Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

510 Charged Particle and Photon Interactions with Matter
The fact that only a few radicals from each path are found has profound implications regarding
hole and electron transfer processes in irradiated DNA, as does the distribution of radicals between
the bases and the deoxyribose sugars. In this section, we focus on the oxidative path and consider
the
location of ionization events in photon irradiated DNA.
For
typical γ-radiation, most of the molecular damage from low LET radiation is effected through
ionizations caused by the cascade of electrons of various energies that follow the initial ionization by
γ-photons (Section 19.1.1). The total yield of ionizations in DNA, in the rst 10
−16
–10
−15
s after irra-
diation, has been estimated to be ca. 1.2μmol/J (Bernhard and Close, 2004). The total yield of free
radicals trapped at 77K for hydrated salmon sperm DNA is ca. 0.25μmol/J (Shukla etal., 2005). This
value is actually dependent on the level of hydration (Wang et al., 1993). For dry salmon sperm DNA
(Γ = 2.5 D
2
O/nucleotide), G = 0.13μmol/J (Wang et al., 1993); for desiccated herring sperm DNA with
residual H
2
O, rather than D
2
O, G = 0.41μmol/J has been reported; and for desiccated calf thymus DNA,
0.29μmol/J (Spalletta and Bernhard, 1992). Although there is some difference in these results, it is quite
clear that most of the initially formed radicals recombine in early spurs, blobs, and short tracks.
Recombinations have the potential to result in excited states, but, unfortunately, little is known
about
the consequences of recombinations for DNA radiation damage.
Relatively
high free radical yields at cryogenic temperatures have been reported for x-ray irradi-
ated hydrated plasmid DNA. For pUC18 DNA, the total yield of trapped free radicals ranged from
G = 0.302μmol/J at Γ = 2.5 H
2
O/nucleotide, to G = 0.718μmol/J at Γ = 22.5 H
2
O/nucleotide and
showed a monotonic increase as hydration levels increased (Purkayastha et al., 2007). For pEC
plasmids, G = 0.71μmol/J was also reported (Purkayastha et al., 2005). These high yields of trapped
free
radicals are ascribed to the dense packing of supercoiled plasmid DNA.
19.2.1.2
the
r
ole
of h
ole
t
ransfer
If it is assumed that the aforementioned recombinations are random, roughly half of the surviving
ionizations will be located on the DNA bases and roughly half on the sugar–phosphate backbone.
However, as can be seen from Table 19.1, ca. 77% of the trapped electron-loss radicals at 77K reside
on the bases, and the remaining 23% originate with backbone radicals. It is quite evident that there
is a substantial hole transfer from the backbone to the bases before the electron loss radicals are
trapped. From the values in Table 19.1, and the assumption that about half the ionizations occur on
the bases and half on the sugar–phosphate backbone (Section 19.1.2), at 77K, there is a signicant
hole transfer from A
•+
, C
•+
, and T
•+
to G to form G
•+
, and, in addition, signicant hole transfer from
the
sugar–phosphate backbone to G to form G
•+
(Scheme 19.3).
A very similar conclusion was reached by Swarts et al., who performed a more detailed analysis
of oxidative path reactions at room temperature, in which the specic fates of holes on the different
bases and sugars were determined by quantication of base release and base damage (Swarts et al.,
1992, 1996). Here, it was found that ca. 13% of the holes on the deoxyribose sugars transferred to
the bases and that 39% of the holes on adenine, cytosine, and thymine transferred to guanine before
Base
•
+
50%
Backbone
•
+
50%
23%
77% G
•
+
ht 27%
77 K
Base
•
+
50%
Backbone
•
+
50%
37%
63%
A
•+
C
•+
T
•+
RT
Base release
A, C, T base damage products
(A) (B)
G
•+
39%
17%
G base damage products
7%
46%
ht
13%
56%
C1
΄
•
C3
΄
•
C5
΄
•
Sugar radicals
sCheme 19.3 Scheme showing the hole transfer and reaction pathways at 77K (A) and room temperature (B).
PhysicochemicalMechanisms of Radiation-Induced DNA Damage 511
guanine base damage products (8-oxo-guanine, fapyguanine) were formed. A scant 7% of the cation
radicals A
•+
, C
•+
, and T
•+
were able to survive long enough to form their own base damage products
(Swarts et al. (1996) should be consulted for details). In summary, the conclusions drawn from two
very disparate experiments at two very different temperatures are remarkably similar. After irradia-
tion, most of the holes formed (63%–75%) end up on the bases, predominantly on guanine, with the
remainder
consigned to the sugars.
