Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

480 Charged Particle and Photon Interactions with Matter
respectively. The (2 + 1) REMPI scheme via the E,F
1
g
∑
+
intermediate state was used to detect the
D
2
(H
2
) products in the ground (X
1
g
∑
+
) state.
The LEEs are typically supplied using pulsed electron beams generated by means of commer-
cially available low-energy (5–1000 eV) electron guns. The typical full width at half maximum
(FWHM) of the electron energy distribution is <0.5eV. The focused beam size is normally ∼1 mm,
and varies little with the electron energy. Most experiments use a variable pulsed (20–200 Hz,
100 ns–25 μs) electron beam that gives electron doses of 10
−5
–10
−3
electrons/surface molecule/s.
This dose is quite small so that all desorption and dissociation events observed are due to direct
electronic transitions and have cross sections greater than ∼10
−23
cm
2
. The electron ux is mea-
sured by picoammeters and is always kept below 10
14
electrons/cm
2
/s to eliminate or greatly
reduce any potential charging effects. The variation of the electron ux is performed by changing
the gun emission current; all desorption and dissociation events discussed in this chapter have a
linear dependence on the electron-beam ux. This indicates that the observed dissociation and
desorption channels arise solely from single scattering events. When necessary, the pulsed electron
beam can be focused on the target and controlled by x–y deection to scan the entire substrate
surface. Since the electron-beam spot size and ux are measured accurately, absolute cross sections
can be obtained.
18.3.3 poStirradiation teMperature-prograMMed deSorption
In addition to the measurement of accurate stimulated desorption and dissociation cross sections,
the subsequent chemistry initiated by the LEE inelastic energy-loss channels can be examined by a
technique known as postirradiation temperature-programmed desorption. This is a straightforward
approach that involves irradiating the sample with a xed dose or ux at a given electron impact
energy. After the irradiation, the lm is heated slowly (typically 8 K/min) and the desorbed prod-
ucts are detected with a QMS as a function of substrate temperature. These experiments are then
carried out for several different electron impingement energies and total doses. The results, when
compared to TPD studies of similar targets that have not been irradiated, yield information regard-
ing the electron-induced chemistry within the ice lms. A disadvantage of this technique is that it
is difcult to examine purely the radiation-driven processes, since the results are a convolution of
radiation and thermally induced reactions. Although this topic will not be discussed in detail in this
chapter, we note that an Fourier transform infra red (FTIR) spectroscopic study of the lms before,
during,
and after irradiation helps to overcome this problem.
18.3.4 poStirradiation gel electrophoreSiS
Another novel technique for the analysis of DNA damage is to remove the irradiated samples from
the UHV chamber and then apply conventional wet chemical methods to determine the extent of
damage. The technique was originally developed by Sanche and coworkers (Boudaiffa etal., 2000).
After electron irradiation, the biological materials (e.g., DNA) are removed from the chamber, redis-
solved into water, and examined ex situ using a technique known as agarose gel electrophoresis.
The supercoiled DNA single-strand breaks (SSBs) and double-strand breaks (DSBs) can easily be
separated spatially based on their different migrating abilities in agarose. Quantitative analysis of
SSBs and DSBs can be determined by integrating the light intensities of corresponding ethidium-
stained DNA bands, thus allowing the measurement of the SSB and DSB probabilities as a function
ofelectron-beam energy and dose. For the water–DNA damage studies, total electron doses of
only ∼10
13
electrons/DNA sample are used. This dose is two orders of magnitude lower than that
known to cause charging in DNA lms (Zheng etal., 2006) and assured a dose response in the
linear regime. The amount of DNA is quantied by measuring absorbance at 260 nm before and
after irradiation. Usually, more than 95% of the deposited mass of DNA is recovered. Three con-
trolled DNA samples are usually analyzed together with postirradiation samples: (1) an original
Low-Energy Electron-Stimulated Reactions in Nanoscale Water Films 481
plasmid DNA solution; (2) a DNA sample that was dried, transferred in vacuum, and recovered in
water
without irradiation; and (3) DSB DNA made by the restriction enzyme Sca I.
