Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.

72 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
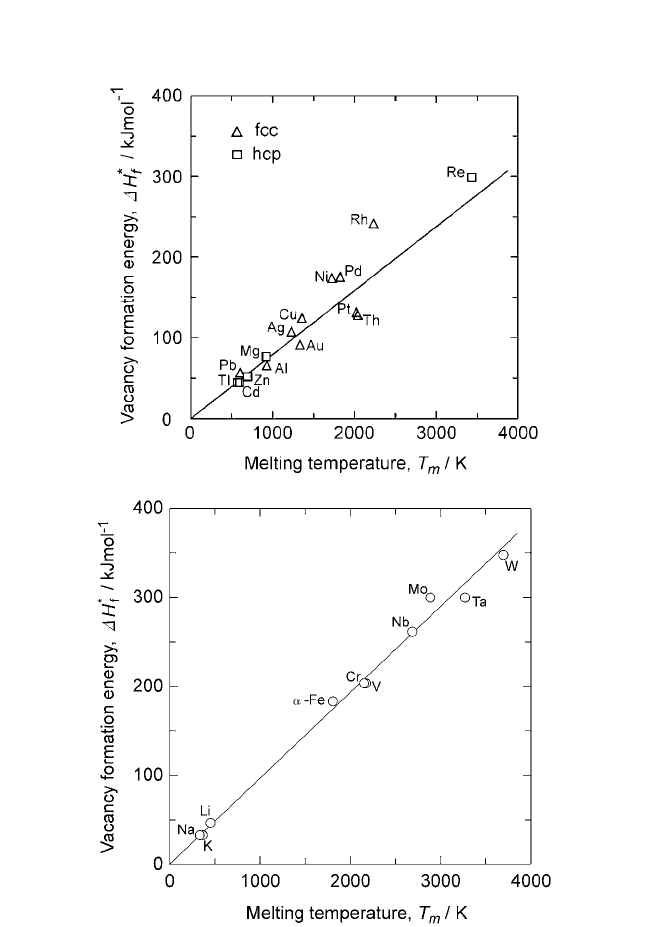
Figure 2.2. (a) Linear correlation between the vacancy formation energy and melt-
ing temperature for FCC and HCP close-packed metals;slope 80.2 J mol
1
K
1
.
(b) Linear correlation between the vacancy formation energy and melting temper-
ature for BCC metals; slope 97 J mol
1
K
1
.
(a)
(b)
Notes: 1. Energies for vacancy formation were obtained from Atomic Defects in Metals, vol. 25
(H. Ullmaier, ed.), Landolt-Börnstein Series, Springer-Verlag (1991).
2. Melting temperatures were obtained from Thermochemical Data of Pure Substances,
vols. I and II (Ihsan Barin, ed.), VCH, Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 72

SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.73
In vacancy formation, the coordination number decides the number of
bonds that are broken. The data points for FCC and HCPmetals lie on the
same line because the coordination number is same in either case.
Equations (3) and (4) also provide an example to show that the propor-
tionality constant between the diffusion parameter and the bulk properties
depends on the crystal structure.
The data on ∆H
f
*
plotted in Fig. 2.2(a) and (b) are listed in Tables 2.1
and 2.2. The tables also give the vacancy migration energy, ∆H
*
m
, as well
as the ratio between the two quantities. Several interesting facts emerge
out of the data in the two tables:
• The vacancy formation energies are generally higher than the
migration energies. However, the ratio of vacancy formation
to the migration energy shows significant variations from
metal to metal. Despite this, the correlation with the melting
point is very well maintained. It shows that the subtle differ-
ences in the process of diffusion itself among different met-
als do not affect the relationship between the melting point
and the vacancy formation energy.
• The ratio of vacancy formation energy to the migration
energy in FCC and HCP lattices, in comparison to BCC, is
generally smaller.
• The same feature is seen in the ratio of vacancy formation
energy to the melting point; namely, Eqs. (3) and (4). The
proportionality constant for closed packed lattices is smaller,
indicating that the vacancy formation in these cases is ener-
getically easier than the BCC structures.
