Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.

neighboring atoms formed at the saddle-point in the path of diffusion has
been designated as relaxion by Nachtrieb and Handler.
[5]
Structurally, this
configuration is hypothesized to be similar to the molten state of the
matrix. Within the relaxion, ions can roll over or squeeze past one another,
and the diffusive jump is facilitated by the absorption of phonons. The
relaxion concept provides a simple explanation for the linear relationship
between diffusion and the melting parameters.
2.2.2 Elastic Constants
The correlation between the elastic constants and diffusion parame-
ters was pioneered by Wert and Zener.
[2]
They first suggested that a frac-
tion, l, of the free energy difference between the equilibrium position and
the saddle-point configuration of a diffusing atom (∆G
*
) arises from the
straining of the surrounding lattice. Consequently, the entropy of activation
82 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
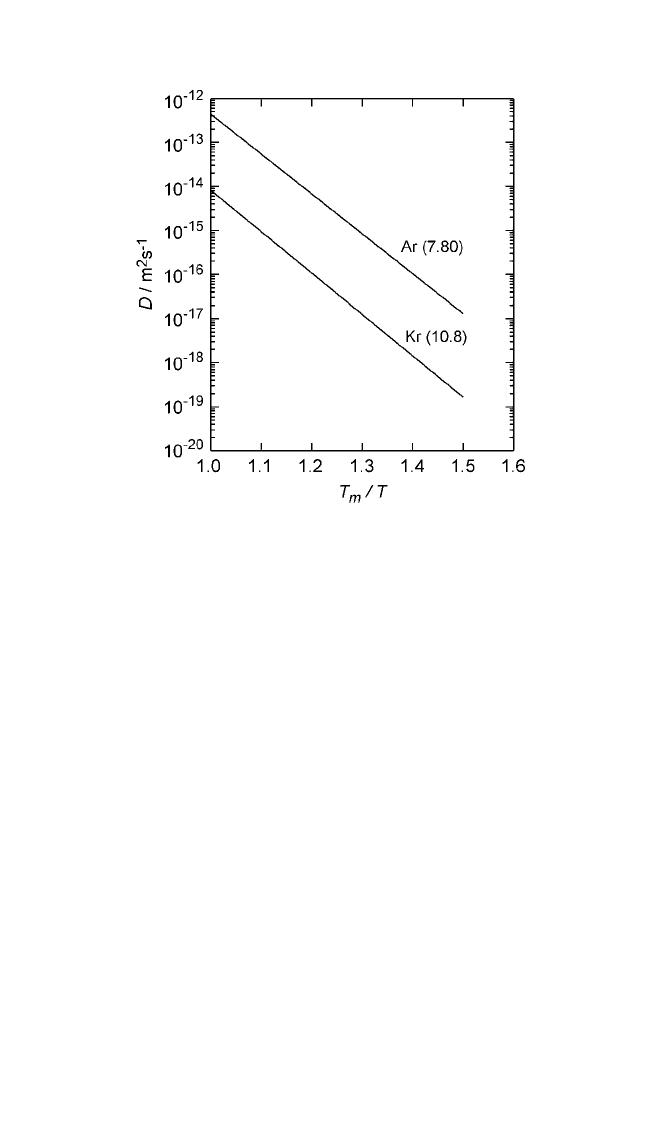
Figure 2.10 Logarithmic plots of self-diffusion coefficients in rare gas solids as a
function of homologous temperature (T
m
T). Numbers in parentheses represent
the magnitude of entropy of fusion in J mol
1
K
1
. On a relative scale, a low value
of the entropy of fusion indicates a higher self-diffusion rate, and vice versa. Self-
diffusion data for rare gas solids are from Chadwick and Sherwood.
[18]
Note: The numbers in parentheses represent the magnitude of entropy of fusion, in units of
Jmol
1
K
1
, from Thermochemical Data of Pure Substances, vols. I and II (Ihsan Barin, ed.),
VCH, Weinheim, FRG (1995).
