Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

430 Charged Particle and Photon Interactions with Matter
at3500cm
−1
due to the physisorbed water decreases on heating to reveal two OH surface bands
at 3780 and 3680cm
−1
. These bands are due to the terminal and bridged OH groups, respectively
(Merle-Mejean
et al., 1998). Absorption at the low energies is usually due to solid lattice modes.
Proton
NMR has been used to identify the OH groups on the surface of SiO
2
. Silica can have
several different OH groups depending on whether silane or siloxane groups are present. Thomas has
used this technique to observe three different OH groups (Thomas, 1993). Ceramic oxide powder
surfaces have also been probed with neutron and Raman scattering to characterize the OH sur-
face sites (Ozawa et al., 1997). However, highly penetrating probes cannot distinguish whether the
observed sites are on the surface or in the bulk. Water trapped in interstitial cavities or co-crystallized
during powder formation will be observed with NMR, EPR, x-ray, or neutron techniques.
The dissociation of water at a particular site on the ceramic oxide is due to the specic activity
at that site. For instance, ZrO
2
possesses acidic, basic, oxidizing, and reducing properties (Tanabe,
1985). Both Lewis and Bronsted acid sites have been identied on ZrO
2
(Hertl, 1989). A wide
variety of ceramic oxides exhibit both acid and basic character (Boehm, 1971). Sustained heat-
ing will drive the water off the surface site leaving a Lewis acid due to the unsaturated cation site.
Attachment of an available anion will quickly follow. Irradiation may also lead to dissociation of
a surface
group leaving a reactive cation site.
Surface-bound
OH groups essentially form a layer of H atoms covering the ceramic oxide. The
next layer of water molecules is highly inuenced by this interface and forms a structured water–ice
layer through hydrogen bonding, Figure 16.1. Infrared observation of Al
2
O
3
surfaces with increas-
ing amounts of relative humidity show that the next 2–3 water layers adsorbed on top of the mono-
layer of OH groups are very ice-like. The infrared spectra show a very structured ice-like layer
due to the hydrogen bonding network. A further increase in relative humidity results in a more
disordered transition layer in which the water is more liquid-like (Al-Abadleh and Grassian, 2003;
Thomas and Richardson, 2006). A somewhat similar result was observed in PuO
2
(Stakebake, 1967,
1981;
Stakebake and Dringman, 1968a,b).
Water
has three degrees of freedom so a typical IR spectrum should reveal two stretching modes
and a bending mode. The two stretching modes are close together and often merge into one broad
peak. Figure 16.2 shows that the absorbances due to the stretching and bending modes are readily
observable in amorphous ice at 3280 and 1680 cm
−1
, respectively (Hudgins et al., 1993). A combina-
tion peak is also observed at 2220cm
−1
and a libration due to hindered rotation is seen at 790cm
−1
.
Many of the features observed in amorphous ice are also observed from water adsorbed on ZrO
2
,
Figure 16.2, and other ceramic oxides. These results suggest that the rst few water layers on these
oxides are very ice-like; that is, they have hindered mobility. The amount of physisorbed water
increases with increasing water content and the spectra reveal more bulk water features. Figure 16.2
shows the DRIFT spectra for ZrO
2
on the addition of a drop of water to the surface to give “wet”
ZrO
2
. The distinctive water adsorption peaks for physisorbed water are broadened compared to that
for ice due to the disorder of the liquid-like phase. The change from the OH surface layer through
the transition ice layer to the bulk water layer is not abrupt. Different ceramic oxides are expected
to have different thicknesses of the transition ice-like layer. However, almost all ceramic oxides can
be expected to have a layered structure of water near its surface. These layers are expected to affect
the radiation chemistry of the associated water molecules and to inuence the reactions of other
molecular
species migrating toward the interface from the liquid phase.
16.4 radiolysis oF aQueous suspensions
The main stable reducing species in the radiolysis of water is H
2
, while the main oxidizing species is
H
2
O
2
. Both products are due to intra-track reactions of species formed by the water decomposition.
At about 1μs following the passage of a gamma ray, the yield of H
2
is about 0.45 molecule/100eV
of energy absorbed in liquid water (Pastina et al., 1999). The yield of H
2
O
2
under similar radiolytic
conditions is about 0.7 molecule/100eV (Pastina and LaVerne, 1999). These yield values are maxima.
Radiation Chemistry of Water with Ceramic Oxides 431
At longer times, the reactions of H
2
with OH radicals and H
2
O
2
with hydrated electrons or H atoms
lead to a series of reactions to reform water (Pastina and LaVerne, 2001). These back reactions can
be suppressed if the H
2
escapes from the liquid water phase, for instance by vaporization into a
headspace or purged away by a gas ow. Selected scavengers are routinely added to aqueous solu-
tions in radiation chemistry studies to stop the back reactions and allow the measurement of stable
product yields. Essentially no H
2
is observed at long times in the radiolysis of closed systems of pure
bulk
liquid water with gamma rays (Allen, 1961).