• The effect of electronic band structure on the ratio of
vacancy formation energy to the migration energy can also
be discerned from Tables 2.1 and 2.2. This ratio is higher for
normal metals, where s and d bands are separated. This rule
is followed without exception by BCC metals. The FCC and
HCP metals show the same trend. However, nickel is one
exception for which the ratio ∆H
f
*
∆H
m
*
is nearly the same as
that for copper.
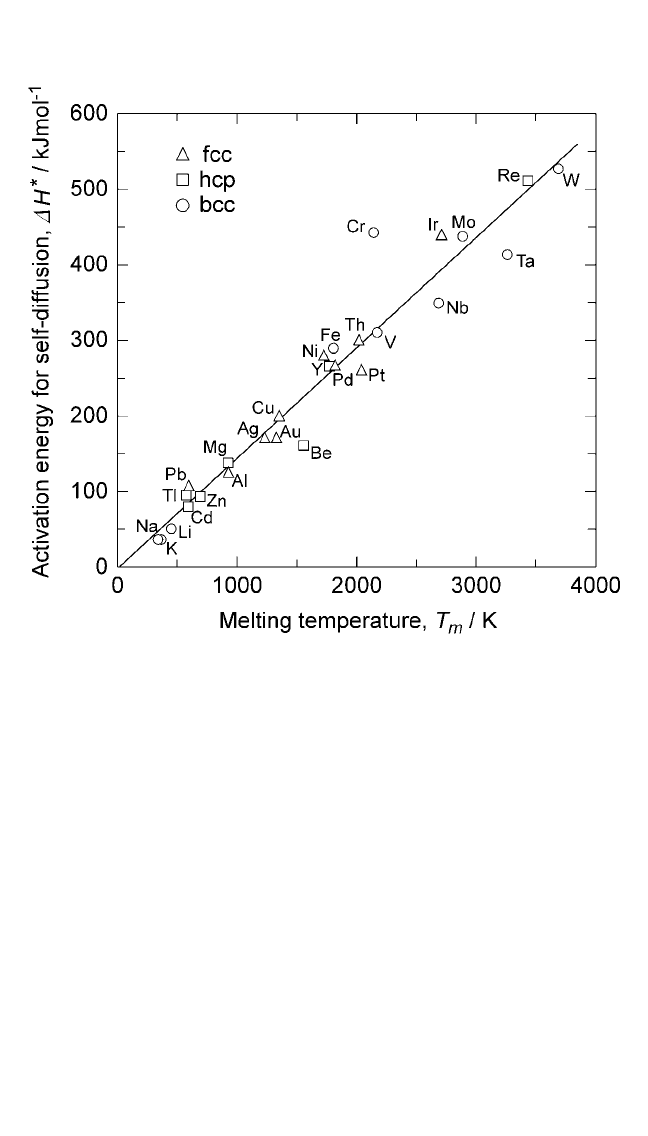
Diffusion parameters have been linked to the melting parameters in
several other ways. Historically, as well as from practical considerations,
the correlations between the melting and diffusion parameters are very
important. The activation enthalpy for diffusion has been related
[3–5]
to the
melting point (T
m
) and the enthalpy of fusion (H
m
) as:
∆H
*
K
1
T
m
(5)
Ch_02.qxd 11/30/04 8:45 AM Page 73

and
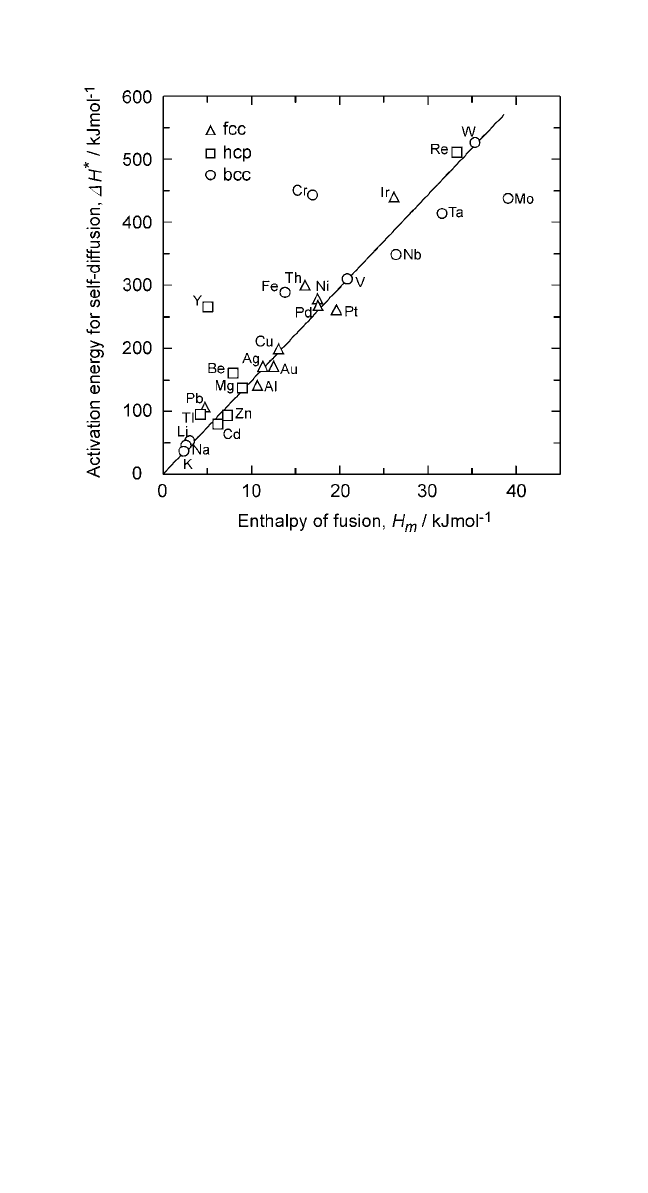
∆H
*
K
2
H
m
. (6)
Here, K
1
and K
2
are constants for any given class of solids. The plots for
Eqs. (5) and (6) are shown in Figs. 2.3 and 2.4. In both cases, the relationship
74 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Table 2.2. Vacancy Formation and Migration Energies in BCC Metals
a
Melting Vacancy Formation Vacancy Migration ∆H*
f
T
m
Metal Point (K) Energy (∆H
*
f
k J mol
1
) Energy (∆H
*
m
k J mol
1
) (J mol
1
K
1
) ∆H
*
f
∆H
*
m
Li 453 46.32 3.67 102.2 12.62
Na 371 32.81 2.90 88.4 11.31
K 336 32.81 3.67 97.6 8.940
V 2173 202.65 48.25 93.2 4.000