Ch_02.qxd 11/30/04 8:45 AM Page 82
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.83
in Zener’s equation
[1]
is represented as:
∆S
*
lb , (14)
where b is defined as
b d(EE
0
)d(TT
m
). (15)
In this formulation, E and E
0
are the values of appropriate elastic constants
at a temperature T and 0K, respectively. Although this equation was orig-
inally proposed for interstitial diffusion,
[2, 29]
it has been extended for self-
diffusion via a vacancy mechanism by Zener,
[30]
LeClaire,
[7]
Buffington
and Cohen,
[8]
and Flynn.
[31]
The extension of Zener’s hypothesis to the
substitutional diffusion is based on the assumption that the free energy
associated with the straining of the lattice during atomic migration bears
H
*
T
m
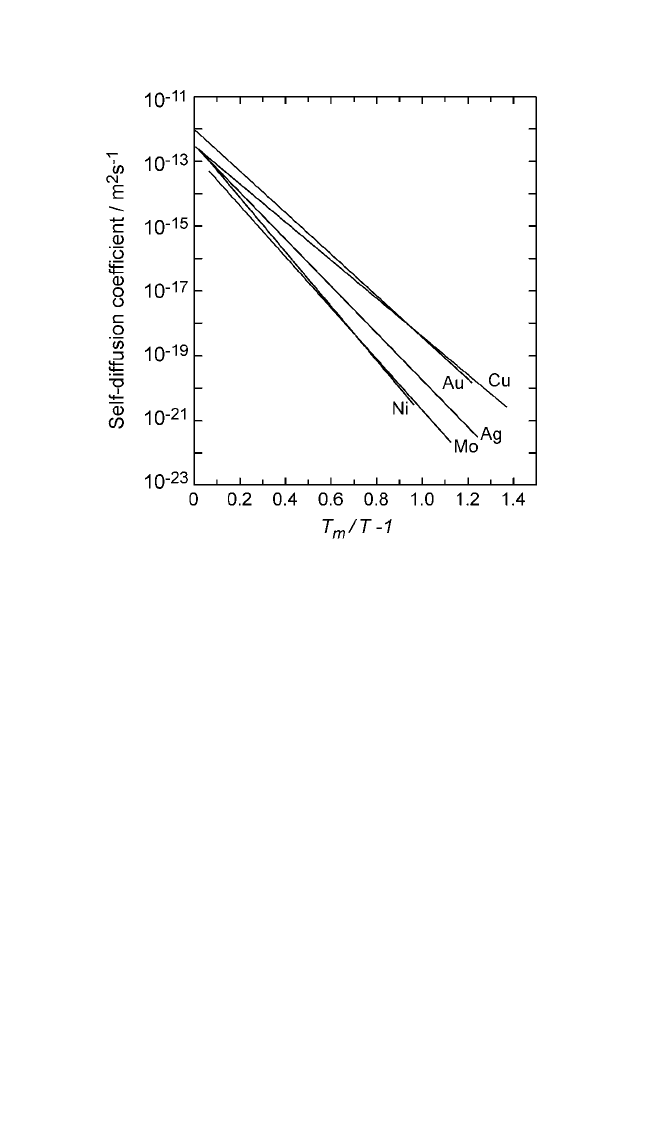
Figure 2.11 Logarithmic plots of self-diffusion coefficients in some common
metals against the parameter [(T
m
T) 1].The linearity of the plots demonstrates
the validity of the relationship between self-diffusion and the entropy of fusion as
given in Eq. (12).
Ch_02.qxd 11/30/04 8:45 AM Page 83

a constant ratio to the total free energy of diffusion. Le Claire,
[7]
as well as
Buffington and Cohen,
[8]
assumed that the appropriate elastic modulus
involved in the straining of the lattice during the diffusion jump is
C
44
12(C
11
C
12
). According to Flynn,
[31]
however, the appropriate
modulus (<C>) is given differently, as follows:
. (16)
The activation energy for vacancy migration, ∆H
m
*
, for FCC and BCC
metals is related to C
[32]
as:
∆H
*
m
K
3
Ca
3
, (17)
where a is the lattice parameter and K
3
isanother constant. The values of
K
3
for FCC and BCC are 0.022 and 0.020, respectively. According to
Erhart et al.,
[32]
these values, though slightly different from the one given
by Flynn,
[31]
provide a better agreement between the experimental values
of ∆H
m
and Eq. (17).