A
large number of the early studies on aqueous suspensions focused on the photolysis of water
with metal colloids because of the importance in solar energy conversion and in environmental
remediation by photooxidation (Rabani et al., 1988). Many of the rst papers on the radiolysis of
aqueous solutions of metals and metal oxides were logical extrapolations of these early systems,
especially the TiO
2
and SiO
2
suspensions. An obvious advantage to examining aqueous suspensions
is that pulsed radiolysis techniques can be utilized to examine transient species. The radiolysis of
acidic solutions of alcohols containing TiO
2
nds that excess electrons can be transferred from
the aqueous phase to the solid. Both the hydrated electron and the alcohol radical promote elec-
trons to the TiO
2
conduction band. Conduction band electrons can then react with added solutes.
These results are important because they prove that the transfer of energy and charge through the
water–oxide interface is possible (Safrany et al., 2000; Gao et al., 2002, 2003). Interestingly, these
studies show no effect due to particle size. The excess electrons in the conduction band can even
reduce aqueous Pt to form Pt clusters on the TiO
2
surface (Kasarevic-Popovic et al., 2004). The Pt
clusters can then reduce aqueous H
+
to H
2
at the surface of the TiO
2
(Behar and Rabani, 2006). The
scavenging of hydrated electrons or radicals by TiO
2
particles is not that surprising. With the right
redox potentials, the particle is just behaving like a typical aqueous solute. However, the potential
for
initiating new chemistry at the surface of the particles makes these systems very interesting.
As
one increases the concentration of particles in aqueous suspensions, more energy will be
deposited initially into the solid phase. A fundamental inquiry concerns whether that energy can
migrate to the surface and become available for initiating chemistry in the aqueous phase. Meisel
and coworkers used pulse radiolysis techniques to examine the hydrated electron yields in the aque-
ous phase with variation of SiO
2
concentration and particle size (Schatz et al., 1998). Some of
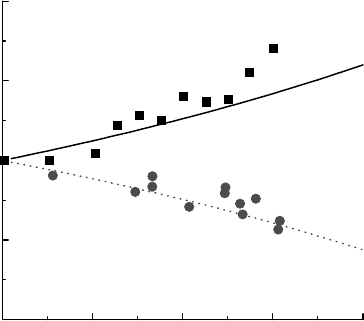
the data of that work for 7 nm SiO
2
is shown in Figure 16.3. The system was probed using pulsed
electron radiolysis techniques with a xed sample size. With increasing concentration of SiO
2
, the
relative electron density of the sample increases and the amount of energy deposited by the electron
beam pulse increases proportionately. In converse, the volume of water in the sample must decrease
with increasing SiO
2
concentration. The upper and lower solid lines of Figure 16.3 show these
values, respectively. Hydrated electrons, which are obviously in the water phase, have yields that
track with the electron density of the total medium. If energy deposited initially within the oxide
remained there, then the expected hydrated electron yields should vary with water content as predicted
by the lower curve in Figure 16.3. The results show that not only do the electrons escape the SiO
2
,
but the ionization yield within the oxide must be very similar to that within water. Monte Carlo track
calculations indeed predict that the average ionization yield of SiO
2
is 4.11 ions/100 eV, which is in
excellent agreement with the measured yield of hydrated electrons in water (Milosavljevic et al.,
2004). The pulse radiolysis experiments found no signicant variation in hydrated electron yields
with particle sizes of 7–22nm, suggesting that the electrons readily escape into the aqueous phase.
No fast time resolved experiments on the observation of the hydrated electron have been published,
but preliminary experiments have shown that the hydrated electron is produced within 8ps in aque-
ous suspensions of up to 40% SiO
2
, suggesting that electrons escape the SiO
2
with more than just
thermal energy (LaVerne et al., 2003). In other words, the carrier of the charge or energy within the
particle
must migrate very quickly to and through the interface with water.
Concurrent
with the production of an electron, a hole must also be formed in the solid phase.
Holes within the solid phase are difcult to examine in aqueous suspensions, but should the holes
migrate to the particle surface, they may react with solutes adsorbed on that surface. The oxidation
432 Charged Particle and Photon Interactions with Matter
of
( )SCN
2
−
and
Fe CN( )
6
3
−
on the surface of SiO
2
by radiolytically generated holes has been exam-
ined using electron pulse radiolysis techniques (Dimitrijevic et al., 1999). The oxidized products
are shown in Figure 16.3 and they can be seen to follow the volume fraction of the water in these
systems. These results suggest that the holes produced in the SiO
2
make it to the surface, but do
not cross into the aqueous phase. At the surface, they can initiate the oxidation of surface-bound
species (Dimitrijevic et al., 1999). Most commercial suspensions have substantial concentrations
of stabilizers that may react at the surface and interfere with the desired chemistry. Long time
studies of the hydrated electron will exhibit a pseudo rst-order decay due to the reaction with
additives.