Nb 2690 260.55 53.10 96.8 4.907
Ta 3269 299.15 67.55 91.5 4.429
Cr 2148 202.65 91.68 94.3 2.210
Mo 2885 299.15 130.28 103.7 2.296
W 3695 347.4 164.10 94.0 2.117
a. H. Schultz, in Atomic Defects in Metals, Landholt-Bornstein New Series, vol. 25 (H. Ullamier, ed.),
Springer-Verlag (1991), p. 115
Table 2.1. Vacancy Formation and Migration Energies in FCC and HCP
Metals
a
Melting Vacancy Formation Vacancy Migration ∆H
*
f
T
m
Metal Point (K) Energy (∆H
*
f
kJmol
1
) Energy (∆H
*
m
k J mol
1
) (J mol
1
K
1
) ∆H
*
f
∆H
*
m
FCC Ag 1233.8 107.12 63.69 86.79 1.682
Al 933.1 64.65 58.86 69.29 1.098
Au 1336 89.75 68.51 67.16 1.310
Cu 1356 123.52 67.55 91.08 1.829
Ni 1726 172.74 100.36 100.06 1.721
Pb 600.4 55.97 41.50 93.27 1.350
Pd 1825 178.52 99.40 97.81 1.796
Pt 2042 130.27 138.00 63.79 0.944
Th 2024 123.52 196.86 61.02 0.627
HCP Cd 594 44.39 38.60 74.7 1.150
Mg 923 77.20 48.25 83.6 1.600
Tl 576 44.39 55.97 77.1 0.793
Zn 692.5 52.11 40.53 75.2 1.286
a. P. Erhart, in Atomic Defects in Metals, Landholt-Bornstein New Series, vol. 25 (H. Ullamier, ed.),
Springer-Verlag (1991), p. 88
Ch_02.qxd 11/30/04 8:45 AM Page 74
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.75
is linear. The linearity of these plots is validated by the high value of their
regression coefficients. The values of K
1
and K
2
are the same, even if FCC,
HCP, and BCC metals are considered separately. From Figs. 2.3 and 2.4,
K
1
146 and K
2
14.8. The validity of Eq. (5) has been demonstrated
for alkali halides as well by Barr and Lidiard.
