Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

770 Charged Particle and Photon Interactions with Matter
A X v J A X v v J J
v J
( , , , ) ( , , , , , )
,
α α=
∑
f f
f f
(28.5)
where
A(X, α, v
f
, v, J
f
, J) is the Einstein A-coefcient for spontaneous transition from the excited state
│
α, v, J〉 back to
│
X, v
f
, J
f
〉 of the X
1
Σ
g
+
ground state
A(X, α, v, J) is the total emission probability (including transitions to lower singlet-gerade states
and
to the continuum levels of the X
1
Σ
g
+
state)
η
P
(α, v, J) is the predissociation + pre-ionization yield
Tr(X,
α, v, J, T) is the transmission function for self-absorption by resonance lines
The
volumetric excitation rate (excitations/s/cm
3
) at that point (x, y, z) is given by
′
=
∑
g v F E( , , ) ( )α σ,
e
J E N
i ij
i
e e
(28.6)
We use the index i to represent the initial electronic state |X, v
i
, J
i
〉, the index j to represent the excited
electronic state |α, v, J〉, N
i
= N
o
× f
x
i
and the excitation cross section, σ
ij
= σ(v
i
, v, J
i
, J). The volu-
metric excitation rate g′(α, v, J, E
e
) is proportional to the population density N
i
of the molecule in
the initial vibrational–rotational level v
i
, J
i
, and the monoenergetic electron-impact ux F
e
due to the
electron beam current (Glass-Maujean etal., 2009). Equations 28.4 and 28.6 can be used to dene
a P, Q, or R branch rotational line emission cross section (without cascade) between two electronic
states
X and α:
σ α
α
α
η σ α
em ex
f f
f f
( , , , , , )
( , , , , , )
( , , , )
( ) ( ,X v v J J
A X v v J J
A X v J
X
J
= −1 ,, , , , , )
,
v v J J E
i i
J
i i
e
ν
∑
(28.7)
The total thermally averaged excitation cross section for an electronic band system is dened by
Glass-Maujean
etal. (2009) as
σ
ex
e
=
(
)
∑
N E
N
i
ij
i j
σ ( )
,
0
(28.8)
and
the corresponding thermally averaged emission cross section is given by
σ
η
em
=
−
∑
N
i
ij
σ ( )( )E
N
i j
je
,
0
1
(28.9)
The individual rovibronic excitation cross sections σ
ij
(E
e
) can be calculated from a known transition
probability and a measured excitation function for H
2
(Liu etal., 1998). For negligible self-absorp-
tion, the total band system volumetric photoemission intensity in Equation 28.4 is proportional to
the total emission cross section, σ
em
, and the neutral gas density, N
0
. The σ
em
is related to σ
ex
as
indicated by comparing Equations 28.8 and 28.9. The ratio σ
em
divided by σ
ex
gives the thermally
averaged emission yields for each of the ungerade Rydberg states in Table 28.1 (Glass-Maujean
etal.,
2009).

UV Molecular Spectroscopy from Electron Impact for Astrophysics 771
The UV wavelength calibration methodology developed at JPL has been used by several space-
craft missions (Hord etal., 1992; Esposito etal., 2004). This technique coupled with a relative
ow or swarm gas calibration developed at JPL (Ajello etal., 1989b) allows the determination
of absolute emission cross sections σ
em
from any of the ESL optical instrument systems. Recent
work by the ESL has established benchmark standards for absolute emission cross sections by
electron-impact uorescence measurements at 100 eV for reference gas H
2
(121.6 nm) σ
em
= 7.03
± 0.47 × 10
−18
cm
2
(McConkey etal., 2008) and reference gas N
2
(120nm) σ
em
= 3.7 ± 0.5 × 10
−18
cm
2
(Malone etal., 2008).