Molecular hydrogen is the most common stable product examined in aqueous suspensions. The
driving force for many of these experiments is the same as in photochemical studies—the conver-
sion of radiant energy into chemical energy. A series of experiments have observed the change in the
production of H
2
in aqueous suspensions as a function of added TiO
2
and Al
2
O
3
(Yamamoto et al.,
1999; Seino et al., 2000, 2001a,b; Chitose et al., 2003). In all cases, an increase in the amount of H
2
generation over that of water alone is observed. Particle size ranged from 7 to 200nm with slurries
of 0.01% to 2% weight of ceramic oxide. The production of H
2
is independent of particle size for
particles less than a few tens of nm, but a substantially reduced yield of H
2
is observed at 200 nm.
Somewhat similar results are observed with SiO
2
suspensions, where above-normal yields of H
2
are
observed for 7 and 12nm particles and a decreased yield is observed for 22nm particles (Meisel,
2003). Yields are found to be linearly dependent on surface area suggesting that H
2
is formed at
the water–oxide interface (Seino et al., 2001a,b). However, large surface areas can be attributed to
small particle sizes and the observed increase in H
2
with decreasing particle size may be due to the
increased probability for H
2
precursor escape to or through the interface. High dose rates decrease
H
2
yields, presumably by increased second order reactions. The slurries of TiO
2
are very efcient
oxidizing agents in phenol degradation (Chitose et al., 2003). Studies with aqueous suspensions con-
taining a variety of ceramic oxides show that the efciency of H
2
production increases with particle
surface area and that the yields are greater than that of normal water (Cecal et al., 2008). In all of
the studies on suspensions, an increase in H
2
yield is found with increasing particle concentration.
Aqueous suspensions of TiO
2
with molar concentrations of methanol are found to give more H
2
0 10 20 30 40
0.6
0.8
1.0
1.2
1.4
Normalized values
SiO
2
(wt%)
Relative electron
density
H
2
O volume
fraction
Figure 16.3 The radiolysis of aqueous SiO
2
suspensions relative to that for pure water as a function of SiO
2
weight percent: (◾) hydrated electron concentration (Schatz et al., 1998); (
⚫
) hole production (Dimitrijevic et al.,
1999); (solid line) electron density; (dotted line) water volume fraction. (Reprinted from Schatz, T. et al., J. Phys.
Chem. B, 102, 7225, 1998; Dimitrijevic, N.M. et al., J. Phys. Chem. B, 103, 7073, 1999. With permission.)
Radiation Chemistry of Water with Ceramic Oxides 433
than without methanol (Jung et al., 2003). However, in these systems, methanol is probably being
sacriced at the surface of the particles and it is the source of H
2
rather than a modication in
the
water chemistry.
16.5 radiolysis oF adsorbed water
The yields of H
2
in the radiolysis of suspensions and slurries of ceramic oxides are enhanced com-
pared to that found in bulk liquid water. They also show a direct dependence on the particle surface
area suggesting that the water–oxide interface has an important effect on the radiation chemistry
of the surrounding water. However, even the most concentrated slurries have far too much water to
observe the chemistry occurring at the water–oxide interface without using special techniques such
as probe molecules on the particle surface. Pulsed radiolysis studies on aqueous suspensions suggest
that many of the electrons formed in SiO
2
migrate into the bulk water while the holes tend to remain
on the particle surface (Schatz et al., 1998; Dimitrijevic et al., 1999). However, the actual yield of
electron-hole pairs formed in ceramic oxides is not known. The recombination of an electron-hole
pair on a water molecule at the surface of a particle can lead to H
2
production by a dissociative pro-
cess (LaVerne and Pimblott, 2000). Even a single low-energy electron can lead to H
2
formation from
water by a dissociative attachment reaction (Rowntree et al., 1991; Kimmel et al., 1994; Corbut et al.,
1996). Such a process has been attributed to the observation of H
2
from water on silicon surfaces
(Klyachko et al., 1997). Exciton (electron-hole pair) formation and migration to the solid surface is
another potential source of H
2
production. The results are conclusive in that chemistry can occur
at the water–oxide interface, but the details remain unresolved. An excellent method to examine
radiation effects occurring on or very near the interface of water and ceramic oxides is to remove
most of the excess water and observe the chemistry at surfaces with a few layers of adsorbed water.
The rst studies on the radiolysis of adsorbed molecules examined the decomposition of organ-
ics on a variety of mineral solids, especially silica gel, in part because of interest in application to
petroleum rening. Allen and coworkers found the decomposition of the organics to be enhanced
in the adsorbed state as compared to the liquid or gas state and attributed the increase in radiolytic
yields to an energy-transfer process involving an exciton (Caffrey and Allen, 1958; Sutherland and
Allen, 1961; Rabe et al., 1964, 1966). They noted that the enhancement effects were greatest with
wide band gap solids and almost nonexistent with semiconductors. Sagert and coworkers noticed
that replacement of silanol by siloxane groups on silica gel had a large effect on radiation yields.