[6]
For inert gas solids and
molecular organic solids, the validity of Eqs. (5) and (6) has been estab-
lished by Chadwick and Sherwood.
[18]
The diffusivity plots for any group of solids having identical physical
and chemical properties scale inversely with the magnitude of the entropy
of fusion.
[19, 20]
In Figs. 2.5 through 2.10, the diffusivity plots for metals as
well as other classes of solids are shown. In the case of alkali halides,
intrinsic conductivities, which are mediated by the ionic diffusion, have
been used. The two quantities are related by the Nernst-Einstein equation.
Figure 2.3 Correlation between the activation energy for self-diffusion in metals
and melting point; slope 146 J mol
1
K
1
.
Notes: 1. Activation energies were obtained from Smithells’Metal Reference Book, vol. VII
(E. A. Brandes and G. B. Brook, eds.), Butterworths Pub. (1992).
2. Melting temperatures were obtained from Thermochemical Data of Pure Substances,
vols. I and II (Ihsan Barin, ed.), VCH, Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 75
Numbers in parentheses represent the entropy of fusion. In each group or
a class of solids, a low value of entropy of fusion is an indication of high
diffusion rates, and vice versa. The relationship between the magnitude of
the entropy of fusion and the relative rates of the diffusion within a group
of solids holds, in general, irrespective of the nature of chemical bonding.
[19,
20]
Similar plots for a larger number of systems are shown elsewhere.
[20]
This phenomenon can be explained on the basis of the assumption that the
free energy of activation for diffusion, ∆G
*
, is in direct proportion to the
free energy of the liquid state. Hence, as first suggested by Dienes,
[9]
we
may write:
[21]
∆G
*
k G
l
, (7)
where k is a constant and G
l
is the free energy of the matrix in the liquid
state. Differentiation of Eq. (7) yields:
∆S
*
kS
m
, (8)