18.4 a useFul multiple sCattering Formalism
The theory we utilize to investigate the LEE-induced damage of water and water–DNA interfaces
was developed to examine diffraction effects in stimulated desorption (Sieger etal., 1999; Orlando
etal., 2004; Oh etal., 2006) and is a modication of the scattering theory used to describe photo-
electron diffraction (Chen etal., 1998) and XAFS (Rehr and Albers, 1990). A brief description of
the most relevant aspects of the calculation is given below. Specically, the electron wavefunction
is given by
φ φ φ φ φ( ) ( ) ( ) ( ) ( )r r r r r= + + + +
0 1 2
N
(18.1)
where
ϕ
0
(r) and ϕ
N
(r) represent the non-scattered
Nth
order scattered components, respectively
The
rst-order scattering component can be represented as
φ π ρ
1
0
4( ) ( )
*
( )r R
r
k R
=
( )
⋅
∑∑
G t Y e
L i
i
i L
i
Li
i
k
ˆ
B
(18.2)
In order to solve the free-space electron propagator, G
LL′
, the separable representation has been
introduced
with the expression
G
e
LL
i
L L
′
′
=
∑
( ) ( ) ( )
ρ
ρ
ρ ρ
ρ
λ
λ
λ
Γ Γ
(18.3)
where λ is the matrix index for quasi-angular momentum. After some mathematical treatment, the
rst-order
scattering component can be expressed as
φ ρ ρ
ρ
λ
λ
λ
ρ
1
( ) ( ) ( , )r M P
r r
r
k R
r
=
∑∑
⋅
i
i
i
i
i
i
e
e
i
i
k
ˆ
(18.4)
where
P
λ λ
ρ π ρ( , ) ( )
*
( )
ji
L
ji
i
L
L
t Yk k
ˆ ˆ
=
∑
4 Γ
(18.5)
is dened as the introduction matrix and M
r rλ λ
ρ ρ( ) ( )
i i
= Γ
0
is the termination matrix (Rehr and
Albers,
1990; Rehr etal., 1995).
18.5 low-energy eleCtron interaCtions with water interFaCes
Before we discuss the primary desorption and dissociation channels involved in LEE interactions with
nanoscale lms of water and water–DNA interfaces, a brief review of the electronic structure and
excited states of condensed-phase water is necessary. A more detailed description of the electronic
structure of water can be found elsewhere (Ramaker, 1983; Sieger etal., 1997; Herring etal., 2004).
482 Charged Particle and Photon Interactions with Matter
18.5.1 electronic Structure of water thin filMS
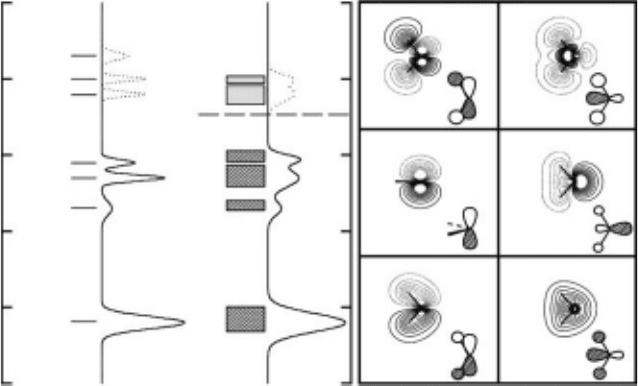
The real-space orbitals of water can be represented as a linear combination of the valence
molecular orbitals: the 1b
2
and 2a
1
orbitals are the primary constituents of the O–H bonds,
while the 1b
1
and a
1
make up the oxygen lone-pair orbitals (Jorgensen and Salem, 1973). The
four lowest unoccupied molecular orbitals are 4a
1
, 2b
2
, 2b
1
, and 5a
1
. The 4a
1
and 5a
1
orbitals
are strongly antibonding, and occupying these states leads to breaking of the O–H bond. The
4a
1
orbital mixes with the 3s Rydberg state in the gas phase, and is sometimes referred to as
3s4a
1
. Calculations and photoemission data show that the electronic structure of condensed
ice retains much of the gas-phase character with some broadening and shifting of the energy
levels. Since the molecular orbitals retain much of their gas-phase character, the peaks in the
ice valence-band density of states are usually labeled by the same spectroscopic notation as
free water. The molecular orbitals, the gas-phase photoelectron spectrum, and condensed-phase
photoemission data are shown schematically in Figure 18.5. The unoccupied 4a
1
orbital is in
the bandgap, spatially extended, and is mixed with the 2b
1
level. The conduction band of ice is
very narrow, with the band minimum less than 1 eV below the vacuum level. The Fermi level is
estimated to be 5 eV above the 1b
1
band maximum. The optical absorption spectra of ice show
a pronounced peak at ∼8.6 eV, corresponding primarily to a 1b
1
→ 4a
1
transition, well below
the photoelectric threshold (Kobayashi, 1983). In the solid state, the unoccupied molecular orbit-
als (i.e., the 4a
1
) maybe best described as Frenkel excitons, in which the Coulomb attraction
localizes the electron–hole pair on the water molecule. This may be particularly important at
surfaces and terminal sites.
