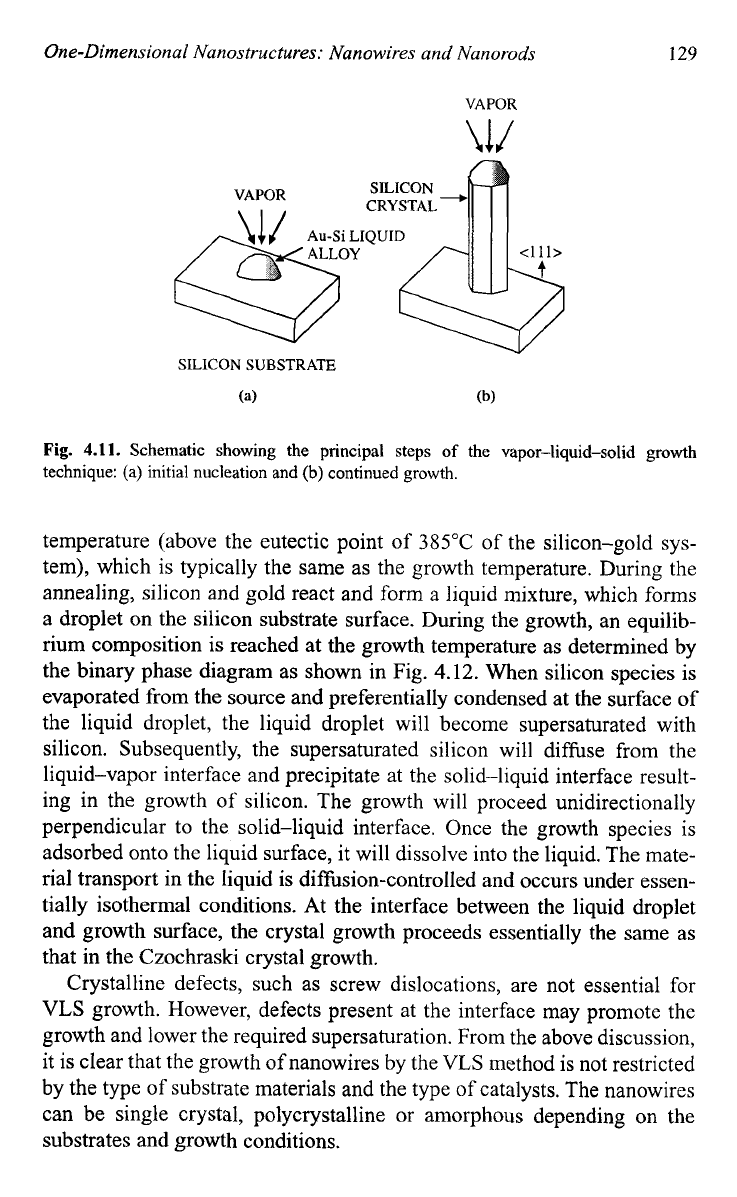
sketched in Fig.
4.1
1.
The growth species is evaporated first, and then dif-
fuses and dissolves into a liquid droplet. The surface of the liquid has a
large accommodation coefficient, and is therefore a preferred site for dep-
osition. Saturated growth species in the liquid droplet will difhse to and
precipitate at the interface between the substrate and the liquid. The pre-
cipitation will first follow nucleation and then crystal growth. Continued
precipitation or growth will separate the substrate and the liquid droplet,
resulting in the growth of nanowires.
Let us take the growth of silicon nanowires with gold as a catalyst as an
example to illustrate the experimental process of the
VLS
growth.
A
thin
layer of gold
is
sputtered on a silicon substrate and annealed at an elevated
One-Dimensional Nanostructures: Nanowires and Nanorods
I29
VAPOR
SILICON
CRYSTAL-
xT4POR
I/
.n
h
\
14
4
Au-Si
LIQUID
/
%ALLOY
SILICON
SUBSTRATE
(a)
Fig.
4.1
1.