19.2.1.3
multiple
o
xidations
Centered on a s
ingle
d
g
•+
In a chemically oxidizing environment, dG
•+
may undergo a series of reactions that might serve as a
radiation protection mechanism (Shukla et al., 2004a, 2007). It is currently thought that the principal
(but not sole) source of direct-type effect radiation-induced strand-break formation is theelectron
loss path (Becker and Sevilla, 1998, 2008; Ward, 2000; Bernhard and Close, 2004; Sevilla and
Becker, 2004; von Sonntag, 2006; Li and Sevilla, 2007; Becker et al., 2007; Close, 2008; Kumar
and Sevilla, 2008a). Any factors that might impede this path might be viewed as being protective,
and, indeed, such factors do exist. In a study of the reactions of dG
•+
using double stranded DNA,
samples were annealed step wise from 77 to 258K and the ESR spectra obtained were analyzed for
possible intermediates (Shukla et al., 2004a, 2007). It was found that at least three one-electron oxi-
dation steps occurred in transforming the original G
•+
to possible diamagnetic molecular products
(Shukla et al., 2004a, 2007). This is shown in Scheme 19.4. InScheme19.4A, the structures of the
actual radical intermediates observed using ESR spectroscopy (G
•+
, GOH
•
, 8-oxo-G
•+
) are shown
along with the reaction sequence that occurs. Scheme 19.4B summarizes the free radical reaction
process found. Formation of GOH
•
results in a damage site that is itself easily oxidized. The oxidiz-
ing species is additional G
•+
(E
red
= 1.29V (Steenken, 1989, 1992, 1997)), which by hole transport
can be collected at such reactive sites in irradiated DNA. Each step thereafter is also downhill and
should be rapid, for example, 8-oxo-G is also easily oxidized (E
red
of one-electron oxidized 8-oxo-
dG at pH 7 at ambient temperature in aqueous solution is 0.74V (Steenken et al., 2000)).
Recalling that the electron loss path can be thought of as a major damage pathway, Scheme 19.4A
shows that four potential damages can be intercepted and channeled via three one-electron oxida-
tion events into a single likely repairable base damage. This mechanism might be very effective in
“simplifying” the complex damage in clusters of proximate radicals. Such clusters are thought to
be particularly harmful since clustering of damage may impede normal cellular repair processes.
Proof
that this mechanism occurs in cells has not yet been found.
19.2.1.4
inuence
of the p
rotonation
s
tate
of the o
ne-e
lectron o
xidized
guanine
in
dna on the m
echanism
of the Formation of 8-oxo-
g
Cation r
adical
It has already been mentioned in Section 19.2.1.3 that ESR studies employing systematic thermal
annealing of γ-irradiated hydrated DNA samples to higher temperatures (195–258 K) (Shukla et al.,
H
2
N
HN
N
N
N
O
H
2
N
dR
+
•
H
HN
N
N
N
O
H
2
N
dR
•
H
+
H
2
O
–H
+
OH
HN
N
N
N
O
H
2
N
dR
–e
–
–H
+
O
H
HN
N
N
N
+
O
dR
–e
–
O
H
•
dG
•
+
dGOH
•
8-oxo-dG
8-oxo-dG
•
+
+
H
2
O
–H
+
–e
–
–H
+
–e
–
dG
•
+
dGOH
•
8-oxo-dG
8-oxo-dG
•
+
–e
–
Diamagnetic products
dG
•
+
dG
dG
•
+
dG
dG
•
+
dG
(B)
(A)
sCheme 19.4 Mechanism of formation of 8-oxo-G
•+
and its further oxidation to diamagnetic products via
multiple
one-electron oxidation.
512 Charged Particle and Photon Interactions with Matter
2004a, 2007) have shown that 8-oxo-G cation radical is formed at ≥200K. Cullis and coworkers
have established that, for the formation of 8-oxo-G, one-electron oxidized guanine must remain in
its cation radical state (G
•+
:C) (Cullis et al., 1996). Only in the protonated state, does the nucleo-
philic addition of water take place at the C-8 atom in the guanine ring—which is the rst step in the
formation
of 8-oxo-G (see Schemes 19.1 and 19.4A).