The largest change in the condensed phase occurs at the levels of a
1
symmetry, which are
signicantly broadened (compared to the 1b
1
and 1b
2
levels), and are most affected by hydro-
gen bonding. Calculations of the ice band structure indicate that the a
1
bands have the most
dispersion, while the 1b
1
and 1b
2
bands are virtually dispersionless. One would then expect
that electronic excitations involving a
1
bands would be the most sensitive to changes in the
hydrogen-bonding environment. Specically, a reduction of the bandwidth should occur if the
10
0
–10
–20
–30
–40
2a
1
2a
1
1b
2
4a
1
CB
E
f
SolidVapor
3a
1
1b
1
1b
2
2b
2
1b
1
1b
2
4a
1
3a
1
2a
1
Energy(eV)
3a
1
1b
1
3s4a
1
2b
2
2b
1
5a
1
Figure 18.5 Left frame: Comparative relationship between the gas-phase and condensed-phase photoelec-
tron spectra of water. Right frame: The similarity allows for extension of the gas-phase state labels to the solid
phase.
Molecular orbital density plots of the valence levels for the water molecule.
Low-Energy Electron-Stimulated Reactions in Nanoscale Water Films 483
hydrogen bonding is weakened, and the lifetimes of electrons and holes in these bands should
increase as a consequence.
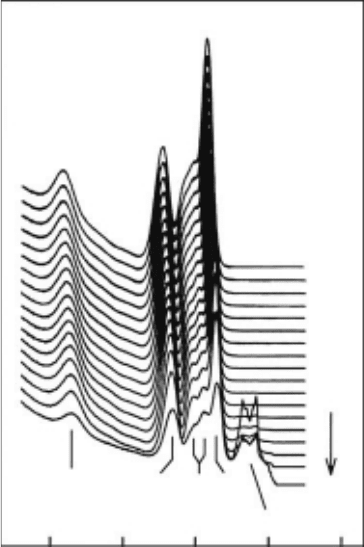
The effect of temperature on the photoemission spectra of ice has been measured and is
shown in Figure 18.6. It is clear that the a
1
bands are the broadest; however, the broadening
associated with the 3a
1
level emerges into a reasonably well-dened splitting as the temperature
is increased. The 3a
1
molecular orbital is composed primarily of the oxygen lone-pair orbitals,
is strongly involved in hydrogen bonding, and is very sensitive to the local geometry of nearby
water molecules. The splitting and narrowing of the 3a
1
level is likely due to the reduced per-
turbation on the a
1
levels and the general return of the atomic P
z
character as the number of
hydrogen bonds drops with increasing temperature. For a symmetric pair of water molecules in a
unit cell of ice, the 3a
1
orbitals directly interact with each other giving rise to a bonding and anti-
bonding combination (Nordlund etal., 2008). Alternatively, this splitting can also be described
in terms of Davydov splitting due to the fact that ice has a nonzero entropy even at 0 K (Petrenko
and Whiteworth, 2002). Thus, adjacent unit cells in the lattice are symmetry-inequivalent and
can have nonzero overlap integrals allowing for mixing of the a
1
bands between cells (Winter
etal., 2004).
Though it is difcult to discern this in Figure 18.6, the bands, particularly the 2a
1
level, shift ∼1eV
to lower energy (relative to the Cu substrate) as the temperature increases. This can be attributed to
the change in the work function. Note that the orientation of water molecules in the surface dipole
layer collectively contributes to the work function. As the temperature increases, the net orientation
of these molecules changes, giving rise to many more water molecules with dangling OH bonds
pointing into the vacuum.
40 ML amorphous D
2
O/Cu(111)
hν = 55 eV
2a
1
1b
2
20 30 40 50
Electron kinetic energy (eV)
60
3b
1
1b
1
180 K
110 K
Cu d-bands
Temperature
Photoemission intensity (arb. units)
2a
1
and 3a
1
narrow
with increasing temp
Figure 18.6 Temperature-dependent photoemission data of 40ML amorphous ice deposited on Cu(111)
from
110 to 180
K.