Schematic showing the principal steps
of
the vapor-liquid-solid growth
technique:
(a)
initial nucleation and
(b)
continued growth.
temperature (above the eutectic point of
385°C
of
the silicon-gold sys-
tem), which is typically the same as the growth temperature. During the
annealing, silicon and gold react and form a liquid mixture, which forms
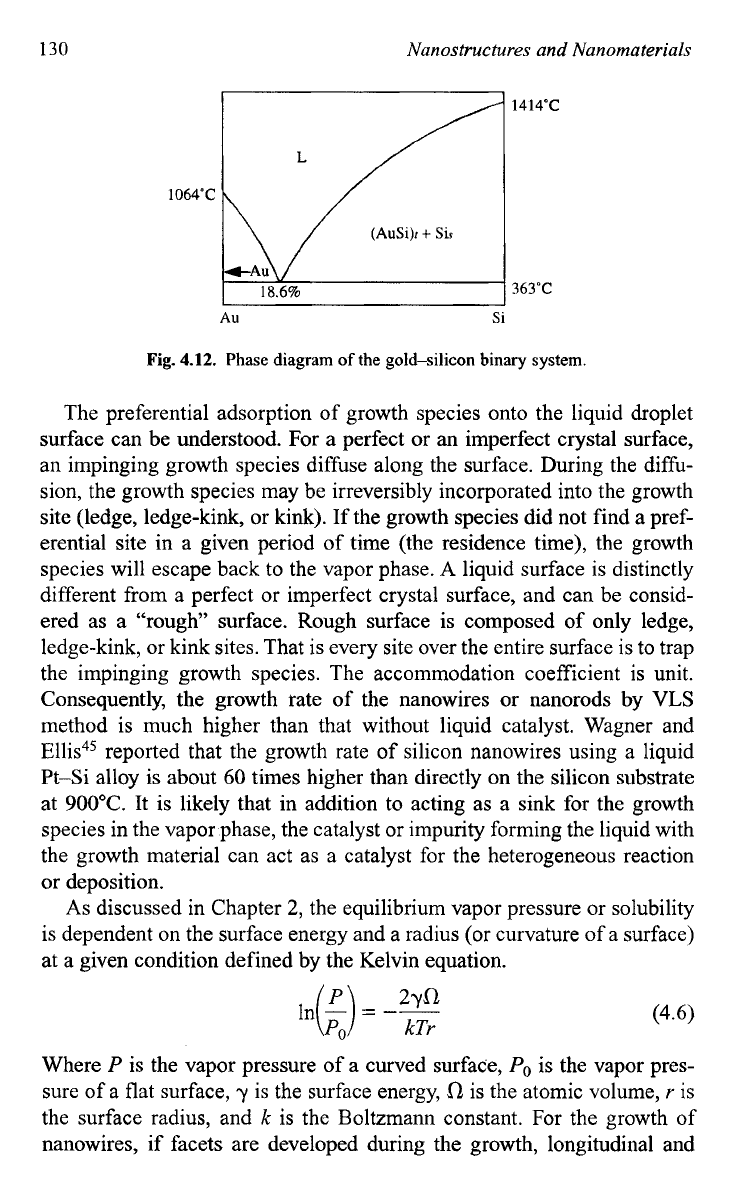
a droplet on the silicon substrate surface. During the growth, an equilib-
rium composition
is
reached at the growth temperature as determined by
the binary phase diagram as shown in Fig.
4.12.
When silicon species is
evaporated from the source and preferentially condensed at the surface of
the liquid droplet, the liquid droplet will become supersaturated with
silicon. Subsequently, the supersaturated silicon will diffuse from the
liquid-vapor interface and precipitate at the solid-liquid interface result-
ing in the growth of silicon. The growth will proceed unidirectionally
perpendicular to the solid-liquid interface. Once the growth species is
adsorbed onto the liquid surface, it will dissolve into the liquid. The mate-
rial transport in the liquid is diffusion-controlled and occurs under essen-
tially isothermal conditions. At the interface between the liquid droplet
and growth surface, the crystal growth proceeds essentially the same as
that in the Czochraski crystal growth.
Crystalline defects, such as screw dislocations, are not essential for
VLS
growth. However, defects present at the interface may promote the
growth and lower the required supersaturation. From the above discussion,
it is clear that the growth
of
nanowires
by
the
VLS
method is not restricted
by the type of substrate materials and the type
of
catalysts. The nanowires
can be single crystal, polycrystalline or amorphous depending on the
substrates and growth conditions.