In the application of Eq. (14), we are concerned with the temperature
dependence of C, not with its actual value. This is usually taken as equal
to the temperature dependence of Young’s modulus, determined at low
temperatures to avoid errors that may be caused by the grain boundary
viscous flow at higher temperatures. Zener
[1]
suggested l to be equal to
0.55 and 1.0, respectively, for FCC and BCC metals. Using these param-
eters, Lazarus
[33]
showed that the experimental values of the temperature-
independent pre-exponential factor in Eq. (1) agreed reasonably well with
the values calculated with the help of Eq. (14).
In practice, the product lb varies between 0.15 and 0.35 for self-
diffusion in metals via a vacancy mechanism, if we take Zener’s value for
l
[1]
and Koster’s value for temperature dependence of Young’s modulus.
[34]
This narrow range of the product lb allows only a small variation in the
frequency factors for the self-diffusion of metals. This is the most impor-
tant conclusion from Zener’s hypothesis. For any group of solids, its value
depends on the mode of diffusion and the crystal structure.
[35]
A violation
of Zener’s equation for ∆S
*
and melting point correlations for ∆H
*
,
namely, Eqs. (3), (4), and (14), in any system is characterized anomalous
and considered worthy of explanation.
[4]
Beke et al.
[36]
have extended
Zener’s hypothesis to the impurity diffusion in metals using dimensional
analysis. Their equation is given as:
ln
F
, (18)
H
0i
*
H
0
*
T
m
D
0i
D
0
1
C
44
2
C
11
C
12
3
C
11
15
C
84 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_02.qxd 11/30/04 8:45 AM Page 84

SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.85
where F is a constant and other terms have the same meaning as before.
The subscripts 0 and 0i refer to self-diffusion and impurity diffusion,
respectively. Beke et al.
[36]
showed that the impurity diffusion data in alu-
minum, copper, and silver are in accord with Eq. (18).
2.2.3 Bulk Modulus
Toth and Searcy
[37]
were first to correlate the activation energy for
vacancy migration with bulk modulus. They stated that an adequate analy-
sis of the elastic distortions undergone by the diffusing atoms is not avail-
able. The elastic distortions associated with the saddle-point configuration
are neither isotropic, as implied by the use of bulk modulus, nor unidirec-
tional, as implied by the use of shear modulus. They are multidirectional.
If only one of them is to be used, then any one can be used. Toth and
Searcy preferred to use bulk modulus because the data are much more eas-
ily available. Following Le Claire
[7]
and Nachtrieb and Handler,
[5]
Toth
and Searcy used Eq. (6), with a slightly different constant, to evaluate
vacancy formation energy. The equation Toth and Searcy developed for
self-diffusion in FCC and HCP metals is:
∆H
*
22.6BV 0.27H
m
, (19)
where Bis the bulk modulus and V is the specific volume. Room-temperature
values were used for bulk modulus and specific volume. The corresponding
equation for BCC metals is similar except that the multiplier for the term BV
is changed to 25.4. Leibfried
[38]
has shown that the quantity BVis propor-
tional to the melting point. Using this proportionality, Toth and Searcy also
gave the following equation for self-diffusion in FCC and HCPmetals:
∆H
*
16.0T
m
0.27H
m
. (20)
For BCC metals, the multiplier for T
m
is altered only marginally to 15.8 in
Eq. (20). Satisfactory agreement with the actual data was obtained for
Eqs. (19) and (20). Using median values, identical expressions were also
developed for substitutional alloys.
Varotsos and Alexopoulos
[39]
suggested that ∆G
*
is a function of the
product of bulk modulus and the specific volume of the matrix as follows:
∆G
*
cBV, (21)
where c is an arbitrary constant found to be independent of temperature
and pressure. However, it is a function of the mechanism of diffusion. The
value of calso varies from one system to another. With the help of Eq. (21),
Ch_02.qxd 11/30/04 8:45 AM Page 85

Eq. (1) for the diffusion coefficient can be written as:
ln D ln(fa
2
u)
. (22)
Further, the activation entropy and energy for self-diffusion can be
expressed as:
∆S
*
c
P
, (23.1)
and
∆H
*
cBV Tc
P
. (23.2)
Equations (22), (23.1), and (23.2) form the basis of the model
proposed by Varotsos and Alexopoulos.