484 Charged Particle and Photon Interactions with Matter
18.6 stimulated dissoCiation and desorption
o
F w
ater
t
hin
Films and i
nter
F
aCes
18.6.1 lee-StiMulated deSorption of anionS
TNI resonances result when the ground or excited electronic states of molecular collision partners
temporarily capture an incident electron during the electron–molecule collision. The simplest
TNI is produced when the incident electron is trapped by the centrifugal potential arising from
the interaction between the incident electron and the neutral molecule in its ground electronic
state. These are referred to as single-particle shape resonances. Shape resonances usually lie at
low energies (∼E
i
< 5 eV) and are energetically above the potential energy surfaces of the neu-
tral molecule ground states. These resonances typically decay via autodetachment leaving the
neutral moleculewith vibrational and rotational excitation. Another important decay pathway is
the DEA, which involves the formation of anionic and neutral fragments. Shape resonances can
also involve electronic excitations, and these core-excited shape resonances involve an attractive
interaction between the incident electron and the excited state of the target molecule. Since the
potential barrier is strongly dependent on the angular momentum value of the excited-state orbitals,
p-, d-, and f-waves are expected. These resonances also lie above the neutral excited-state ener-
gies and decay by autodetachment or DEA.
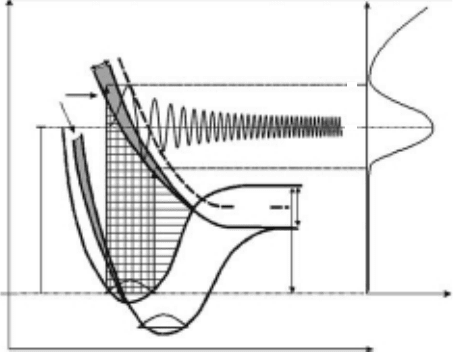
Another type of a core-excited resonance is referred to as the Feshbach (Type-I) resonance. This
involves coupling of the kinetic energy of the captured electron to nuclear motion. If the incident
electron excites a valence or shallow core level, a dynamic polarization is produced and the electron
is temporarily trapped in the dipole eld of the excited target molecule. Electron correlation and
reduced screening allow the incoming electron to couple to the excited electron and a slightly posi-
tive core, producing a one-hole, two-electron core-excited Feshbach resonance. These resonances
lie below the corresponding excited states (i.e., they have a positive electron afnity). The energy
gained in this correlation is referred to as the Feshbach decrement, and is typically ∼0.5eV. As
depicted in Figure 18.7, the transition is typically mediated by Franck–Condon factors and the cross
section is mediated by lifetimes against autodetachment and dissociative attachment (Ingolfsson
etal., 1996). The negative-ion fragments are often produced with an excess kinetic energy, which
can be partitioned into reactive scattering events with coadsorbed molecules or surface terminal
Potential energy
AB*
–
AB*
Γ
A + B
A + B
–
EA(B)
D(AB)
ε
AB
AB
–
0
Anion yield
AB bond coordinate
r
c
Incident electron energy
Figure 18.7 Schematic of the potential energy surfaces accessed during DEA processes. Inelastic electron
scattering results in a transition to an excited (dissociative) potential surface that is asymptotic to the anionic
decay
channels.
Low-Energy Electron-Stimulated Reactions in Nanoscale Water Films 485
groups. As illustrated in Figure 18.7, the conservation of energy and momentum yields the most
probable
kinetic energy of the departing anion fragment, E
K
−
, as
E E
nK
−
= − + − − −( ) [ ]1 EA(B) D(AB) D(AX)β ε
(18.6)
where
β
is the ratio of the mass of B
−
to that of AB
ε
is the captured electron’s energy
EA(B) is the electron afnity of B
D(AB)
is the bond energy of AB
E
n
is the excitation energy of the fragments (Christophorou etal., 1984)
It
is apparent that DEA favors the loss of the light fragment.
The
effects of the condensed phase on the DEA and TNI resonances have been studied and several
aspects are well understood. Specically, the DEA and TNI resonances can be drastically altered due
to third-body interactions that open pathways for energy dissipation (Bass and Sanche, 1998, 2003a,b;
Sanche, 2000). These interactions can have an effect during the lifetime of the TNI as well as alter
products after the TNI dissociates. Most notably, dissociation from the low-energy shape resonances
is suppressed, while dissociation from the Feshbach resonances can be enhanced by the surrounding
medium.