76 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 2.4 Correlation between the activation energy for self-diffusion in metals
and the latent heat of fusion; slope 14.8.
Notes: 1. Activation energies were obtained from Smithells’Metal Reference Book, vol. VII
(E. A. Brandes and G. B. Brook, eds.), Butterworths Pub. (1992).
2. Melting temperatures were obtained from Thermochemical Data of Pure Substances,
vols. I and II (Ihsan Barin, ed.), VCH, Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 76
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.77
where S
m
is the entropy of fusion. Further,
G
l
H
m
TS
m
. (9)
Therefore, from Eqs. (7) and (9), we get:
∆G
*
kH
m
kTS
m
. (10)
A comparison of temperature-independent parameters between Eqs. (2.2)
and (10) shows that:
∆H
*
kH
m
.(11)
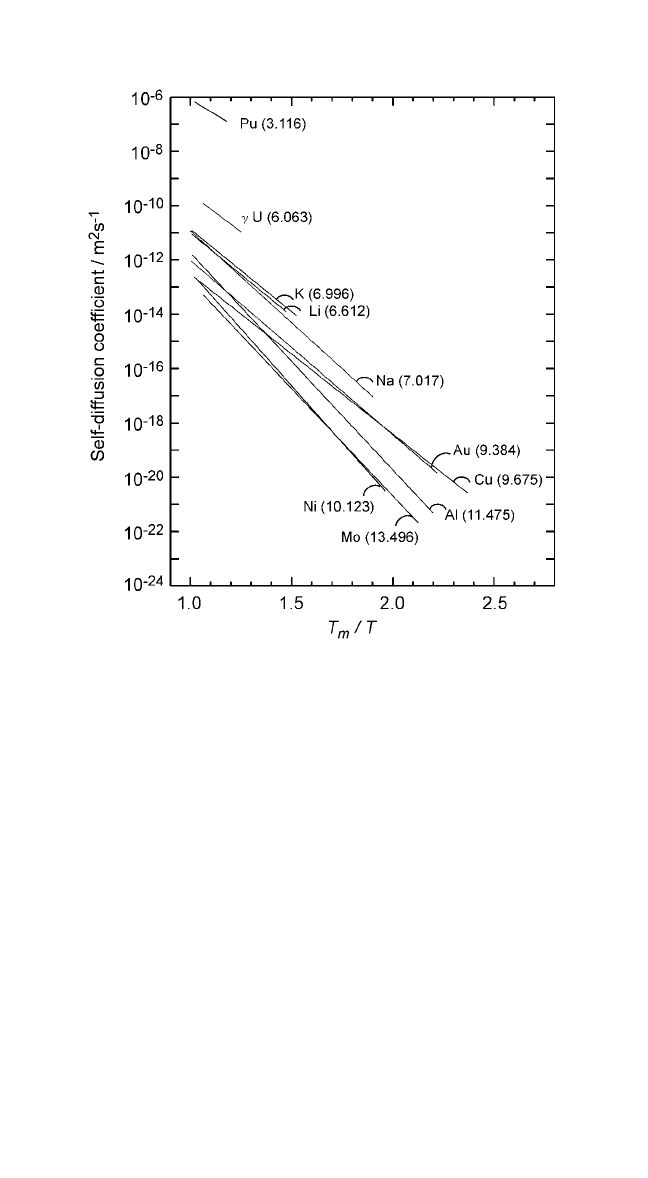
Figure 2.5 Logarithmic plots of self-diffusion coefficients in metals plotted against
the homologous temperature (T
m
T), showing that the self-diffusion rates vary
inversely with the entropy of fusion.Numbers in parentheses represent the entropy
of fusion in J mol
1
K
1
.
Note: Numbers in parentheses represent the entropy of fusion, in units of Jmol
1
K
1
, from
Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.), VCH,
Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 77
It is obvious that Eqs. (6) and (11) are identical. Hence, k K
2
. By sub-
stitutions from Eqs. (8) and (11) in Eq. (1), we have:
D fa
2
n exp
1
. (12)
According to Eq. (12), D should vary inversely with the entropy of
fusion at a constant value of T
m
T. A plot of Eq. (12) for some common
T
m
T
K
2
S
m
R
78 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
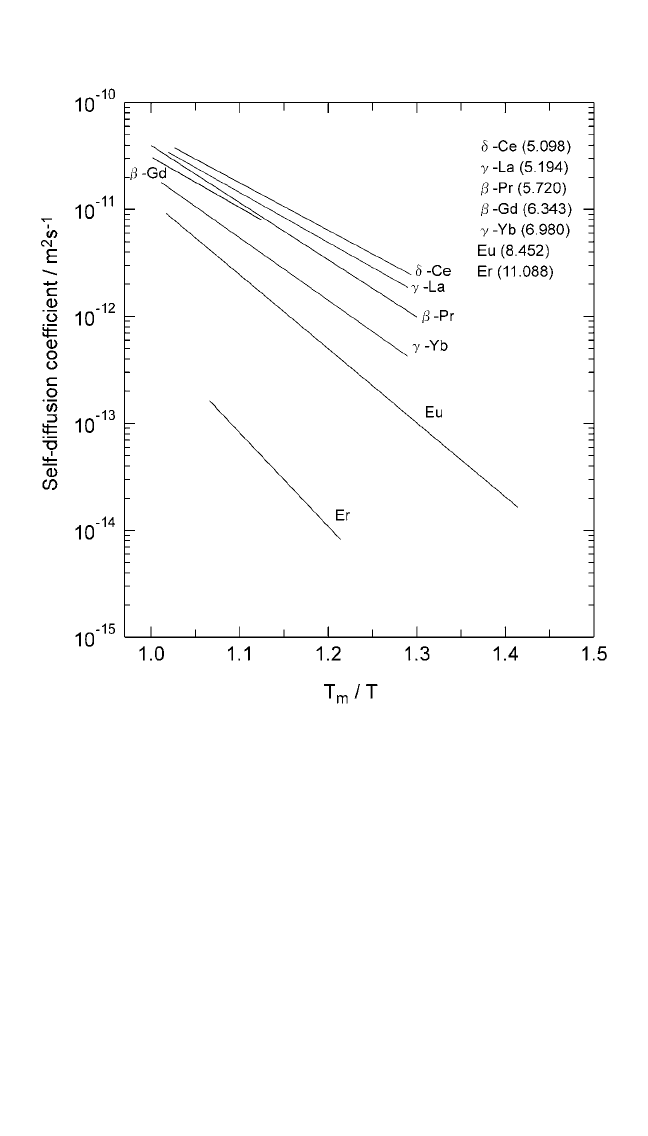
Figure 2.6 Logarithmic plots of self-diffusion coefficients in lanthanide metals plot-
ted against the homologous temperature (T
m
T ), showing that the self-diffusion
rates vary inversely with the entropy of fusion. Numbers in parentheses represent
the entropy of fusion in J mol
1
K
1
.