Recent
work employing double stranded DNA-oligomers incorporated with C-8-deuterated gua-
nine at specic locations have clearly established that, at 77K, the protonation state of the one-electron
oxidized guanine in double stranded DNA is entirely the proton transferred radical (C(+H
+
):G(-H)
•
)
(discussed in Section 19.5.5). This means, the proton transferred state (C(+H
+
):G(-H)
•
) is the thermo-
dynamically stable state (Adhikary et al., 2009). From this work (Adhikary et al., 2009), and also from
published work on electron and hole transfer by thermal annealing in DNA (Spalletta and Bernhard,
1992; Becker and Sevilla, 1998, 2008; Bernhard and Close, 2004; Cai and Sevilla, 2004; Sevilla and
Becker, 2004; Shukla et al., 2004a, 2007; von Sonntag, 2006; Becker et al., 2007), it is concluded that
barrier to the reverse intra-base pair proton transfer from C(+H
+
):G(-H)
•
to G
•+
:C is small. In agree-
ment, a recent theoretical report suggests the barriers to forward and reverse proton transfer are 1.4
and 2.6kcal/mol, respectively (Kumar and Sevilla, 2009a). Thus, at elevated temperatures (≥200K),
the prototropic equilibrium between C(+H
+
):G(-H)
•
and G
•+
:C is established (see Section 19.5.5). At
these temperatures, the presence of G
•+
:C allows for the nucleophilic addition of H
2
O, thereby provid-
ing the rst step as shown in Scheme 19.4 toward the formation of 8-oxo-G in DNA.
19.2.2 dna electron-gain trapped radicalS after low let irradiation at 77 k
19.2.2.1 base radicals Found after irradiation at 77 k
Table 19.1 and Scheme 19.2 show the principal electron-gain radicals found at 77K after low LET
radiation. As can be seen from Table 19.1, the preponderance of the trapped radicals is on the
pyrimidine bases, in the form of T
•−
and C(N3)H
•
. There is no evidence as yet that these two radi-
cals are responsible for strand-break formation, and much evidence to the contrary. However, they
are not biologically meaningless. On warming to room temperature, T
•−
forms T(C6)H
•
, and ulti-
mately, dihydrothymine (DHThy), and C(N3)H
•
forms dihydrocytosine (DHCyt). Both these lesions
may occur in signicant amounts in anaerobic cells subjected to radiation (Dawidzik et al., 2004).
Although both lesions are typically repairable through a base excision process (Cadet et al., 2000),
when in a damage cluster, they are not easily repaired and can prevent repair of nearby strand breaks
(Hada
and Georgakilas, 2008).
19.2.2.2
low
e
nergy
electron (
lee):
d
issociative
electron a
ttachment
(
dea)
The two reductive path backbone radicals found, RO
2
PO
•−
and
C
dephos
′
3
i
, are present in only small
amounts with low LET radiation, and in larger relative concentrations with ion-beam irradiations
(Section 19.4). However, they are signicant because they are immediate strand-break radicals,
that is, they are the direct result of a strand break. Early ESR investigations of small phosphate
esters and diphosphate esters concluded that radicals of this type originate with DEA (Nelson and
Symons, 1977; Nelson et al., 1993; Sanderud and Sagstuen, 1996). In DNA, the two radicals origi-
nate from divergent DEA paths as illustrated in Scheme 19.5. The LEE (low energy electron) is a
thermal
electron (≤
ca.
20
eV)
(Sanche, 2008).
The
percentages given for each path are derived from the approximate relative concentrations of
C3′
•
dephos
(95%) and
ROPO
2
i−
(5%) found in ion-beam irradiated samples (Becker et al., 2003).
A direct and striking proof that LEE cause induced DNA strand breaks was rst reported in a clas-
sic paper by Sanche and coworkers (Boudaïffa et al., 2000). In this work, pGEM 3Zf(–) DNA isolated
from E scherichia coli and hydrated to Γ = 2.5 H
2
O/nucleotide was subjected to a beam of LEE in
the 3–20eV range. Subionization (<ca. 7.5eV) electrons were able to cause substantial strand breaks,
including both single and double strand breaks. The yield information in this work (ca. 10 × 10
−4
sb
total/e
−
for a 10eV electron) suggested a small but signicant probability for strand-break formation.