130
Nanostructures and Nanomaterials
1414°C
363°C
Au
Si
1064‘C
Fig.
4.12.
Phase
diagram
of
the gold-silicon binary system
The preferential adsorption of growth species onto the liquid droplet
surface can be understood. For a perfect or an imperfect crystal surface,
an impinging growth species diffuse along the surface. During the diffu-
sion, the growth species may be irreversibly incorporated into the growth
site (ledge, ledge-kink, or kink). If the growth species did not find a pref-
erential site in a given period of time (the residence time), the growth
species will escape back to the vapor phase.
A liquid surface
is
distinctly
different from a perfect or imperfect crystal surface, and can be consid-
ered as a “rough” surface. Rough surface is composed
of
only ledge,
ledge-kink, or kink sites. That is every site over the entire surface is to trap
the impinging growth species. The accommodation coefficient is unit.
Consequently, the growth rate of the nanowires or nanorods by
VLS
method is much higher than that without liquid catalyst. Wagner and
Ellis45 reported that the growth rate of silicon nanowires using
a
liquid
Pt-Si alloy is about
60
times higher than directly on the silicon substrate
at 900°C. It is likely that in addition to acting as a sink for the growth
species in the vapor phase, the catalyst or impurity forming the liquid with
the growth material can act as
a
catalyst for the heterogeneous reaction
or deposition.
As
discussed in Chapter
2,
the equilibrium vapor pressure or solubility
is
dependent on the surface energy and a radius (or curvature of a surface)
at a given condition defined by the Kelvin equation.
(4.6)
Where
P
is the vapor pressure
of
a curved surface,
Po
is the vapor pres-
sure of a flat surface,
y
is the surface energy, is the atomic volume,
Y
is
the surface radius, and
k
is
the Boltzmann constant. For the growth
of
nanowires, if facets are developed during the growth, longitudinal and

One-Dimensional Nanostructures: Nanowires and Nanorods
131
lateral growth rates of nanowires or nanorods will be solely determined
by the growth behavior of individual facets. However, if nanowires were
cylindrical in shape, the lateral growth rate would be significantly smaller
than the longitudinal one, assuming all surfaces have the same surface
energy. Convex surface (the side surface) with very small radius
(<
100
nm) would have a significantly higher vapor pressure as compared
with that of a flat growing surface.
A
supersaturated vapor pressure or
concentration of the growth species for the growing surface may be well
below the equilibrium vapor pressure of the convex surface of thin
nanowires. For the growth of uniform high quality crystalline nanowires
or nanorods, in general supersaturation should be kept relatively low,
so
that there would be no growth on the side surface.
A
high supersaturation
would result in growth of other facets, just as in the vapor-solid growth
discussed before. Further high supersaturation would lead to secondary
nucleation on the growth surface or homogeneous nucleation, resulting in
termination of epitaxial growth.