Note: Numbers in parentheses represent the entropy of fusion, in units of Jmol
1
K
1
, from
Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.), VCH,
Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 78
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.79
metals shown in Fig. 2.11 bears out this expectation. The linearity of the plots
in Fig. 2.11 validates Eq. (12) and the assumption made in its derivation.
The diffusion coefficient is pressure-dependent in view of the contri-
bution of the term P∆V
*
to the Gibbs free energy (∆G
*
), which is the
controlling thermodynamic factor in its entirety. Consequently, in the pres-
sure-dependent diffusion measurements at constant temperature, the acti-
vation volume, ∆V
*
, becomes an analogous parameter to ∆H
*
. Nachtrieb
et al.
[22]
have correlated these two parameters with the pressure depend-
ence of the melting temperature (dT
m
dP) as:
∆V
*
(∆H
*
T
m
)(dT
m
dP). (13)
Equation (13) predicts that ∆V
*
is controlled by the sign and magnitude
of dT
m
dP. In fact, it shows excellent agreement with the experimental
data.
[23]
In general, dT
m
dP is positive for metals. However, it is negative
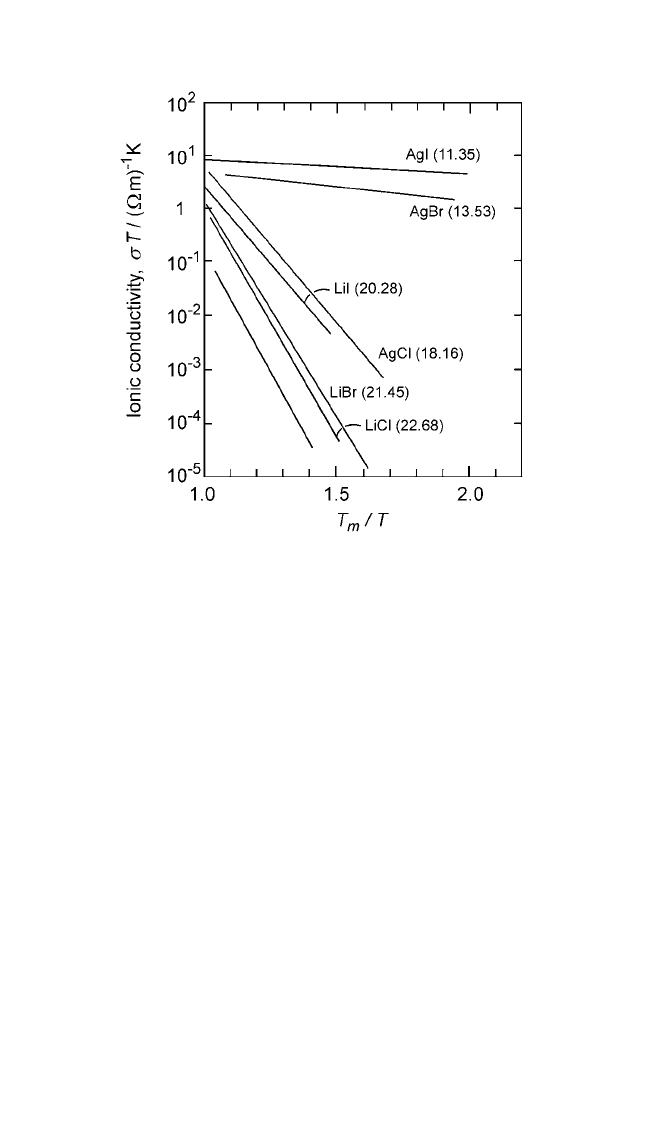
Figure 2.7 Logarithmic plots of ionic conductivity (s ) multiplied by temperature
(T) as a function of homologous temperature (T
m
T ) for silver and lithium halides.