[40]
In this model, all the infor-
mation about the process of diffusion is contained in the parameter
cBV. The constant c varies with the change of host lattice as well as
with the change in the mode of diffusion. Its magnitude is estimated
with the help of Eqs. (21) and (22) at one particular temperature, usu-
ally at T = 0 K. The temperature and pressure variations of the diffu-
sion coefficient or any other diffusion parameter are governed by the
corresponding variations in the product BV. The model has been
applied extensively to self-diffusion in metals, alkali halides, and rare
gas solids.
[40-43]
It has also been applied to the estimation of the forma-
tion and migration volumes of vacancies, solute diffusion in metals,
and nonlinear/curved diffusivity plots for self-diffusion in FCC and
BCC metals.
[43]
There seems to be little doubt that a reasonable numerical agreement
exists between the model and the experimental results, and these equa-
tions can be used to estimate the diffusion data when actual data are lack-
ing. However, one basic difficulty with this method of data treatment is
that Eq. (21) equates free energy with enthalpy. This thermodynamic
approximation is strictly valid only at absolute zero temperature. In fact,
this condition is used by the authors to evaluate the constant c. Secondly,
the basis of this model is the same as Zener’s hypothesis.
[1,29,30]
However,
in Zener’s model, the constant l has a simple and direct interpretation. A
similar interpretation cannot be attributed to the constant c in the model
proposed by Varotsos and Alexopoulos.
[39]
∂(BV)
∂T
∂(BV)
∂T
cBV
RT
86 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_02.qxd 11/30/04 8:45 AM Page 86

SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.87
Patil and Tiwari have given an equation correlating the vacancy for-
mation energy with the compressibility.
[44]
Applying thermodynamics to
the continuum models of lattice, the equation:
(24)
was independently derived by Keys
[45]
and Lawson.
[46]
Here, a is the coef-
ficient of volumetric thermal expansion, and b is the compressibility.
2.2.4 The Debye Temperature
From semi-empirical considerations, Mukherjee
[10]
derived a relation-
ship between the vacancy formation energy and Debye temperature (q) as:
q K
4
12
, (25)
where M is the atomic weight, V is the specific volume, and K
4
is another
constant. This equation connects vacancy formation energy with a
dynamic property of the perfect lattice. The Mukherjee’s equation was
later derived by March,
[47]
somewhat more rigorously, on the basis of
screening theory. Subsequently, Tewary