The absolute cross section for the emission of a particular spectral line λ induced by electron
impact on a target species M in a swarm gas experiment at 90° to the electron beam can be mea-
sured
at low pressure as (Johnson etal., 2003a,b)
σ
ξ
λ
λ λ
λ
( )
( / ))
M =
1 (
e
KS p
b PI
− 3
(28.10)
table 28.1
e
lectronic-
band
Cross s
ections
and e
mission
y
ields
of h
2
singlet-Ungerade
s
tates
a
state
present
σ
ex
previous
σ
ex
present
σ
em
previous
σ
em
present
e
mission
y
ield
(%)
previous
e
mission
y
ield
(%)
B
1
Σ
u
+
264
b
262
c
263 262
c
99
b
100
C
1
Π
u
244
b
241
c
249
b
241
c
98
b
100
B′
1
Σ
u
+
36
b
38
d,e
21 21
d
53 56
D
1
Π
u
+
25 24
d
11 11
d
43 46
D
1
Π
u
−
21 18
d
21 18
d
100 100
B″
B
1
Σ
u
+
11 >4
d
2.2 1.6
d
20 <40
D′
1
Π
u
+
9.3 7.1
d
1.6 1.0
d
18 14
D′
1
Π
u
−
7.3
≥5.3
d
5.7 5.3
d
78
≤100
D″
1
Π
u
3.2 >0.6 0.9 0.6 28 —
5pσ
1
Σ
u
+
— — 1.1 — — —
6pσ
1
Σ
u
+
— — 0.6 — — —
6pπ
1
Π
u
— — 0.9 — — —
7pπ
1
Σ
u
+
— — 0.6 — — —
Source: Glass-Maujean,
M. etal., Astrophys. J. Suppl., 180, 38, 2009.
With
permission.
a
E = 100 eV and T = 300K. Unit is 10
−19
cm
2
. σ
ex
and σ
em
denote excitation and emission
cross sections, respectively. Certain numbers may not add up due to roundings. See Section
28.5.3
(Glass-Maujean etal., 2009) for estimated errors in cross sections.
b
Excitation cross sections include the excitation into the H(1s) + H(2l) continuum, which is
estimated from the calculation of Glass-Maujean (1986). Emission cross sections exclude
emission from the H(1s)+H(2l) continuum levels, but include continuum emission from the
excited discrete levels into the continuum levels of the X
1
Σ
g
+
state. Transitions to the X
1
Σ
g
+
continuum contribute 27.5% and 1.5%, respectively, to total emission cross sections of B
1
Σ
u
+
− X
1
Σ
g
+
and C
1
Π
u
− X
1
Σ
g
+
(Abgrall etal. 1997). Note correction to B′
1
Σ
u
+
present σ
ex
,
as suggested by Liu (private communication, 2010).
c
From Liu etal. (1998).
d
From Jonin etal. (2000).
e
Include excitations into the continuum levels of the B
1
Σ
u
+
state.
772 Charged Particle and Photon Interactions with Matter
where
S
λ
is the photon signal
K
is a constant related to the geometry of the detector
b
λ
is the sensitivity of the detector
ξ
is the instrumental polarization sensitivity of the system
p
λ
is the polarization of the emitted radiation
P
is the gas pressure
I
e
is the electron beam current
The trapping of resonance radiation can reduce the emission rate signicantly at high gas pressure.
To avoid this complication, the gas pressure range is maintained at a sufciently low level. K is
determined from an intensity measurement of the known N
2
120 nm or H
2
121.6nm emissions, since
the instrumental factors are common to both target species (standard and target gas M). At 100 eV,
the atomic multiplets from dissociative excitation are unpolarized since many repulsive states con-
tribute
to the emissions.
28.4 present status oF h
2
, n
2
, Co, and so
2
28.4.1 h
2
–uv
Hydrogen is by far the most abundant element in the universe, playing a pivotal role in many physi-
cal and chemical processes. For example, in diffuse molecular clouds of the ISM and stellar atmo-
spheres, hydrogen chemistry permeates astronomical changes and provides the markers of stellar
evolution (Dalgarno, 1993, 1995). Over the past two decades, the observations of the ISM have
shown that H
2
is an active component of star formation. The changes that a star undergoes during
the formation and dying process are truly dramatic. These result in the most important interactions
between a star and its environment. Indeed, it is in this area of research that some of the most chal-
lenging astrophysical problems remain unanswered. UV and near-IR emissions from H
2
are among
the principal ways the interstellar gas cools following gravitational collapse during the star forma-
tion (Lepp and Dalgarno, 1996; Lepp etal., 2002). During the last 10 years, the observations of the
distribution of H
2
gas throughout the galaxy by FUSE have contributed to our understanding of stel-
lar evolution (Moos etal., 2000). Hence, H
2
has a unique and extraordinary position in astronomy
by
virtue of its UV spectroscopic signature of diverse energetic environments.