Numbers in parentheses represent the entropy of fusion in J mol
1
K
1
.The prod-
uct sT is directly proportional to the self-diffusion rates. Ionic conductivity data for
alkali halides are from Uvarov et al.
[12]
Note: The entropy of fusion is indicated by the numbers in parentheses, in units of Jmol
1
K
1
,
from Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.), VCH,
Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 79
for plutonium and, as expected from Eq. (13), ∆V
*
is negative for this
element.
[24]
Acritical test of this equation was performed by Zanghi and Calais,
[25]
who measured the activation volume for self-diffusion in the Pu-Zr sys-
tem and showed that the magnitude and sign of ∆V
*
are controlled by
dT
m
dP. The work of Zanghi and Calais
[25]
provides support for extending
the correlation between the diffusion and melting parameters to alloys as
well. As pointed out earlier by Vignes and Birchenall
[26]
for systems
80 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
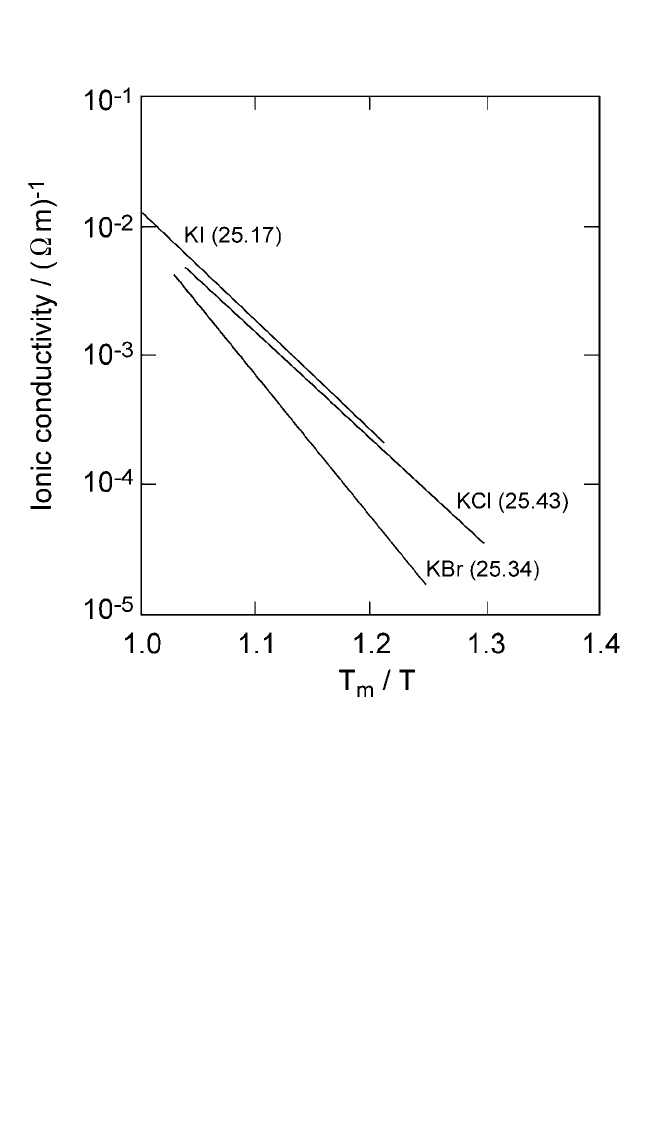
Figure 2.8 Logarithmic plots of ionic conductivity (s) multiplied by temperature (T)
as a function of homologous temperature (T
m
T ) for potassium halides. Numbers
in parentheses represent the entropy of fusion in J mol
1
K
1
. The product sT is
directly proportional to the self-diffusion rates. Ionic conductivity data for alkali
halides are from Uvarov et al.