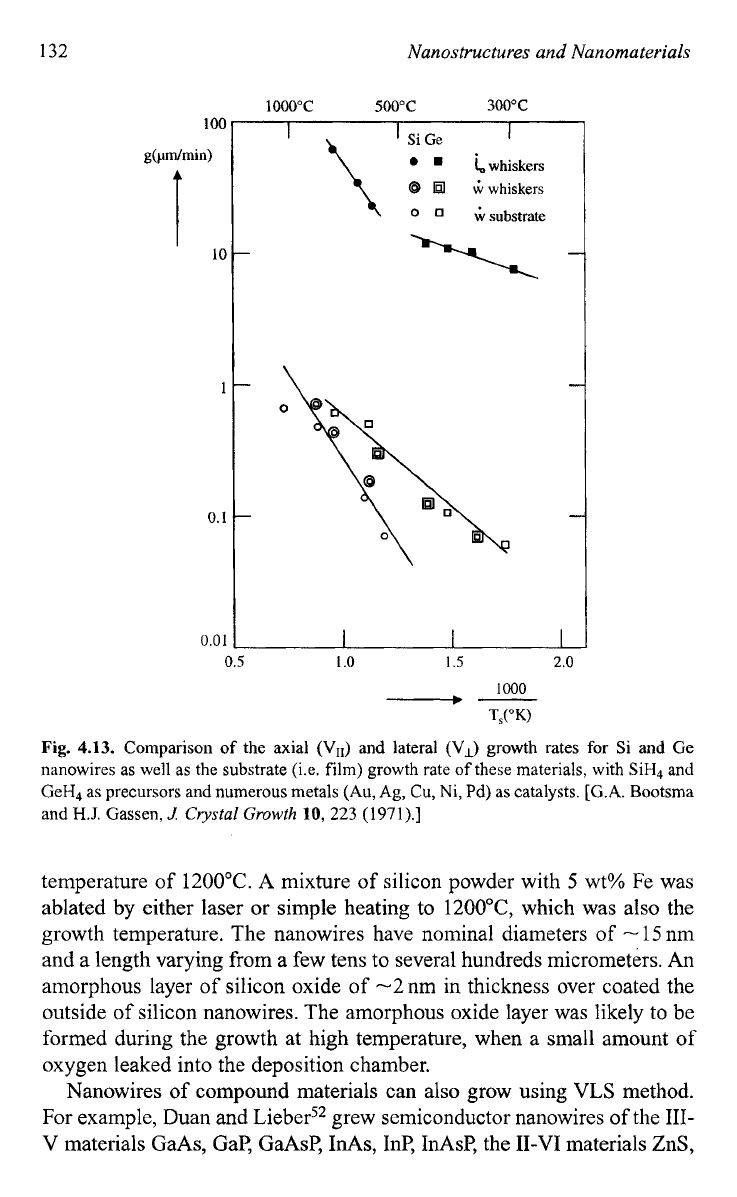
Figure
4.13
compared the axial (VII) and lateral
(V,}
growth rates for
Si and Ge nanowires as well as the substrate (i.e. film) growth rate of these
materials, with
SiH4
and GeH4 as precursors and numerous metals (Au,
Ag, Cu, Ni, Pd) as catalysts.46 This figure indicates that the lateral and
the substrate growth rates are essentially the same, whereas the axial rate
by the VLS process for both Si and Ge nanowires, are approximately
two orders of magnitude higher than the
VS
growth rates under the same
conditions.
The enhanced growth rate can also be partly due to the fact that the
condensation surface area for the growth species in the VLS growth is
larger than the surface area of the crystal growth. While the growth sur-
face is the interface between the liquid droplet and the solid surface, the
condensation surface is the interface between the liquid droplet and vapor
phase. Depending on the contact angle, the liquid surface area can be sev-
eral times of the growth surface.
4.2.2.2.
VLS
growth
of
various nanowires
Growth of elementary Si and Ge nanowires has been well established.4749
Figure
4.14
shows typical Si nanowires grown by the VLS method.48
Although gold was initially used for the growth of silicon nanowires with
the VLS method, other catalysts have been found to be effective in the
formation of nanowires of various materials. For example, Si nanowires
can be synthesized using Fe as a cataly~t~~~~' at a relatively high growth
132
Nanostructures
and
Nanomaterials
100
g(pm/rnin)
T
10
1
0.
I
0.01
i
1
ooooc
500°C
3009c
1
'
SiGe
I
-\
0
-
I
I
1
I
1
.o
1.5
2.0
Fig.
4.13.
Comparison
of
the axial
(VII)
and lateral
(V,)
growth rates for Si and
Ge
nanowires as
well
as the substrate (is.
film)
growth rate
of
these materials, with SiH4 and
CeH4 as precursors and numerous metals
(Au,
Ag,
Cu,
Ni, Pd) as catalysts.
[G.A.
Bootsma
and
H.J.
Gassen,
J:
Cvystal Growth
10,
223
(1971).]
temperature
of
1200°C.
A
mixture
of
silicon powder with
5
wt% Fe was
ablated by either laser or simple heating to
1200"C,
which was also the
growth temperature. The nanowires have nominal diameters of
-
15
nm
and a length varying from a few tens to several hundreds micrometers. An
amorphous layer of silicon oxide
of
-2nm in thickness over coated the
outside
of
silicon nanowires. The amorphous oxide layer was likely to be
formed during the growth at high temperature, when a small amount
of
oxygen leaked into the deposition chamber.