Molecular
hydrogen is the simplest molecule from a structural point of view, but its band spectra
are quite complex and extend from UV to near-IR wavelengths due to the relatively large values of
rotational constants for all the electronic states. An accurate model of the H
2
spectrum has been
a fundamental building block for understanding the chemistry of the solar system and ISM. Until
recently, a 50%–200% uncertainty existed for some of the excitation cross sections and transition
probabilities of the singlet-gerade (even) states of H
2
and HD, i.e., the states that provide VOIR
cascade excitation to the Lyman and Werner bands (Ajello etal., 2005b; Aguilar etal., 2008). The
recent study of H
2
emission cross sections (Aguilar etal., 2008) is the rst of the VOIR wave-
length range (300–1100nm) in 50 years, since the pioneering work of Dieke and coworkers (Dieke,
1958; Dieke and Cunningham, 1965; Crosswhite, 1972) who demonstrated the existence of over
100,000 rotational lines and transitions in this wavelength region involving 15 electronic states of
H
2
(Crosswhite, 1972). The complete single-scattering VOIR spectra of the H
2
and HD gerade–
ungerade band systems had never been studied in the laboratory, nor have the oscillator strengths
been accurately calculated until recently. The theoretical oscillator strength study by the Meudon
Observatory (Aguilar etal., 2008 and references there in) involves detailed calculations of emission
transition probabilities and line positions of individual rotational lines of the nine coupled EF, GK,
HH, K, P
1
Σ
g
+
states and I, R
1
Π
g
and J, S
1
Δ
g
+
states. All of these coupled states contribute heavily to
the UV spectrum through cascading. Comparing the laboratory spectra to model calculations based
UV Molecular Spectroscopy from Electron Impact for Astrophysics 773
on the theoretical oscillator strengths, many irregularities (intensity and wavelength positions) in
the VOIR were explained (Aguilar etal., 2008), although many remain unaccounted for and are
being
reevaluated.
The
ESL has provided the same molecular parameters for the singlet-ungerade (odd) states of
H
2
and HD (and even of D
2
) to an accuracy of 10% (i.e., states that lead to the direct excitation of
the Lyman and Werner bands) (Liu etal., 1995; 1998; 2000; 2002; 2003; Abgrall etal., 1999; Ajello
etal., 2005b; Glass-Maujean etal., 2009). These will aid in the studies of the ISM and planetary
atmospheres,
where both types of electron-excited transitions take place.
Very
accurate synthetic spectral models of H
2
for UV astronomy have been developed recently
(Dols etal., 2000; Jonin etal., 2000; Liu etal., 2003; Gustin etal., 2004; Glass-Maujean etal.,
2009). These models properly account for cascade, predissociation, and resonance effects by utiliz-
ing high-resolution measurements of spectra and cross sections (Glass-Maujean etal. 2009). We
now understand that the complexity of the H
2
band system arises from intense conguration interac-
tion, predissociation, and autoionization that are present in the 11–16eV electronic energy region of
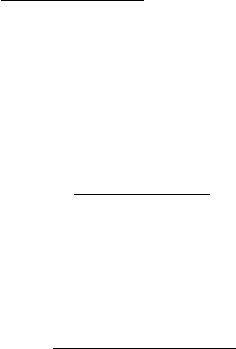
the Rydberg and valence (RV) states. A simplied adiabatic energy level diagram of H
2
exhibiting
the strongest allowed excitation process producing the B → X (Lyman bands) and the strongest
optically
forbidden process producing the EF → B (Lyman band cascade) is shown in Figure 28.7.