This work and several others attributed the increase in the decomposition of the organics to
electron-transfer processes from the solid to the adsorbants (Rojo and Hentz, 1966; Wong and
Willard, 1968). Khare and Johnson used an inorganic matrix system to denitively show that the
transfer of energy can depend on the band gap and illustrated the role of excitons in the process
(Khare
and Johnson, 1970).
Most
of the early studies on the radiolysis of adsorbed water were performed in the former Soviet
Union. The rst published studies on the radiolysis of adsorbed water were performed on semicon-
ductors and silica gel (Bubyreva et al., 1966; Krylova and Dolin, 1966). This work has been followed
by others examining the radiolysis of water adsorbed on a variety of surfaces and the production
of H
2
has been observed (Garibov et al., 1982a,b, 1984, 1987a,b, 1990, 1991a,b, 1992; Rustamov
etal., 1982; Garibov, 1983; Nakashima and Tachikawa, 1983, 1987; Nakashima and Aratono, 1993;
Nakashima and Masaki, 1996). All of these studies found that the type of oxide has a huge effect
on H
2
yields. The production of H
2
is by far the most common process examined in the radiolysis
of adsorbed water. This product is stable and relatively easy to measure. The main stable oxidizing
species produced in the radiolysis of water, H
2
O
2
, is known to react thermally at ceramic oxide
surfaces (Hiroki and LaVerne, 2005). Few studies have been reported on the radiolysis of H
2
O
2
in
the presence of ceramic oxides even though the process may be extremely important in nuclear
power
reactors.
434 Charged Particle and Photon Interactions with Matter
A very thorough examination of the effects of various types of ceramic oxides on the production of
H
2
from adsorbed water has been performed (Aleksandrov et al., 1991; Petrik et al., 1999, 2001).
These studies nd that most ceramic oxides fall into three categories when the yield of H
2
is deter-
mined with respect to the energy deposited in the water phase alone. These categories are as
follows: oxides in which the yield of H
2
is lower than that of water (MnO
2
, Cr
2
O
3
, Co
2
O
3
, and ZnO)
with yields of 0.001–0.01 molecule of H
2
per 100eV; oxides in which the yield of H
2
is comparable
to that for water (SiO
2
, V
2
O
5
, NiO, CuO, CdO, TiO
2
, and the alkaline earth oxides) with yields of
0.1–1 molecule of H
2
per 100eV; and oxides in which the yield of H
2
is greater than that of water
(La
2
O
3
, MgO, ZrO
2
, and Er
2
O
3
) with yields of 50–100eV per 100eV. The researchers conclude that
the physisorbed water has little effect on H
2
production and that surface OH groups also have little
effect. They claim that most of the H
2
is due to chemisorbed water, the highly structured ice-like
layer
between OH group surface layer and the physisorbed liquid-like layer.
A
few observations should be made about the determination of H
2
yields from adsorbed water.
The previous section on the radiolysis of aqueous suspensions noted that the density of the sys-
tem, and thereby the energy absorbed, increases with increasing oxide concentration. The dose in
aqueous suspensions is usually reported with respect to the energy deposited to the total system,
oxide and water. Product yields are expected to change with variation in oxide concentration simply
because of density and volume effects. The yields from the radiolysis of adsorbed water often report
the dose with respect to the energy absorbed by the water alone. This method is often favored in sys-
tems
of adsorbed water because a direct representation of energy transfer between the water–oxide
phases is obtained. A yield of H
2
lower than that observed in liquid water suggests energy or charge
transport into the oxide from the liquid or some sort of quenching process by the oxide surface. A yield
of H
2
greater than that observed in liquid water indicates energy transfer from the solid to the liquid or
other
enhancement in water decomposition.
The
amount of water on an oxide surface is usually only a few weight percent at most and difcult
to measure accurately (LaVerne and Tandon, 2002). A major problem in dosimetry with respect to
the energy deposited directly into the water is quantitatively determining the amount of adsorbed
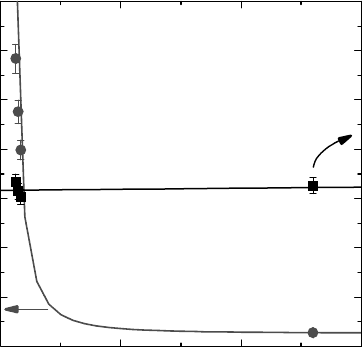
water for each type of ceramic oxide and in each experimental condition. Figure 16.4 shows a typical
result for the yield of H
2
from water adsorbed on ZrO
2
as determined by the energy deposited in the
0.0 0.5 1.0 1.5
0
25
50
75
100
125
150
175
ZrO
2
γ-rays
G(H
2
) (molecules/100 eV in water)
Water loading (wt%)
0.000
0.025
0.050
0.075
0.100
0.125
0.150
0.175
G(H
2
) (molecules/100 eV in total)
Figure 16.4 Yield of H
2
in the gamma ray radiolysis of the water adsorbed on ZrO
2
as a function of the
water weight percent: (
⚫
) yield relative to the energy deposited in the water alone; (◾) yield relative to the energy
deposited
in both the water and oxide.