Nanowires of compound materials can also grow using
VLS
method.
For example, Duan and Lieber5* grew semiconductor nanowires
of
the III-
V
materials GaAs, GaP, GaAsP,
Ids,
InP, InAsP, the 11-VI materials ZnS,
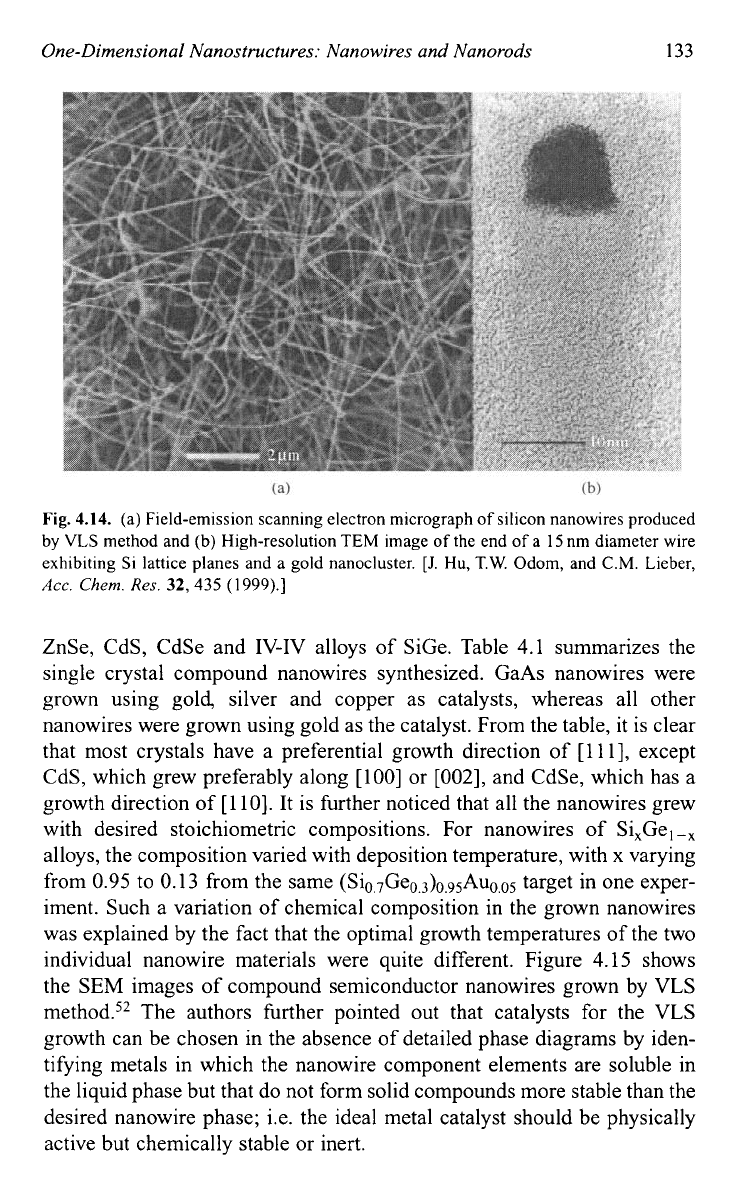
One-Dimensional Nanostructures: Nanowires and Nanorods 133
Fig.
4.14.
(a) Field-emission scanning electron micrograph
of
silicon nanowires produced
by
VLS
method and (b) High-resolution
TEM
image
of
the end
of
a
15
nm diameter wire
exhibiting Si lattice planes and a gold nanocluster.
[J.
Hu,
T.W.
Odom, and
C.M.
Lieber,
Acc.
Chem.
Res.
32,435
(1
999).]
ZnSe, CdS, CdSe and IV-IV alloys of SiGe. Table 4.1 summarizes the
single crystal compound nanowires synthesized. GaAs nanowires were
grown using gold, silver and copper as catalysts, whereas all other
nanowires were grown using gold as the catalyst. From the table,
it
is clear
that most crystals have a preferential growth direction of
[
1
1
11,
except
CdS, which grew preferably along
[loo]
or
[002],
and CdSe, which has a
growth direction of
[
1
101.