τ = 21 ns
τ = 99 ns
τ = 200 ns
τ = 1.1 μs
τ = 2 ns
0
10
15
14
13
12
11
5
4
3
2
1
0
0 0.8 1.6
Internuclear distance (Å)
Potential energy (eV)
2.4 3.2
5
x
1
Σ
g
+
B
1
Σ
u
+
E, F
1
Σ
g
+
G, K
1
Σ
g
+
0
0
0
5
5
2
hν
(Visible-IR)
hν
(MUV)
hν
(Lyman bands)
H
1
Σ
g
+
H
2
Figure 28.7 A partial energy level diagram for H
2
showing the energy regions for the VOIR (gerade-
ungerade) singlet transitions and the UV (ungerade-gerade) transitions (Aguilar etal., 2008). The lowest-lying
ungerade–gerade transition is B
1
Σ
u
+
→ X
1
Σ
g
+
(direct excitation of the Lyman bands) and the lowest-lying
gerade–ungerade transition is E, F
1
Σ
g
+
→ B
1
Π
u
→ X
1
Σ
g
+
(cascade excitation of the Lyman bands). The
approximate lifetimes for some of the direct (dipole-allowed) and cascade (optically forbidden) vibronic
states are listed.
774 Charged Particle and Photon Interactions with Matter
Shemansky and Ajello (1983) identied, for the rst time, the presence of the two most impor-
tant UV emissions in the observations by the Voyager I, II spacecraft for application to the outer
planets. These are the Rydberg series of H
2
, namely,
1
Σ
u
+
1sσnpσ (B, B′, B″, n = 2, 3, 4) → X
1
Σ
g
+
and
1
Π
u
1sσnpπ (C, D, D′, D″, n = 2, 3, 4, 5) → X
1
Σ
g
+
through n = 5, along with optically forbidden exci-
tation of X
1
Σ
g
+
→ EF
1
Σ
g
+
followed by dipole-allowed cascade of EF
1
Σ
g
+
→ B
1
Σ
u
+
, which were found
to be the indicators of electron energy and were the source of the principal (overlapping) spectral
contributions to the Voyager UV spectrum (Shemansky and Ajello, 1983; Ajello et al., 1984; Jonin
etal., 2000). Prior to this work, the Lyman and Werner band systems (n = 2) were thought to be the
only bands involved in the Voyager analysis (Broadfoot etal., 1979, 1981a,b), as shown in Figure
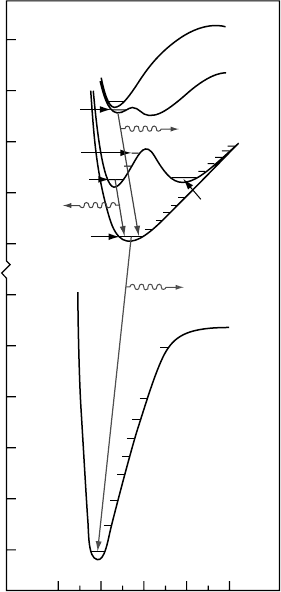
28.3a. In Figure 28.8, we show (to scale) a composite of the rst four (n = 2,3) of the 15 singlet-state
band
systems contributing directly to the H
2
UV spectrum (Liu etal., 1995; Ajello etal., 2001).
Over the last 20 years, a study has been carried out of the remaining singlet-ungerade states
in the high-resolution EUV emission spectra of molecular hydrogen excited by electron impact
at 100eV under optically thin, single-scattering experimental conditions (Jonin etal., 2000; Liu
etal., 2000; Glass-Maujean etal., 2009). A portion (94.5–96 nm) of the high resolution spectrum
(FWHM = 0.0085nm) spanning the wavelength range of 80–115nm is shown in Figure 28.9. The
total emission cross sections for D
1
Π
u
, D′
1
Π
u
, D″
1
Π
u
, B′
1
Σ
u
+
, B″
1
Σ
u
+
states for the n = 3 and four
transitions to the ground state were obtained at 100eV by measuring the emission cross section
of each rotational line. The Lyman and Werner bands have the largest emission cross sections at
100 eV with values of 2.64 × 10
−17
cm
2
and 2.44 × 10
−17
cm
2
, respectively (Liu etal., 1998; Jonin
etal., 2000; Glass-Maujean etal., 2009). Glass-Maujean etal. (2009) now give the 100eV electronic
Rydberg band system emission and excitation cross sections through n = 7. We list these cross sec-
tions in Table 28.1.