Radiation Chemistry of Water with Ceramic Oxides 435
whole system (ceramic oxide and water) or deposited in the water alone (LaVerne and Tandon, 2002).
The amount of water in this example is equal to the physisorbed water as determined by the weight
gain following adsorption from constant humidity chambers. The yield of H
2
with respect to energy
deposited directly into the water is on the order of a few hundred molecules per 100eV of energy.
This yield is huge and it is too large to be due only to water decomposition by the energy directly
deposited in that phase. A yield of 100 molecules/100eV corresponds to 1eV for the formation of one
H
2
molecule. This amount of energy is much too small to account for bond breakage and atomic rear-
rangement and indicates that energy is being transferred from the solid phase to the water. A yield of
100 molecules/100eV would seem to imply the prolic production of H
2
, remember that the yield for
bulk water is 0.45 molecules/100 eV. However, the amount of water in these systems is extremely
small. On an absolute scale, the yield of H
2
is only about 0.08 molecules/100 eV when the dose is
determined with respect to the energy deposited in the whole system (ceramic oxide and water),
Figure 16.4. Dosimetry in the radiolysis of adsorbed water is commonly reported using both
methods for energy deposition, in the water alone or in the water and ceramic oxide, depending
on the point being made.
The yields of H
2
exhibit interesting trends as a function of the amount of water loading that can
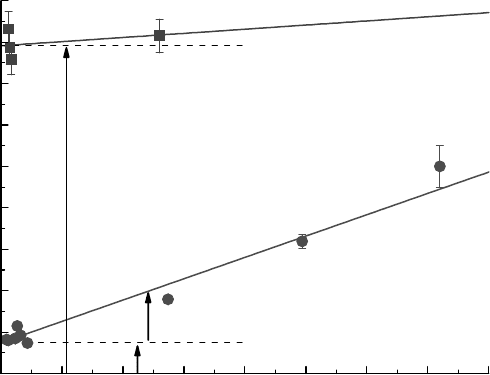
be used to show the effects due to the type of ceramic oxide. Figure 16.5 gives the yields of H
2
from
water adsorbed on ZrO
2
and CeO
2
as a function of the water weight percent (LaVerne and Tandon,
2002). Dosimetry in this example is with respect to the total energy deposited in both the water and
the ceramic oxide. The results show a slight increase in H
2
yield with increasing water fraction, and
the yields with ZrO
2
are distinctly higher than that observed with CeO
2
. Another interesting feature
in this presentation of the data is the extrapolated yield of H
2
at zero water fraction. Obviously, no
H
2
can be produced without some form of water on the particle surface. The water in this example,
Figure 16.5, is determined from the weight gain of physisorbed water. A dissociated water layer and
an ice-like layer are strongly bound to the oxide surface beneath the physisorbed water layer. These lay-
ers of water near to the particle surface appear to be responsible for a large portion of the observed
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0
0.00
0.01
0.02
0.03
0.04
0.05
0.06
0.07
0.08
0.09
γ-rays
CeO
2
ZrO
2
Surface effect
Yield from increasing water layers
Surface effect
G(H
2
) (molecules/100 eV in total)
Water loading (wt%)
Figure 16.5 Production of H
2
relative to the amount of total energy (water + oxide) deposited by gamma
rays as a function of the weight percent of physisorbed water on the oxides: (
⚫
) CeO
2
, (◾) ZrO
2
(LaVerne and
Tandon, 2002). The extrapolated H
2
yields at zero water loading represent the production due to dissociated
water
and ice-like water at or near the oxide surface.
436 Charged Particle and Photon Interactions with Matter
difference in the yields of H
2
. Water in the physisorbed layer is probably behaving like normal bulk
water when irradiated. Clearly, the water–oxide interface is playing an important role in water
decomposition
and in the production of H
2
.
Much of the literature on the radiolysis of adsorbed water has focused on the effects of the type
of oxide on the production of H
2
at the water–oxide interface. Following an extensive investigation
of
many types of oxides, the observation was made that oxides within a narrow band gap energy at
about 5eV can lead to a signicant increase in H
2
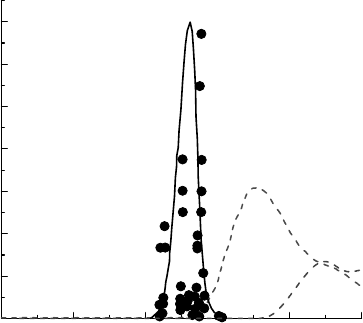
(Petrik et al., 1999, 2001). The dose in these stud-
ies was determined with respect to energy deposited in water alone and some of those results are
reproduced in Figure 16.6. The narrow band gap energy response for the large increase in H
2
yield
suggests that a resonance process is responsible. An exciton may possibly migrate to the particle
surface and resonantly couple to a specic state of a water molecule that is favorable toward the pro-
duction of H
2
. However, not all oxides within this band gap lead to an increase in H
2
yields, so the
band gap cannot be the sole characteristic for enhanced H
2
production. Furthermore, a resonance
process implies a coupling between the oxide and the water. Figure 16.6 shows the dissociative
electron absorption (DEA), cross section, and the differential dipole oscillator distribution (DIOS)
for water ice as functions of the absorbed energy (LaVerne and Mozumder, 1986; Klyachko et al.,
1997). The energy dependence of neither of these processes matches well with the proposed band
gap resonance energy. Special states of water may be formed when they are in the vicinity of certain
ceramic oxides. Of course, resonance and a variety of other processes may be contributing in vari-
ous magnitudes to the radiolytic formation of H
2
from adsorbed water. Further work is required to
understand
this important phenomenon.