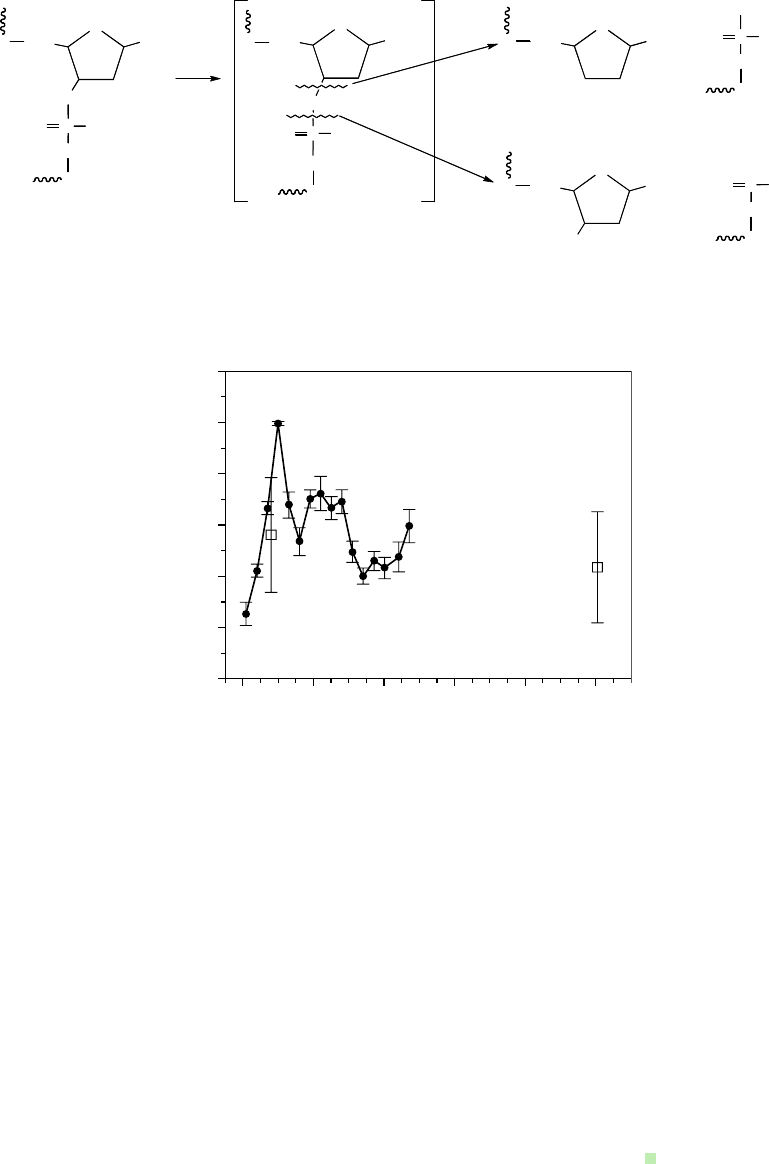
PhysicochemicalMechanisms of Radiation-Induced DNA Damage 513
Sanche and coworkers later rened their studies by showing that very low energy electrons, down
to near 0eV, could also cause single strand breaks with yields similar to those at far higher energies
(Figure 19.1) (Burrow et al., 2006; Pan and Sanche, 2006; Sanche, 2008). For example, the yield of
ssb at 1eV was even greater than that at 10eV (Burrow et al., 2006). Furthermore, in more recent
efforts, a base sequence dependence on sites of the LEE induced cleavage in DNA oligomers have also
been demonstrated (Sanche, 2008). Furthermore, other groups have recently elucidated mechanisms
of LEE damage (Bald et al., 2006, 2008; Swiderek, 2006; Sanche, 2008). Overall, these results suggest
that LEE addition to both the base and the phosphate group are pathways to DNA damage.
19.2.2.3 theoretical
t
reatment
of lee
-induced
s
trand
b
reaks
A great deal of theoretical work has focused on the mechanisms for LEE-induced strand-break forma-
tion, and at least three mechanisms have been proposed in the literature (Simons, 2006; Kumar and
Sevilla, 2007, 2008a,b, 2009b; Li and Sevilla, 2007). In the rst mechanism, Simons suggestedthat
a shape resonance results from an LEE initially attaching to a LUMO (a π*-MO localized on the
DNA base). Subsequently, during C3′–O3 bond elongation (upper path Scheme 19.5), the electron
transfers to the phosphate group and a strand break results (Simons, 2006). Simons suggested that this
O
CH
2
O
O
P O
–
O
O
CH
2
Base
LEE
O
CH
2
O
O
P
O
–
O
O
CH
2
Base
•2–
O
CH
2
O
–
O
O
–
O
O
CH
2
Base
O
CH
2
O
O
–
P O
–
O
O
CH
2
Base
•
+
+
•
95%
5%
ssb
ssb
C3
΄
•
ROPO
2
•
–
P
dephos
sCheme 19.5 Low Energy Electrons (LEE) induce cleavage of DNA backbone resulting in specic
strand-break radicals. (Adapted from Becker, D. et al., Radiat. Res., 160, 174, 2003. With permission.)
30
25
20
15
10
5
0
0 2 4
Eective cross section for the production
of SSBs in DNA
E
o
(eV)
σ (10
–15
cm
2
)
6 8 10
Figure 19.1 Effective cross sections for formation of single strand breaks in pGEM 3Zf(–) plasmid DNA,
determined using two different analytical methods. See reference Panajotovic for details. (From Panajotovic,
R.
et al., Radiat. Res., 165, 452, 2006. With permission.)