Although there is no detection of anions from the low-energy shape resonance, dissociation
could still occur where the products simply do not gain sufcient kinetic energy to escape the surface
potential. Mechanisms for enhancement in dissociation can be explained by the effects of the medium
on the electron autodetachment probability. One mechanism for dissociation enhancement through the
Feshbach resonance is the lowering in energy of the anionic potential energy surface via the substrate
and surrounding medium polarization interaction (Balog etal., 2004). Due to the lowering of the ionic-
state potential, the wavepacket on the TNI potential spends less time traversing it, thus lowering the
autodetachment probability. Another explanation for enhanced dissociation in the condensed phase is
the more efcient conversion of an open-channel resonance into a Feshbach resonance due to interac-
tions with the medium (Balog etal., 2004). An open-channel resonance lies higher in energy than
the Feshbach resonance and only requires a one-electron transition to return to the associated neutral
molecule compared to the two-electron transitions needed for the Feshbach resonance. The electron
autodetachment probability is higher for the one-electron transition compared to the two-electron
transitions; thus, the Feshbach resonance is more likely to dissociate than autodetach.
The formation of H
−
(D
−
) from the DEA of water H
2
O (D
2
O) was studied extensively in the gas
phase (Compton and Christophorou, 1967; Melton, 1972; Jungen etal., 1979; Curtis and Walker,
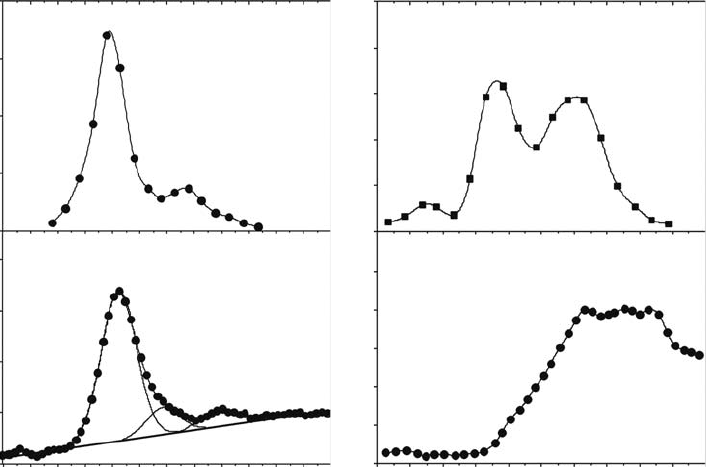
1992; Fedor etal., 2006), and some of these recent results from Fedor etal. are shown in Figure
18.8a (Fedor etal., 2006). There is a strong resonance with a threshold energy near ∼5 eV, a peak at
6.7eV, and a weak resonance with a peak near 9.7eV. The yield of H
−
(D
−
) from ASW (i.e., nanoscale
multilayers of water) condensed on Pt(111) has also been studied over the past several years. Our
results from these experiments are shown in Figure 18.8b (Simpson etal., 1997). The main feature
peaking near 6.7eV remains although the peak shifts to ∼7.3eV in the condensed phase. The width
of
this feature is also broader, and there is a resolvable weak structure near 10
eV.
The rst feature observed in Figure 18.8a involves excitation of the 1b
1
electron into the mixed
3s4a
1
level, giving rise to a
2
B
1
, one-hole, two-electron (…1b
1
−1
3s4a
1
2
) core-excited Feshbach reso-
nance. Since the 4a
1
level is dissociative, this leads to facile bond breakage. The detailed dynamics
of the
2
B
1
DEA channel has been calculated using accurate potential surfaces (Haxton et al.,
2007a,b). The second, much weaker feature, may be due to the excitation of the 3a
1
electron into
the mixed 3s4a
1
level, giving rise to a
2
A
1
, one-hole, two-electron (…3a
1
−1
1b
1
2
3s4a
1
2
) core-excited
Feshbach resonance. This state mixes with the lower energy
2
B
1
state via nonadiabatic curve
crossings, andalso dissociates due to the 4a
1
antibonding level and probably some admixture
containing the 2b
1
character.