[48]
gave a formal derivation based
on Fourier-transform of two-body pair potential in a crystal. Vacancy
formation energy is treated as equal to half the sum of pair potential at a
lattice site. It is shown that in an isotropic Debye approximation, this sum
is proportional to the Debye temperature.
According to Tewary,
[48]
the constant in Eq. (25) is defined by:
K
4
(hk)(9N4p)
13
(2J)
12
, (26)
where h is Planck’s constant, N is Avogrado’s number, and J is the
mechanical equivalent of heat. The relationship between q and ∆H
*
as
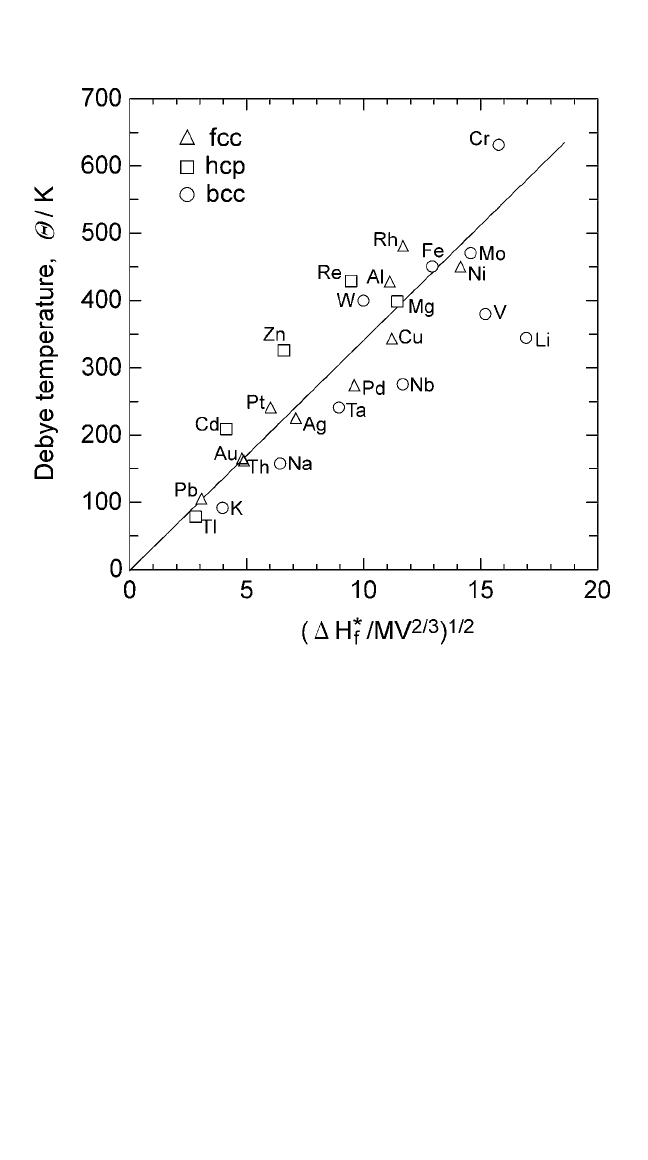
given in Eq. (25) is shown graphically in Fig. 2.12. The value of K
4
given
by Eq. (26) is equal to 34.2, which is the same as that obtained from
Fig. 2.12. The agreement between the theory and the experiment is indeed
very satisfactory. Equation (25) has been extended to alkali halides by
Sastry and Mulimani.
[49]
H
f
*
MV
23
a
b
S
*
V
*
Ch_02.qxd 11/30/04 8:45 AM Page 87
2.2.5 Valence Bond Parameter
Valence bond parameter for a metal is defined as the ratio of cohesive
energy (E) to its most prominent chemical valence (Z). An interesting rela-
tionship between the valence bond parameter and ∆H
*
is discussed here.
[50]
The kinetic energy, U
o
, of an atom of mass mvibrating in a crystal is given by:
U
o
m gv
2
. (27)
Here, g and v are the maximum displacement and the corresponding
vibration frequency, respectively. Assume that U
o
is some constant fraction,
1
2
88 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 2.12 Debye temperatures versus vacancy formation energies;slope 34.7.
Notes: 1. Debye temperatures were obtained from C. Kittel, Introduction to Solid State Physics,
VII ed., Wiley (1986).
2. Vacancy formation energies were obtained from Atomic Defects in Metals, vol. 25
(H. Ullmaier, ed.), Landolt-Börnstein Series, Springer-Verlag (1991).
Ch_02.qxd 11/30/04 8:45 AM Page 88
SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.89
r, of cohesive energy per valence bond, that is, the valence bond para-
meter, EZ.
[51]
Hence, we may write:
v
m
1
g
12
12
. (28)
Further, in the harmonic approximation, v is the central frequency of the
atomic vibration and is equal to the Debye-frequency evaluated from the
specific heat. Hence, Eqs. (25) and (28) can be combined to yield:
∆H
f
*
A
1
, (29)
where A
1
is a proportionality constant. Amore useful relationship for pre-
dicting the activation energy for self-diffusion can be derived from
Eq. (29) on the basis that the ratio between the activation energies for
vacancy formation and self-diffusion is a constant for elements that
belong to the same crystal class.