It
is further noticed that all the nanowires grew
with desired stoichiometric compositions. For nanowires of %,Gel
--x
alloys, the composition varied with deposition temperature, with x varying
from
0.95
to 0.13 from the same (Sio,,Geo~3)o~95Auo,05 target in one exper-
iment. Such a variation of chemical composition in the grown nanowires
was explained by the fact that the optimal growth temperatures of the two
individual nanowire materials were quite different. Figure 4.15 shows
the
SEM
images of compound semiconductor nanowires grown by VLS
method.52 The authors further pointed out that catalysts for the VLS
growth can be chosen in the absence
of
detailed phase diagrams by iden-
tifying metals in which the nanowire component elements are soluble in
the liquid phase but that do not form solid compounds more stable than the
desired nanowire phase; i.e. the ideal metal catalyst should be physically
active but chemically stable or inert.
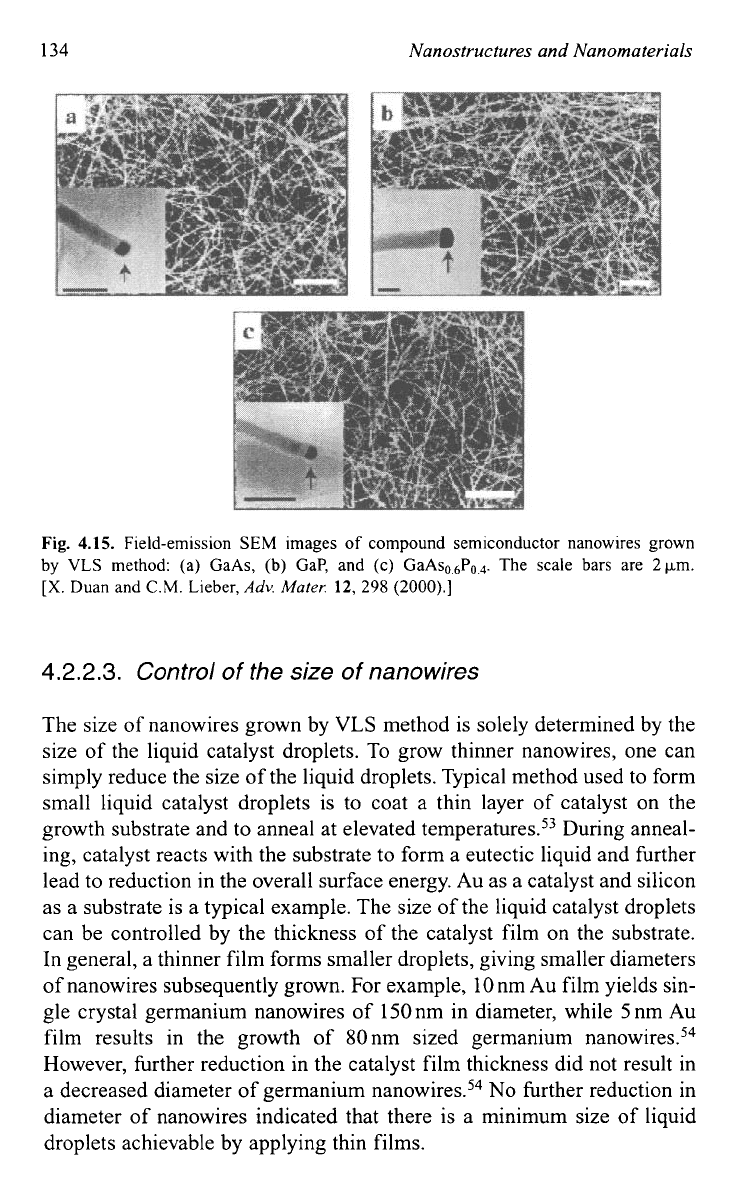
134
Nanostructures and Nanomaterials
Fig.
4.15.