486 Charged Particle and Photon Interactions with Matter
As can be seen in Figure 18.8, the yields of H
−
(D
−
) in both the gas phase and the condensed
phase are very low for excitation energies above 10eV (Simpson etal., 1997). This is largely due to
the fact that the higher-energy resonances associated with excitations from the 3a
1
and 1b
2
levels
decay to primarily form O
−
and H
2
. The O
−
yield has been measured in the gas phase (Compton
and Christophorou, 1967; Melton, 1972; Jungen etal., 1979; Curtis and Walker, 1992; Fedor etal.,
2006), whereas the neutral molecular hydrogen channel was measured from multilayer water sam-
ples condensed at 90K. These are also shown in Figure 18.8c. The two resonances dominating
the O
−
yields in Figure 18.8 are one-hole, two-electron core-excited Feshbach resonances, and are
formally assigned to the
2
A
1
and
2
B
2
states. We have demonstrated that the molecular hydrogen
produced from water multilayers via the
2
A
1
and/or
2
B
2
resonances contains only even quanta of
vibrational energy (Kimmel and Orlando, 1996). Since the vibrational and rotational quantum-state
distributions of the departing H
2
are identical from E
i
= 8–14eV, it is very likely that the
2
A
1
and/or
2
B
2
resonances are strongly mixed, particularly in the condensed phase.
Based upon the β parameter discussed above, the O
−
is not expected to leave the surface of mul-
tilayer water ice. However, it is very probable that this product, as well as the OH product, reacts
within the ice or water directly at an interface or surface. In the case of water at a DNA interface,
this
can lead to facile DEA-induced damage of DNA.
18.6.2 lee-StiMulated deSorption of cationS
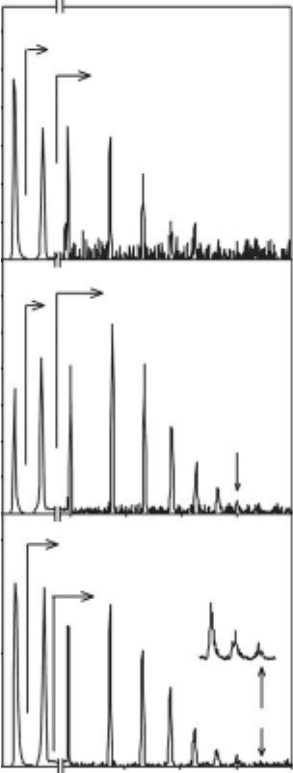
Figure 18.9 shows the cations produced and desorbed during electron impact of pristine ice.
Adetailed description of the cation yields from pristine ice can be found elsewhere (Herring-
Captain etal., 2005). Briey, the cation yield is dominated by H
+
with a much smaller yield of H
2
+
.
The H
+
and H
2
+
yields increase with increasing growth and substrate temperature with ratios of
1:2:3 (PASW:ASW:CI) for H
+
and 1:1:1.5 for H
2
+
. There is also a very reproducible protonated
8
6
4
2
0
8
6
4
2
0
3 4 5
2
B
1
2
B
1
2
B
1
2
A
1
2
A
1
2
A
1
2
B
2
2
B
2
2
B
2
10
(a)
(b)
8
6
4
2
0
10
8
6
4
2
0
2
B
2
6 7 8 9
(c)
(d)
D
–
from condensed D
2
O D
2
from condensed D
2
O
D
–
from gas-phase D
2
O O
–
from gas-phase D
2
O
10 11 12 13
Incident electron energy(eV) Incident electron energy(eV)
14 15 5 6 7 8 9 10 11 12 13 14 15
Figure 18.8 Comparison of DEA resonances for gas-phase water (Fedor etal., 2006) and ice lms (Simpson
etal., 1997). (a) and (b) show a highly correlated resonance structure for D
−
desorption. (c) and (d) show that
D
2
desorption from a condensed phase correlates with excitation to the
2
B
2
state (Kimmel and Orlando, 1996).
Low-Energy Electron-Stimulated Reactions in Nanoscale Water Films 487
cluster ion signal from PASW, ASW, and CI. The cluster yield from PASW is typically ve to
six times larger than CI, and the ASW cluster yield is about two to three times larger than CI
(Herring-Captain etal., 2005).