[12]
Note: The entropy of fusion is indicated by the numbers in parentheses, in units of Jmol
1
K
1
,
from Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.), VCH,
Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 80
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.81
exhibiting extended solubility, the variations in the activation energy for
interdiffusion scale uniformly with the solidus temperature. More inter-
estingly, Roux and Vignes
[27]
and Ablitzer
[28]
have shown that the solute
diffusion rates vary systematically with the slope of the solidus curve.
Close relationships between diffusion and melting parameters are
depicted in Eqs. (3) through (6). Furthermore, the inverse scaling of
the diffusion coefficient with the entropy of fusion and the magnitude of
the activation volume are satisfactorily predicted by Eqs. (12) and (13).
Although not yet fully understood, these features are common to all types
of solids, irrespective of the type of crystal structure and nature of chem-
ical bonding.
[12, 18, 19, 20]
The configuration consisting of a vacancy and its
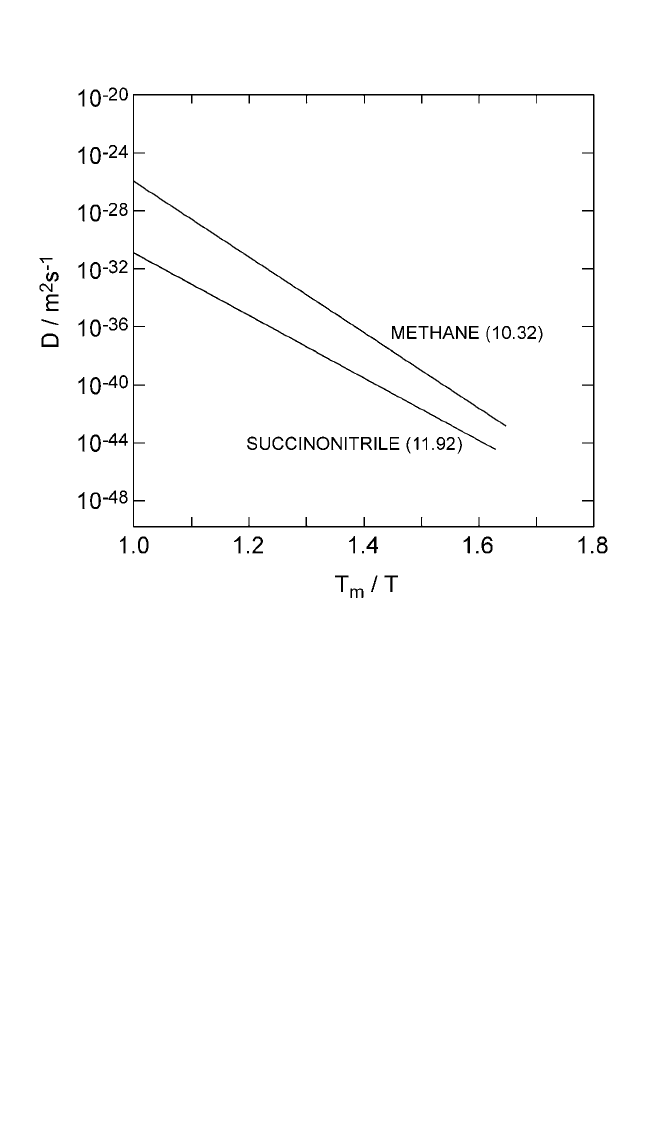
Figure 2.9 Logarithmic plots of self-diffusion coefficients in organic plastic solids
as a function of homologous temperature (T
m
T). Numbers in parentheses repre-
sent the magnitude of entropy of fusion in J mol
1
K
1
. On a relative scale, a low
value of the entropy of fusion indicates a higher self-diffusion rate, and vice versa.
Self-diffusion data for organic solids are from Chadwick and Sherwood.
[18]
Note: The numbers in parentheses represent the magnitude of entropy of fusion, in units of
Jmol
1
K
1
, from Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.),
VCH, Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 81