Field-emission
SEM
images
of
compound semiconductor nanowires grown
by
VLS
method: (a) GaAs,
(b)
Gap, and
(c)
GaAso,6Po,4. The scale bars are
2km.
[X.
Duan and
C.M.
Lieber,
Adv.
Muter.
12,
298 (2000).]
4.2.2.3.
Control
of
the size
of
nanowires
The size of nanowires grown by
VLS
method is solely determined by the
size of the liquid catalyst droplets.
To
grow thinner nanowires, one can
simply reduce the size of the liquid droplets. Typical method used to form
small liquid catalyst droplets is to coat a thin layer of catalyst on the
growth substrate and to anneal at elevated
temperature^.^^
During anneal-
ing, catalyst reacts with the substrate to form a eutectic liquid and further
lead to reduction in the overall surface energy. Au as a catalyst and silicon
as a substrate is a typical example. The size of the liquid catalyst droplets
can be controlled by the thickness of the catalyst film on the substrate.
In general, a thinner film forms smaller droplets, giving smaller diameters
of nanowires subsequently grown. For example,
10
nm Au film yields sin-
gle crystal germanium nanowires of
150
nm in diameter, while
5
nm Au
film results in the growth of 80nm sized germanium nan~wires
However, hrther reduction in the catalyst film thickness did not result in
a decreased diameter of germanium nan~wires.~~
No
further reduction in
diameter of nanowires indicated that there is a minimum size of liquid
droplets achievable by applying thin films.
One-Dimensional Nanostructures: Nanowires and Nunorods
135
Further reduction of diameters of nanowires could be achieved by
dispersing monosized catalyst colloids on the substrate surface, instead
of a thin film of ~atalyst.~~,~~ GaP nanowires were grown
by
laser catalytic
growth synthetic method49 using gold colloids.56 Gold colloids or nano-
clusters were supported on a silica substrate and the reactants Ga and P
were generated from a solid target of GaP by laser ablation. Single crystal
GaP nanowires show a growth direction of
[
11 11 and have a stoichiomet-
ric composition of 1
:
0.94 confirmed by EDAX. The diameters of GaP
nanowires were determined by the size of the catalyst gold nanoclusters.
GaP nanowires grown from
8.4,
18.5
and 28.2nm diameter gold colloids
were found to
be
1
1.4,20
and
30.2
nm, respectively. Similar technique was
applied to the growth of InP nan~wires.~~ The growth substrate tempera-
ture was controlled to be approximately 500-6OO0C, and a constant flow
of Ar at 100 standard cubic centimeter per minute under a pressure of
200 torr was maintained during the growth. The laser for ablation used was
an ArF excimer laser with a wavelength of 193 nm. InP nanowires were
found to be single crystal and grew along the
[
1 1
11
direction. Figure 4.16
shows the general concepts of control of the diameters and length of
nanowires grown by growth time and the size
of
catalyst colloids.55
Detailed analysis further revealed an amorphous oxide layer of
2-4
nm in
thickness presented on all nanowires. The existence of an amorphous
oxide layer was explained by the overgrowth of an amorphous InP on the
side faces and subsequent oxidation after the samples were exposed to air.
The overgrowth on side faces is not catalyst activated and implies super-
saturated vapor concentrations of growth constituents in the system.
Since the diameters of nanowires grown by
VLS
method is solely con-
trolled by the size of the liquid catalyst droplets, thinner wires can be
grown by using smaller liquid droplets. However, this approach has its
limit. From
Eq.
(4.6), we already know that the equilibrium vapor pressure
of a solid surface is dependent on the surface curvature. The same depend-
ence is found for a solubility of a solute in a solvent.
As
the size of the
droplets was reduced, the solubility would increase. For the growth of
very thin nanowires, a very small droplet is required. However, a convex
surface with a very small radius would have a very high solubility. As a
result, a high supersaturation in the vapor phase has to be generated.
A
high supersaturation in the vapor phase may promote the lateral growth
on the side surface of nanowires with the vapor-solid mechanism.
Therefore, a conical structure may be developed instead of uniformly
sized nanowires. Further, a high supersaturation may initiate homoge-
neous nucleation in the gas phase or secondary nucleation at the surface
of nanowires.