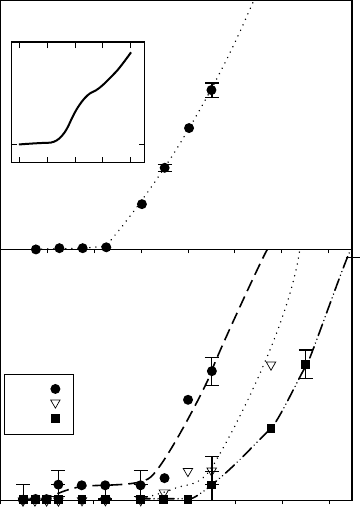
The threshold energies for electron-stimulated production and desorption of H
+
and H
2
+
from CI,
and H
+
and H
+
(H
2
O) from PASW and ASW are 22 ± 3 eV. There is also a H
2
+
yield increase near
40± 3 eV and an ∼70eV threshold for H
+
(H
2
O)
n=2–8
for all the phases of ice studied. These threshold
measurements are shown in Figure 18.10 and are very useful in determining the dominant excited
states
and excitations involved in cation desorption.
18.6.2.1
mechanisms
for lee
-stimulated
Cation d
esorption
Of the two-hole and two-hole, one-electron congurations, at least four are known to produce protons
from ice with kinetic energies ranging from 0 to >7eV (Sieger etal., 1997; Sieger and Orlando, 2000;
×50
×300
×150
×40
10 15
Time of flight (μs)
20 25 30
×20
×50
80 K PASW
H
+
(H
2
O)
n
E
i
= 250 eV
110 K ASW
H
+
(H
2
O)
n
E
i
= 250 eV
140 K CI
H
+
(H
2
O)
n
E
i
= 250 eV
H
+
H
+
H
+
H
2
+
H
2
+
H
2
+
n = 1
n = 1
n = 2
n = 3
n = 2
n = 3
n = 4
n = 5
n = 4
n = 3
n = 2
n = 1
n = 6
n = 7
n = 7
n = 6
n = 5
n = 8
n = 8
n = 5
n = 4
n = 6
Figure 18.9 Cation ion yields from crystalline, amorphous, and porous amorphous ice lms. Pulsed irradia-
tion stimulated desorption using a 250eV incident electron energy produce protonated water clusters H
+
(H
2
O)
n
.
The yields dramatically increase with the disorder in the lm structure.
488 Charged Particle and Photon Interactions with Matter
Herring etal., 2004; Herring-Captain etal., 2005). These congurationally mixed two-hole excited
states and two-hole, one-electron excited states have excitation energies from 21 to 70eV. The linear
dependence of the proton yield on dose as well as the threshold energy of 21–25eV indicated that
low-kinetic-energy (∼4eV) proton desorption is the result of two-hole states occurring at the surface
of the ice. These have been assigned to (3a
1
)
−1
(1b
1
)
−1
(4a
1
)
1
nal states of terminal water molecules
with
dangling bonds. There is a secondary threshold energy of ∼40
eV,
which may correspond to the
(3a
1
)
−2
(4a
1
)
1
and (1b
1
)
−2
(4a
1
)
1
states of water, the latter producing protons with kinetic energy up to 7eV.
These energetic protons (>7eV) can also be produced from the (2a
1
)
−2
conguration, which can be
produced at incident electron energies of >70eV.
All of these two-hole excited states and two-hole, one-electron excited states involve bands of a
1
symmetry. As previously discussed, levels with a
1
character should be sensitive to the local bond-
ing environment of the water molecule (Herring-Captain etal., 2005). Therefore, the temperature
dependence of the proton yield (not shown) gives insight into the local conguration of the surface
of the ice and the dangling bonds responsible for proton production. At low dosing temperatures, the
random orientation of the surface-water molecules leads to a low proton yield for PASW compared
to CI, most likely because the proton undergoes reactive scattering across the ice surface or into
the bulk (Sieger and Orlando, 1997). As the temperature increases, the degree of coupling between
water molecules decreases and the mobility of surface-water molecules increases (i.e., they have
sufcient energy to rotate and start to diffuse) (Akbulut etal., 1997). The reduced coupling and
emergence of a split 3a
1
level discussed above lead to band narrowing, increased excited-state life-
times,
and increased proton emission (Sieger etal., 1997; Herring etal., 2004).
H
2
+
desorption has similar threshold values compared to H
+
, but the yields are much less. Although
the branching ratio is small, some of the above-mentioned two-hole states and two-hole, one-
electron states responsible for proton desorption can decay to form H
2
+
. This has been observed in the
10 20 30 40
H
+
140 K Cl
80 K PASW
H
+
(H
2
O)
n
0 20 40 60 80 100
Electron energy (eV)
Ion count(arb. units)
120 140
(a)
n =2
=3
=4
(b)
50
Figure 18.10 Threshold energies for electron-stimulated desorption of protonated water cation clusters
from
crystalline and amorphous ice.