The
mechanism for the transport of energy to the surface of the ceramic oxide is still in ques-
tion. The transport medium in the radiolysis of ZrO
2
has been suggested to be excitons, which are
electron-hole pairs (Petrik et al., 2001). One method for identifying the nature of the energy carrier
is by the addition of dopants that can trap the transient species within the particle. An increase in
the number of trapping sites should decrease the probability of excitons reaching the surface and
thereby decrease the observed H
2
production. The substitution of Nb
5+
for Zr
4+
sites in ZrO
2
at an
amount of only 0.1% by mass gives rise to a dramatic decrease in H
2
yields to essentially zero.
Substitution of a small amount of Li
+
or Rb
+
ions in ZrO
2
is found to increase the H
2
production
0 2 4 6 8 10
0
20
40
60
80
100
120
140
Relative yield or intensity
Energy (eV)
H
2
DEA
DIOS
Figure 16.6 The relative yield of H
2
(
⚫
) (Petrik et al., 2001) determined from the energy deposited in the
water alone as a function of the band gap energy for several oxides. The dashed lines are the relative cross
sections for dissociative electron attachment (Klyachko et al., 1997) and the differential dipole oscillator
strength distribution for amorphous ice at specic energies (LaVerne and Mozumder, 1986). (Reprinted
from Petrik, N.G. et al., J. Phys. Chem. B, 105, 5935, 2001. With permission.)
Radiation Chemistry of Water with Ceramic Oxides 437
by a factor of 2 (Petrik et al., 2001). These results suggest that the precursor to H
2
production is
due to an electronic excitation such as an exciton that diffuses through the solid to the interface.
The attenuation of secondary electrons would require much larger concentrations of dopants than
observed. However, these results have to be resolved with the radiolysis of suspensions of SiO
2
,
where there is strong evidence that nonthermal electrons directly escape the particle. A competition
must exist between exciton transport to the surface and trapping by defect sites within the particle.
Excitons are self-trapped in SiO
2
within 250fs so they obviously cannot travel a large distance
through the particle (Saeta and Greene, 1993; Zhang et al., 1997). The different energy carriers may
exist in most of the ceramic oxides, but they may have dissimilar degrees of importance and future
studies
will have to resolve the issue.
Studies
on the production of H
2
from SiO
2
of different surface areas suggest that the transport
is limited to the outer layer of 100nm thickness (Aleksandrov et al., 1991). Other studies with SiO
2
suggest
that particles smaller than 22
nm
have complete transfer of energy to or through the water–
oxide
interface (Milosavljevic et al., 2004). Thomas and coworkers found a dramatic decrease in
H
2
yields on variation of SiO
2
particles size from 6 to 100nm (Zhang et al., 1997). Exciton migra-
tion distance is estimated to be only 5nm in ZrO
2
(Petrik et al., 2001). Nonthermal electrons may
travel relatively large distances in solid media, whereas excitons must be formed very near to the
surface in order to react with adsorbed molecules. The transport layer thickness may also depend
on the morphology of a given ceramic oxide. Differences in electronic transport are observed in
the monoclinic and tetragonal forms of ZrO
2
(Lemaignan, 2002). These differences may explain
the increased production of H
2
observed in tetragonal ZrO
2
as compared to the monoclinic form
(LaVerne, 2005). This latter dependence of yields on morphology is especially important in nuclear
reactor technology as radiation is known to change the microscopic crystalline structure of certain
ceramic oxides (Lemaignan, 2002). Radiation aging of reactor components could modify the chem-
istry at the water–oxide interface and thereby greatly increase the difculty in making long-term
predictions.
16.6 radiolysis oF ConFined water
Water can be in intimate contact with surfaces while conned to pores or cavities within the ceramic
oxide. This environment is different than that of adsorbed water in that the chemistry of the nor-
mally reactive species such as the hydrated electron, H atom, and OH radical can be modied due
to the conned space. Diffusion-controlled reactions of radicals can be restricted within a cavity
because of the small number of molecules. The passage of species in and out of cavities may be
limited by the diameter of the cavity or the size of the openings to the cavity. Reaction of species
between cavities can be prohibited or predetermined by some characteristic such as size or local
acidity. Many natural materials such as the zeolites have distinctive cavity sizes and shapes and
were among the rst to be studied using radiolysis (Zhang et al., 1998). The interest in these materi-
als followed naturally from their use as catalysts in a wide variety of circumstances especially in the
petroleum industry. Recent fabrication techniques have led to an array of porous materials speci-
cally
designed for research and industrial applications.