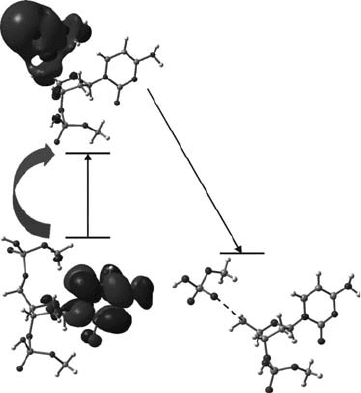
514 Charged Particle and Photon Interactions with Matter
process would likely be too slow by thermal activation alone to explain the effect of LEE on DNA,
and recent theoretical work (Pan and Sanche, 2006; Sanche, 2008) has conrmed that a strand break
from this mechanism is unlikely because a signicant activation barrier is found, ca. 19kcal/mol
for the transient anion radical and nearly 30kcal/mol for the adiabatic anion radical (Kumar and
Sevilla, 2007, 2008a). Although LEE attachment to the LUMO connes the electron to the base, LEE
addition to higher energy unoccupied molecular orbitals (UMOs) (<4eV) form electronically excited
anion radical states, which have energy in excess of the activation energy toward DEA. These higher
energy UMOs can be localized on the phosphate group, the base, or both (Kumar and Sevilla, 2008b,
2009b). Thus, the second mechanism proposed occurs through a shape resonance formed by an LEE
addition to an available UMO of higher energy than the LUMO. The excited anion radical state (likely
a π* state) thus formed can couple with the dissociative σ* state on phosphate (see Figure 19.2) and
lead directly to C–O bond cleavage (Burrow et al., 2008; Kumar and Sevilla, 2008b, 2009b). A third
mechanism was briey presented by Li and Sevilla, who suggested that a vibrational excitation of the
C–O bond could itself capture an electron and lead to a strand break (see Li and Sevilla, 2007, p.80).
Along these lines, vibrational Feshbach resonances that couple dipole-bound and valence-anion states
in LEE interactions with DNA bases have been implicated in bond fragmentation via σ* pathways
(Burrow et al., 2006). In each of the mechanisms proposed above, the captured electron must couple
to the σ* dissociative state involved in the C–O bond cleavage of the sugar–phosphate DNA backbone
(shown in Scheme 19.5 upper pathway). There are mechanisms available at higher energies (>4eV)
that involve other pathways. For example, LEE induced radical anion excited states known as core
excited states (e.g., LEE interaction with a molecule to form an UMO lled with two electrons and
an inner shellhole (core hole)) are available at energies above 4eV (Panajotovic et al., 2006; Sanche,
2008; Kumar and Sevilla, 2009b). Because of their higher energies and reactive nature, core excited
states lead to molecular fragmentation reactions resulting in H
−
, O
−
, and OH
−
in model systems and
single and double strand breaks in DNA (Panajotovic et al., 2006; Sanche, 2008).
19.2.3 Strand breakS froM direct-type effectS
Strand breaks from direct-type effects at low LET have been measured in a variety of DNA
and DNA model compounds by a variety of methods (Table 19.2). Table 19.2 is not meant to be
e
–
π
σ*
<4 eV
–
SOMO
•
Figure 19.2 Low energy electrons interact with DNA to create various excited anion radical states. Here
the excited anion radical of the DNA model system thymidine results in a σ* state that leads to strand-break
formation. For details of the calculation, see Kumar and Sevilla (2009b). (From Kumar, A. and Sevilla, M.D.,
Chem. Phys. Chem.,
10, 1426, 2009b. With permission.)

PhysicochemicalMechanisms of Radiation-Induced DNA Damage 515
comprehensive, but rather to give a general feel for these phenomena; the references cited should
be consulted for details and further elaboration regarding strand-break phenomena under different
hydration
levels, and for the effect of enzyme sensitive and heat labile sites of strand breaks.
Strand
breaks have also been measured for the following oligomers at a hydration of Γ = 8−9 H
2
O/
nucleotide: d(CGCG)
2
, G = 0.11μmol/J (Razskazovskiy et al., 2003b); d(CGCGCG)
2
, G = 0.085μmol/J
(Sharma et al., 2007); d(CGCACG):d(CGTGCG), G = 0.13μmol/J (Razskazovskiy et al., 2003b); and
d(CACGCG):d(CGCGTG), G = 0.055μmol/J (Razskazovskiy et al., 2003b).
19.2.3.1 direct-type
e
ffect
s
trand
b
reaks
and Free r
adical
y
ields
It has been a long standing assumption of DNA radiation chemistry that prompt strand breaks from
ionizing radiation originate with free radicals (von Sonntag, 2006). This has been one of the principal
motivations underlying the need to measure and detect the free radicals that form after irradiation.