[52]
Therefore, we have:
∆H
*
A
2
A
3
, (30)
where A
2
and A
3
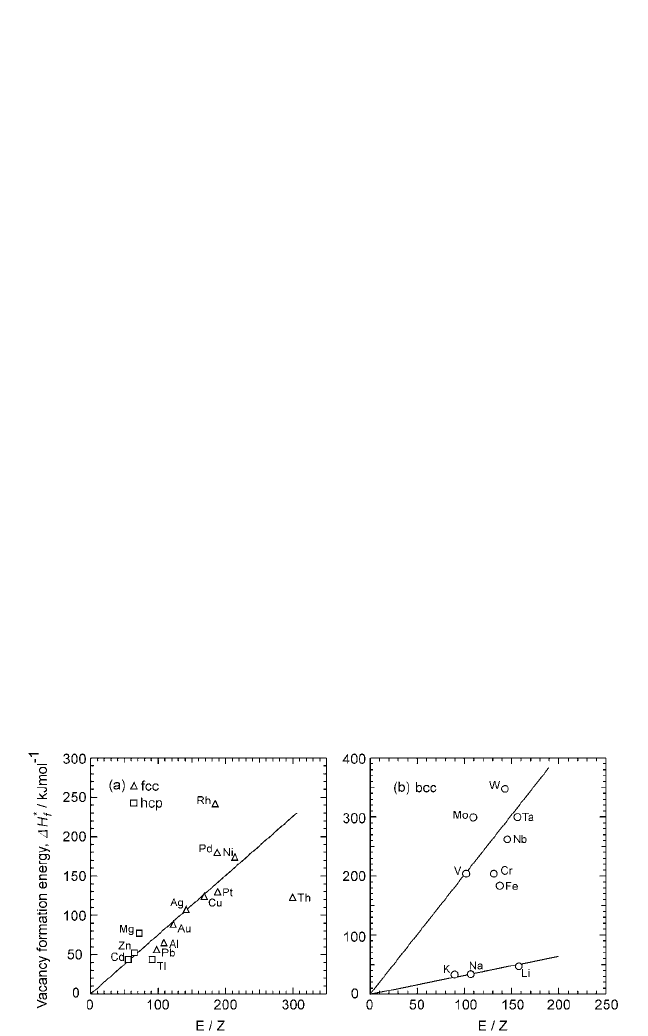
are other constants. Figure 2.13 shows a plot of ∆H
f
*
versus EZ. As in the case of Eqs. (3) and (4), the data points for FCC and
E
Z
E
Z
2rE
Z
Figure 2.13 Vacancy formation energy shown as a function of valence bond
parameter (E/Z); see Eq. (30).
Note: Vacancy formation energy was obtained from Atomic Defects in Metals, vol. 25 (H.Ullmaier,
ed.), Landolt-Börnstein Series, Springer-Verlag (1991).
Ch_02.qxd 11/30/04 8:45 AM Page 89
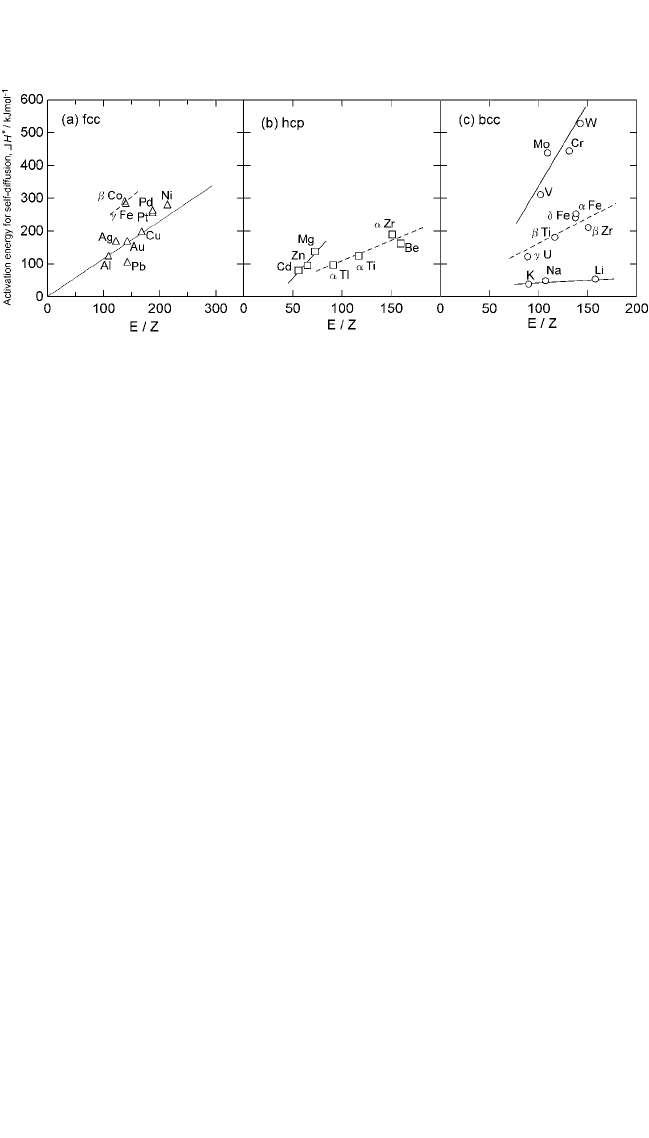
HCPmetals lie on the same line. For BCC metals, the data points for tran-
sition and the alkali metals lie on different lines. This is in contrast to
Fig. 2.2(b), where the data points for all BCC metals lie on the same line.