The
rst instances of the radiolysis of conned water made use of wet silica gel, alumina, and
quartz (Emmett et al., 1962; Weeks and Abraham, 1965). Gamma radiolysis of these materials
at 80K is relatively easy to perform and results in the trapping of the H atoms produced by the
water decomposition. Electron paramagnetic resonance (EPR) techniques give a readily identi-
able H atom signal because of their huge coupling constant. Interstitial sites have been identied as
Bronsted
or Lewis acid sites.
The
radiolytic production of the H atoms observed in the EPR experiments is derived from the
water incorporated in the cavities. Specically, the adsorbed water is in the form of siloxyl groups
(≡≡Si–OH). EPR studies on silica have provided extensive knowledge on the formation, migra-
tion, and reaction of H atoms (Shkrob and Trifunac, 1996, 1997; Chemerisov and Trifunac, 2001;
438 Charged Particle and Photon Interactions with Matter
Chemerisov et al., 2001). The solid phase makes up most of the mass of these systems so the initial
radiolysis process in silica is the production of the electron and hole, SiO
2
→ e
−
+ h
+
, followed by
e
−
+ h
+
→
3
exciton. Combination reactions of the electron and hole lead to the triplet exciton forma-
tion with a mean lifetime of about 150fs (Audebert et al., 1994). The excitons, electrons, and holes
are the main mobile species on the very short timescale. They will be trapped by interstitial sites or
diffuse to the surface where they may initiate chemical reactions with the conned water. As men-
tioned before, self-trapping of the exciton in SiO
2
occurs within 250fs, so excitons will not move
far (Saeta and Greene, 1993). On the other hand, many porous materials have very large surface-to-
volume
ratios so excitons can readily reach the water–oxide interface.
At
low temperatures, excitons produced by the radiation migrate to the silanol group giving the
H
atom and an oxygen hole center, ≡≡SiOH +
3
exciton → ≡≡SiO
•
+ H
•
. The H atoms are trapped at
temperatures below about 150K and are observable using EPR. As the temperature is raised, H atoms
become very mobile in silica and they decay mainly by recombining with oxygen hole centers or
polarons in silica without added water (Shkrob and Trifunac, 1996). H atoms may be produced by
reactions of thermal electrons with interstitial protons at higher temperatures, ≡≡SiOH
+
Si≡≡ +
e
−
→ ≡≡SiOSi≡≡ + H
•
, or ≡≡SiOH
2
+
+ e
−
→ ≡≡SiOH + H
•
. Even “dry” silicates produce a signicant
amount of H
2
due to reactions of H atoms produced from the hydroxyl surface groups (Rotureau
et al., 2005). H atoms and excitons can migrate to cavity surfaces and initiate chemical reactions
with conned water (Shkrob et al., 1999). Electrons and holes are more likely to recombine in the
bulk solid unless they are formed very near the surface. Obviously, energy directly deposited in the
conned water will also lead to product formation. Competition reactions leading to product forma-
tion seem to be controlled by migration of species between the phases as well as pore size, shape,
and
water fraction.
Further
evidence that free electrons are involved in the radiolysis of interstitial water is shown
by the production of N
2
from zeolites with N
2
O (Nakazato and Masuda, 1986; Aoki et al., 1988).
More importantly for water radiolysis, the yield of H
2
is found to decrease with the addition of
N
2
O and increase with the addition of ammonia. The N
2
O scavenges the electron precursors to H
2
,
while the ammonia scavenges the positive hole centers to decrease recombination reactions. Zeolite
slurries are observed to give H
2
yields of about 1–2 molecules of energy adsorbed in the system
(zeolite + water), which is a considerable increase from the 0.45 value for pure water (Cecal et al.,
2008). Smaller-sized cavities were found to give higher H
2
yields than larger ones, suggesting that
cavity size and not just surface area is important. Kinetic studies to determine the reactivity of the
electrons in the cavities suggest that the electrons are not hydrated (Nakazato and Masuda, 1986).
However, spectroscopic studies have identied hydrated electrons in the radiolysis of water in cer-
tain
types of zeolites (Liu et al., 1995).