A recent series of papers reports that, with direct-type effects, the yield of trapped radicals at 4.2K
is lower than the yield of strand breaks found when the same samples are warmed and strand breaks
measured (Purkayastha et al., 2006b). This, of course, is not necessarily inconsistent with the para-
digm that strand breaks largely originate with free radicals when low LET radiation is employed.
Itdoes suggest that some signicant chemistry occurs, even at 4.2K. From earlier investigations, it was
determined that the yield of frank strand breaks, as measured by loss of supercoiled pUC18 plasmid
DNA, exceeded the yield of free radicals at low hydration levels (Table 19.3); later work by the same
group expanded this to all hydration levels studied (vide infra) (Purkayastha et al., 2005).
As can be seen, the yields of prompt strand breaks at room temperature (RT) are higher than the
yield of trapped free radicals (4.2K) for hydration levels of Γ ≤ 11.5 H
2
O/nucleotide. To explain these
results, a double oxidation mechanism is proposed, in which for the initial oxidation, ionization at
table 19.2
strand
b
reaks
in h
ydrated
dna
dna hydration G(ssb, frank) G(dsb, frank) fr G(ssb)/G(dsb) references
pEC
plasmid 22 0.08 0.01 8 Purkayastha et al. (2005)
pUC18
plasmid 22 0.12 0.006 20 Purkayastha et al. (2005)
pUC18
plasmid 24 ± 7 0.071 0.006 12 Yokoya et al. (2002)
B-DNA 22 0.09
a
— — Swarts et al. (1992, 1996)
a
Base release.
table 19.3
Free
r
adical
vs. s
trand-break
y
ields,
puC18
p
lasmid
dna
Γ
a
G(sugar Free radicals)
b
G(ssb)
c
G(dsb)
c
2.5 0.033 0.069 0.0052
7.5 0.040 0.058 0.0046
11.5 0.056 0.060 0.0044
15.0 0.069 0.063 0.0047
22.5 0.079 0.054 0.0039
a
(H
2
O/nucleotide).
b
μmol/J, 4.2K.
c
μmol/J, room temperature frank strand breaks, from loss of
supercoiled DNA.
516 Charged Particle and Photon Interactions with Matter
a deoxyribose sugar followed by rapid deprotonation occurs to form a neutral sugar radical (S
•
).
Instead of being trapped and observed at 4.2K, it is presumed that a nearby base cation radical
oxidizes S
•
, producing a carbocation, which then forms a strand break on warming. Thus, a double
oxidation mechanism produces a strand break without producing any trapped radicals. The authors
correctly
note that at hydration levels above Γ ≥ 11.5, some
•
OH will be formed. At 4.2K, however,
the
•
OH will not mobilize to from DNA radicals. On warming to measure strand breaks, a fraction
of
these will form strand breaks due to the indirect effect.
The
same researchers, in a later report, noted a puzzling feature regarding base damage yields
in pUC18 plasmids as a function of hydration (Purkayastha et al., 2007). The damage was detected
using the base excision repair enzymes endonuclease III (Nth) and formamidopyrimidine-DNA
glycolase (Fpg), which convert most base damage sites on purines (Nth) and pyrimidines (Fpg) to
single strand breaks. In the hydration range Γ = 2.5 H
2
O/nucleotide to Γ = 22.5 H
2
O/nucleotide, the
base damage yields (RT) decreased by a factor of 3.2 as the yield of trapped DNA radicals (4.2K)
increased by a factor of 2.4, contrary to the expected relationship. The hypothesis that this discrep-
ancy is caused by an undercounting of single strand breaks using standard methods of analysis (loss
of supercoiled DNA and Poisson statistics) was then tested by using base release, G(fbr) in μmol/J,
to measure single strand-break formation and assuming that G(fbr) ≈ G(sb) (Sharma et al., 2008).
For this determination, the multiplicity of single strand breaks m(ssb), dened as the number of ssb
formed per super coiled DNA lost, was determined. The formation of strand breaks from
•
OH at
higher hydrations was subtracted so that direct-type effects only were considered, and measuring
base release scores a single dsb as two ssb. At Γ = 2.5 H
2
O/nucleotide, m(ssb) = 1.4 ± 0.2, and at
Γ = 22.5 H
2
O/nucleotide, m(ssb) = 2.8−3.0 ± 0.5, indicating that, for pUC18 under the conditions
used, there is more than one ssb per supercoiled DNA lost. In addition, this work rectied the afore-
mentioned conundrum regarding strand breaks and trapped radicals, since both now increased in
going from Γ = 2.5 H
2
O/nucleotide to Γ = 22.5 H
2
O/nucleotide. Most importantly, these results now
indicate that the excess of strand breaks over trapped free radicals, G(diff) = G(ssb) – G(fr), persists
at high hydration levels, contrary to the results obtained if sb are scored by loss of supercoiled DNA.