Low-Energy Electron-Stimulated Reactions in Nanoscale Water Films 489
time-correlated dissociation of doubly ionized states of water, most likely through a dissociative triplet
state (Tan etal., 1978). Photoionization near the O 1s → 2b
2
resonance (Piancastelli etal., 1999) was
reported to produce H
2
+
. The absorption of four photons to produce H
2
+
X
1
g
∑
+
( )
was also reported in
a recent multiphoton ionization study of gas-phase water (Rottke etal., 1998). The H
2
+
yield observed
from these ices is large relative to the gas phase studies. This indicates that many body interactions
and orientational defects may play a role in its production. Reactive scattering of the energetic protons
may also be a source of H
2
+
.
The mechanism for cluster ion desorption involves the production of two holes and a Coulomb
explosion resulting from these holes in neighboring water molecules. A detailed description of this
model can be found elsewhere (Herring-Captain etal., 2005). Briey, the weak ∼25eV threshold for
H
+
(H
2
O) formation is most likely due to the reactive scattering of a proton (Bernholc and Phillips,
1986). The primary 70eV threshold for clusters with 1–7 water molecules may initially correspond
to the 2a
1
−2
state. Coupling of this doubly ionized water molecule to a neighboring water molecule
leads to a nal state containing one positive charge on each of the two water molecules, most likely
with a OH
+
…H
+
(H
2
O) conguration. The surrounding water molecules respond to the charges
by reorienting in an attempt to solvate the charges, and nally the unstable conguration undergoes
a
Coulomb explosion, resulting in desorption of a protonated cluster.
The
temperature/phase dependence of the cluster ion yield provides insight into the local hydro-
gen bonding in the terminal water layers. While the proton yield reects the behavior of the surface
molecules in a direct line of sight to the vacuum, the clusters reect the behavior of the terminal
1–3 water layers. At low dosing temperatures, the water molecules are randomly oriented and there
is no long-range order. The localization of two holes can cause dissociation and proton transfer
(or hole hopping) along the hydrogen bond, forming a hydronium ion. The holes are then localized
on neighboring water molecules. Due to the lack of an ordered hydrogen-bonding network in PASW,
the holes are less likely to be able to hop to a screening distance (1–2nm) before a Coulomb explo-
sion occurs. This “localized hole hopping,” where a hole hops to a neighboring water molecule but
not far enough to be screened, occurs in the terminal 1–3 water layers of PASW and results in a
large cluster yield. As the temperature increases, a more crystalline structure is formed (Smith etal.,
1996; Sieger etal., 1997) and the holes are more likely to hop further away from each other through
the hydrogen-bonding network. CI has the most extensive hydrogen-bonding network of the samples
studied
here and the lowest cluster yield, due to the efcient hole hopping through this network.
18.6.3 lee-StiMulated deSorption of neutral fragMentS and productS
It is well known that ion desorption accounts for only a small fraction of the total mass loss during
electron bombardment of materials and surfaces. In ice and more strongly coupled materials, this
is due to hole hopping, efcient autoionization, and electron–hole recombination. In the latter case,
the lowest-energy “excitons” formed as a result of hole (ion)–electron recombination can localize at
the surface or in the near surface zone, and dissociate to produce atomic fragments such as H (
1
S) and
O(
3
P,
1
D, etc.) (Kimmel and Orlando, 1995; Orlando and Kimmel, 1997) and molecular products
such as hydrogen (Orlando etal., 1999) and OH. The threshold energies for producing the neutral
atomic hydrogen and oxygen fragments are shown in Figure 18.11. The experimentally observed
value of ∼6.5eV (relative to the vacuum level) is lower than the 9.5 and 11.5eV thermodynamic ener-
gies required to produce O(
3
P
J
) + 2D(
2
S) and O(
1
D) + 2D(
2
S), respectively. The low threshold value
indicates that the formation of atomic oxygen must occur via a pathway that involves a molecular
elimination step. Since the molecular hydrogen is in the
X
1
g
∑
+
( )
ground state, the observation of
both singlet and triplet atomic oxygen demonstrates that singlet and triplet excitons are involved.
This is consistent with exciton formation via hole–electron recombination and the fact that the
triplet states can be directly excited during electron scattering since the transitions are not governed
by
optical selection rules.