This feature is also repeated in Fig. 2.14, which shows the plots between
the valence bond parameter and the activation energy for self-diffusion.
The variations from one group to another in Figs. 2.13 and 2.14 can be
attributed to the term r in Eq. (28). Thus, the constants A
1
, A
2
, and A
3
vary
with the nature of chemical bonding and the crystal structure.
Anovel feature of correlation depicted in Figs. 2.13 and 2.14 is that it
distinguishes between the allotropic and nonallotropic matrices among
FCC, HCP, and BCC metals. Incidence of allotropy enhances the overall
diffusion rates in a matrix. The influence of allotropy on the diffusion
characteristics is discussed elsewhere.
[53]
An interesting aspect of Figs. 2.13 and 2.14 is that Cu, Ag, Au, and Pt
correlate satisfactorily with other metals only when their Z values are
taken as 2, 2, 2, and 3, respectively. This suggests that in some cases, the
number of electrons contributing to the cohesion are different from those
effective in chemical reaction. Considering the configuration of their out-
ermost shell, Cu, Ag, and Au are regarded as monovalent metals. Their
most prominent chemical valencies are 2, 1, and 3, respectively. Similarly,
the prominent valencies of Pt are 2 and 4. It has been clearly demonstrated
for copper and silver that the agreement between calculated and experi-
mental values of cohesive energy is poor if only a single electron is
allowed to take part in the bonding.
[54]
In the noble metals group, s-d
hybridization contributes to the bonding and alloying behavior in a sig-
nificant way, and thus indirectly controls the number of effective bonding
electrons.
[55]
This number could be different from their prominent chemical
90 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 2.14 Activation energy for self-diffusion in metals as a function of valence
bond parameter (E/Z). This correlation makes a distinction between allotropic
(dotted lines) and nonallotropic metals; see Eq. (30).
Ch_02.qxd 11/30/04 8:45 AM Page 90

SOLID STATE DIFFUSION AND BULK PROPERTIES, TIWARI ET AL.91
valencies. This factor seems responsible for the behavior exhibited by
these elements in Figs. 2.13 and 2.14.
2.2.6 Electron-to-Atom Ratio
Fumi
[56]
has shown that the vacancy formation energy is a simple
function of Fermi energy [E
F
] as:
∆H
*
f
txE
F
, (31)
where t is a constant less than unity and x represents the valence number
of the matrix element. In the rigid-band approximation, this equation can
be extended to alloys, if x is identified as the electron-to-atom ratio. Then,
x is the average number of electrons contributed by each atom to the con-
duction band.
Fermi energy is a function of the number of electrons per unit volume:
E
F
(h
2
8m
*
)(3p)
23
(n
*
V)
23
. (32)
Here, m
*
and n
*
are the electronic mass and number of free electrons pres-
ent in a crystal of volume V. If c is the volume occupied by a single atom,
then x is given by:
x (n
*
V)c. (33)
In an FCC lattice,
c a
3
4. (34)
Combining Eqs. (31) through (34), and assuming, as before, that the ratio
of the activation energies for vacancy formation and the self-diffusion is
the same for any one system, we get:
[57]
∆H
*
K
5
x
53
a
2
, (35)
where K
5
is a constant. A plot of solvent diffusion in silver-base alloys
based on Eq. (35) is shown in Fig. 2.15. Although the plots are linear, the
slopes are different for different solutes. If ∆H
*
depends only on x, then the
slopes for different solutes should be identical. It is generally presumed
that the variations in the solvent diffusion rates at a fixed value of x for dif-
ferent solutes are caused by the differences in the electro-negativity of the
solutes. This parameter controls transfer of electrons from electropositive
Ch_02.qxd 11/30/04 8:45 AM Page 91