A
recent series of papers have thoroughly examined the decomposition of water conned in a
variety of porous silica and mesoporous molecular sieves (Foley et al., 2005; Le Caer et al., 2005a,b;
Rotureau et al., 2005). The formation of H
2
in large cavities lled with water mainly occurs by the
same mechanisms as in bulk water radiolysis, that is, by reactions of H atoms and hydrated elec-
trons. The systems were examined as a function of water content within the cavities and also as a
function of cavity size. As water is depleted from the cavity, a void is formed in the center of the
cavity. The remaining water adsorbed on the walls is more stable due to the ice-like structure dis-
cussed in the previous section. H
2
yields were found to increase dramatically with decreasing water
content. Yields of H
2
are as high as 30 molecules/100 eV energy initially deposited in the water
phase (Rotureau et al., 2005). The production of H
2
from the adsorbed water appears to be much
more efcient than in bulk water, even bulk water conned to a cavity. Hot electrons or H atoms
may pass through the interface and produce H
2
by dissociative attachment reactions (Rotureau
etal., 2005). Excitons at the surface may also transfer energy to the conned water leading to
H
2
production by excited-state dissociation. A variety of mechanisms may occur, but no experi-
mental evidence exists on the relative importance of these reactions leading to H
2
production.
Interestingly, EPR and IR techniques show more defects in irradiated dry silica than when
Radiation Chemistry of Water with Ceramic Oxides 439
water is present (Le Caer et al., 2005a,b). Transfer of energy from the solid phase to the water is
preferred,
even to the point of depletion of the available water molecules.
In
normal bulk water radiolysis, OH radical scavengers such as bromide anion or alcohol are
added to prevent depletion of the H
2
yield. The addition of bromine anion, formate, or isopropanol
has no effect on the production of H
2
from water in silica cavities (Rotureau et al., 2005). Radicals
may be separated by the pores thereby decreasing combination reactions or the OH radicals may be
more reactive with something else in the cavity than with H
2
. OH radicals are produced in cavities
as evidence by their scavenging with coumarin (Foley et al., 2005). As with H
2
yields, OH radical
yields increase with decreasing water content of the cavities suggesting that adsorbed water is very
efcient in their production. In fact, the scavenger studies suggest that a signicant amount of OH
radicals are produced at the silica–water interface. Higher yields of OH radicals are observed with
increasing areas of the interface, inferring the transfer of energy from the silica to the interface is
responsible for at least part of the OH production. Some OH radicals may be scavenged by wall
sites, but a signicant fraction combines to form H
2
O
2
. The yield of H
2
O
2
has been determined using
scavenger techniques and found to agree well with H
2
yields (Le Caer et al., 2007). The results sug-
gest that the complementary reducing and oxidizing stable species of H
2
and H
2
O
2
are formed in
equal yields in the radiolysis of conned water. If so, then both oxidizing and reducing species must
be
transported through the silica–water interface, leading to the decomposition of water.
16.7 ConClusions
The radiolysis of water in association with interfaces is becoming one of the new challenges in asso-
ciation with nuclear technology. Understanding the radiolytic decomposition of water adsorbed on
or very near to solid surfaces can be even more demanding than bulk water because of the transport
of charge and energy to and through the water–solid interface. Many studies have shown that the
radiolysis of water is different at a solid interface than in the bulk liquid, although the mechanisms
are still not resolved. The nature of the energy carrier and the mode of transport in the solid are still
in question. Of particular importance is to identify the specic properties of the solid responsible
for the transport of energy and charge. The nature of the water at or near solids and the effect of the
interface
on its radiolytic decomposition still require investigation.
aCknowledgments
This contribution is NDRL-4810 from the Notre Dame Radiation Laboratory, which is supported by
the
Ofce of Basic Energy Sciences of the U.S. Department of Energy.
reFerenCes
Al-Abadleh, H. A. and Grassian, V. H. 2003. FT-IR study of water adsorption on aluminum oxide surfaces.
Langmuir
19: 341–347.
Aleksandrov,
A. B., Byakov, A. Y., Vall, A. I., Petrik, N. G., and Sedov, V. M. 1991. Radiolysis of adsorbed
substances
on oxide surfaces. Russ. J. Phys. Chem. 65: 847–849.
Alig,
R. C. and Bloom, S. 1975. Electron-hole-pair creation energies in semiconductors. Phys. Rev. Lett. 35:
1522–1525.
Allen, A.
O. 1961. The Radiation Chemistry of Water and Aqueous Solutions. New
York: Van
Nostrand.
Aoki,
M., Nakazato, C., and Masuda, T. 1988. Hydrogen formation from water adsorbed on zeolite during
gamma-irradiation.
Bull. Chem. Soc. Jpn. 61: 1899–1902.
Audebert,
P., Daguzan, P., Dos Santos, A., Gauthier, J. C., Geindre, J. P., Guizard, S., Hamoniaux, G., Krastev, K.,
Martin, P., Petite, G., and Antonetti, A. 1994. Space-time observation of an electron gas in SiO
2
. Phys.
Rev. Lett.
73: 1990–1993.
Behar,
D. and Rabani, J. 2006. Kinetics of hydrogen production upon reduction of aqueous TiO
2
nanoparticles
catalyzed by Pd
0
, Pt
0
, or Au
0
coating and an unusual hydrogen abstraction; steady state and pulse radiolysis
study.
J. Phys. Chem. B 110: 8750–8755.