G(diff) was relatively constant in the range of 0.090–0.110μmol/J for the two hydration levels used.
19.3 Charge transFer proCesses indna:summary
o
F the m
eChanisms
In irradiated DNA, both DNA-cation and -anion radicals are produced and the transfer of these spe-
cies through DNA is of signicance regarding the ultimate location of DNA damage (Bernhard, 1981;
Becker and Sevilla, 1993, 1998, 2008; Ward, 2000; Bernhard and Close, 2004; Sevilla and Becker,
2004; von Sonntag, 2006; Becker et al., 2007; Li and Sevilla, 2007; Close, 2008; Kumar and Sevilla,
2008a). A number of experimental studies based on UV–vis photoexcitation of dsDNA-oligomers with
specic sequences having donors and acceptors at xed distances, followed by damage analysis by gel
electrophoresis at ambient temperatures have suggested that charge, that is, holes (orcation radicals) and
electrons, can migrate through long distances (Arkin et al., 1997; Henderson etal., 1999; Giese, 2000,
2002; Lewis, 2005; Lewis et al., 2000, 2001; Schuster, 2000; Drummond etal., 2003; Wagenknecht,
2003). In addition, ultrafast (from pico- to femtoseconds) laser ash photolysis of DNA with donors and
acceptors has further elucidated the time dependence of these charge transfer processes in DNA (Wan
et al., 1999, 2000; Lewis, 2005; Lewis et al., 2000, 2001; Wagenknecht, 2003).
While the transfer of charge in DNA is a complex process, it can be simplied to two principal
mechanisms:
tunneling and hopping.
The
tunneling mechanism involves the direct (i.e., single step) charge transfer between the donor
and the acceptor due to the electronic coupling (π-way) between the donor and the acceptor via
the intervening base sequence. This step is relatively temperature independent and is effective at
PhysicochemicalMechanisms of Radiation-Induced DNA Damage 517
even4K. The tunneling rate constant can be described by the relation, k = k
0
e
−βD
, which gives the
fall off in tunneling rate constant with distance decay constant (β) and distance (D) between the
donor and the acceptor. β is dependent upon the nature of the bridge (i.e., the intervening sequence)
and the electronic coupling between the donor and acceptor. For tunneling of hole and excess elec-
tron transfer within DNA, β values ranging from 0.6–1.1 Å
−1
have been observed in low temperature
work, which isolate this process and in fast kinetic studies for short distances (Lewis et al., 2000,
2001; Lewis, 2005) where this process dominates. Note that, higher β values (ca. 1–1.5 Å
−1
) are
observed
for charge transfer processes in proteins (Nocek et al., 1996).
While
tunneling is always in effect, it is rapid only for short distances. For long distance
action, the tunneling-hopping mechanism is needed (Jortner et al., 1998; Grozema and Siebbeles,
2007). In the “hopping” mechanism, charge transfer along DNA has been proposed to occur in
discrete thermally activated steps from the donor through intervening way stops to the acceptor
(Bernhard and Close, 2004; Cai and Sevilla, 2004; Sevilla and Becker, 2004; von Sonntag, 2006;
Becker et al., 2007; Becker and Sevilla, 2008; Close, 2008; Kumar and Sevilla, 2008a). This
process is not effective in DNA systems until approximately at 200 K. This thermal activation is
necessary to overcome the stabilization by polarization of the medium around the hole and excess
electron adduct as well as proton transfer processes within DNA (Cai and Sevilla, 2004). These
create a potential barrier to transfer via hopping. Indeed, polarization of the media alone by the
trapped charges can be a limiting factor in the hopping process (Cai and Sevilla, 2004). Another
related mechanism for transfer involving polarization is “polaron transport” in DNA (Henderson
et al., 1999). If the polaron is only a single relaxed ion radical with media polarization around it,
then charge transfer via this polaron is the usual hopping case. However, if the hole or electron is
delocalized within a stack of bases (such as A-stacks), then this larger entity can migrate. Usually,
the size of the polaron in DNA is limited to 3–4 base pairs because the polarization stabiliza-
tion is strongest for smaller ionic species and drops off as the ion radius increases (Conwell and
Rakhmanova, 2000; Conwell, 2005). Therefore, delocalization decreases the stabilization of the
polarization. Theoretical studies have shown that solvent polarization and nuclear reorganiza-
tion prevent extensive base-to-base hole delocalization for bases other than adenine (Adhikary et al.,
2008a), and as a result, such polaron formation, in the case of holes, occurs only in A-stacks
(Adhikary et al., 2008a). For hopping or polaron-assisted hopping, the tunneling equation men-
tioned above is not strictly applicable, as a very weak distance dependence is expected and the
rate of charge transfer process should not necessarily decay exponentially with distance. For
such cases, an apparent low value of β (e.g., 0–0.2 Å
−1
) has been reported from various labo-
ratories (Arkin et al., 1997; Fink and Schönenberger, 1999; Henderson et al., 1999; Ly et al.,
1999; Porath et al., 2001; Drummond et al., 2003). Much effort has shown that base sequence is
especially signicant for such long range tunneling (Giese, 2002). As an example, a G placed in
between every several base pairs allows for long distance hole “hopping” from G to G; tracks of
A show little or no distance dependence (Giese et al., 2001; Giese, 2002; Shao et al., 2004; Kawai
and Majima, 2005; Joy et al., 2006; Augustyn et al., 2007; Lewis et al., 2008). Intra-base pair
proton transfer processes have been suggested to play a role of “gating switch” to both hole and
electron transfer through DNA (Steenken, 1989, 1992, 1997; Adhikary et al., 2009; Kumar and
Sevilla, 2009a). Ultimately, the transfer distances of both holes and electrons are limited by
irreversible protonation of thymine and cytosine anion radicals and reaction of guanine cation
radicals with water described in Schemes 19.1 and 19.2.
In cellular systems, DNA is found in higher levels of organization in chromatin, in the form
of nucleosomes, solenoids, and bers, which place DNA double strands in close proximity and
excludes much of the bulk water from the vicinity of DNA. The close packaging increases the possi-
bility of inter-duplex charge transfer in addition to charge transfer within the duplex. Low tempera-
ture ESR work using crystalline DNA (Debije and Bernhard, 2000), or hydrated DNA pellets and
DNA-ice samples (Pezeshk et al., 1996; Cai and Sevilla, 2000, 2004) have provided evidence for
518 Charged Particle and Photon Interactions with Matter
such inter-duplex transfer. Cai and Sevilla have proposed a three-dimensional model that accounts
for both the intra- and inter-duplex charge transfer processes within nucleohistone (Cai et al., 2001;
Cai and Sevilla, 2004). The works of Barton and coworkers employing isolated nuclei (Núñez etal.,
2001) and nucleosome core particles (Núñez et al., 2002) indicate formation of oxidative damage
products
via charge transfer.
In
the last 10 years, a number of reviews as well as books have described both the hole and the
excess
electron transfer processes in DNA (Schuster, 2004; Wagenknecht, 2005).
19.4 linear energy transFer eFFeCts
19.4.1 introduction
It is well established that higher LET radiations usually produce more severe and less repairable
biological damage than do lower LET radiations (Terato et al., 2008). For α particles, it has been
suggested that up to 70% of the lesions in V79-4 Chinese Hamster cells are caused by direct-type
effects (de Lara et al., 1995). It has also been noted that the track structure of radiation is important
in determining the resulting radiation damage to DNA (Hill, 1999). With the development of the
International Space Station, the contemplation of manned space missions to Mars, and the increas-
ing use of ion beams in radiation therapy, a full understanding of the physicochemical track struc-
ture, that is, the physical and chemical events that occur in the track of radiations of various quality,
and
the spatial organization of these events, is quite relevant.
19.4.2 free radical yieldS at 77 k
Ion-beam irradiation of hydrated DNA at low temperature results in substantial differences in the
free radical yields and dose–response behavior for DNA free radicals relative to that found using
low LET radiation. These effects depend not only on the radiation quality, but also on the hydration
level and the identity of the irradiating ion. Typically, however, the overall yield of radicals is lower
for high LET radiation than for low LET radiation. Table 19.4 shows this for dry DNA using various
quality radiations, but a similar phenomenon occurs for hydrated DNA.
Signicantly, the relative and/or absolute amount of neutral sugar radicals (C1′
•
, C2′
•
, C3′
•
,
C
dephos
′
3
i
) and phosphorus-centered radicals are also much higher in ion-beam irradiated DNA rela-
tive to γ-irradiated samples (Becker et al., 1996, 2003). As an example, Table 19.5 shows the yields
of radicals for DNA hydrated to Γ = 12 ± 2 D
2
O/nucleotide after argon ion irradiation with an ion
energy
of 75
MeV/u
and initial LET of 600
keV/μm.
For this particular experiment, the total yield of base radicals [G
•+
, C(N3)H
•
, T
•−
] equals about
0.10 μmol/J. For a γ-irradiated sample under identical conditions, the total yield of radicals is
table 19.4
Free
r
adical
y
ields
in d
ry
dna
irradiation let G (
- 2008 — 2025 «СтудМед»