X4
X4
D
1
Π
u
1sσnpπ n = 3
C(Werner)
1
Π
u
1sσnpπ n = 2
800 1000 1200
Wavelength (Å)
Intensity (arb. units)
1400 1600 1800
B
΄
1
Σ
u
1sσnpσ n = 3
B(Lyman)
1
Σ
u
1sσnpσ n = 2
Figure 28.8 Model line spectrum of the B
1
Σ
g
+
→ X
1
Σ
g
+
, C
1
Π
u
→ X
1
Σ
g
+
,
′
∑ → ∑
+
+
B
1 1
u g
X ,
and D
1
Π
u
→ X
1
Σ
g
+
band systems of H
2
at 300K and 100 eV without self-absorption. The model is based on the line positions and
transition probabilities of Abgrall etal. (1993a,b,c, 1994, 1997) and the electron emission cross sections of
Liu
etal. (1998).
UV Molecular Spectroscopy from Electron Impact for Astrophysics 775
For J values of 1–4 at laboratory temperatures of ∼300 K, the emission yields of each rovibronic
level of the npσ
1
Σ
u
+
and npπ
1
Π
u
states are determined by comparing observed and calculated rota-
tional spectra (Glass-Maujean etal., 2009). Since Jovian aurora take place at elevated rotational
temperatures of 500–1200K (Gustin etal., 2004), models of the EUV require predissociation yields
to high J-value (∼J = 5–10) (Glass-Maujean etal., 2009). In summary, the mean emission yields of
the B
1
Σ
u
+
, C
1
Π
u
, B′
1
Σ
u
+
, D
1
Π
u
+
, D
1
Π
u
−
, B″
1
Σ
u
+
, D′
1
Π
u
+
, D′
1
Π
u
−
, D″
1
Π
u
states, dened previously,
are 99%, 98%, 53%, 43%, 100%, 20%, 18%, 78%, 28%, respectively, at 100eV and 300K (Glass-
Maujean
etal., 2009) (see Table 28.1).
Using
the Q1(1,4) Werner rovibronic line and the P1,2,3(8,14) Lyman rovibronic line, we devel-
oped an accurate modied Born model for the excitation cross section (without cascade) of the
Lyman and Werner bands for use in the electron transport codes of planetary atmospheres and
astrophysics. The model for 0–1.2keV electron impact energy is shown in Figure 28.10 with the
updated Lyman and Werner 100eV cross sections (Liu etal., 1998; Glass-Maujean et al., 2009).
Glass-Maujean
etal. (2009) give estimates for excitation functions for the B″ and D′-states.
An
accurate model of a 100 eV high-resolution laboratory electron-impact-induced uorescence
spectrum, based on the calculated transition probabilities and predissociation yields of Abgrall
etal., (1993a,b,c) and Glass-Maujean etal., (2007a,b,c,d, 2008), veries adiabatic transition proba-
bilities for RV rovibronic states of n = 4–8 and nonadiabatic transition probabilities for n = 2–4. The
synthetic spectrum is capable of modeling over 98% of the laboratory e + H
2
emission spectrum at
room temperature 300K and 100eV electron energy. Furthermore, the Lyman and Werner emission
cross section energy dependence from Liu etal. (1998) for the B and C states, and the rotational line
positions from Roncin and Launay (1994) have allowed us to generate an accurate (∼15% accuracy
from 79 to 90nm and ∼5% from 90 to 175nm) synthetic high-resolution rotational line spectrum of
the singlet-ungerade states in the UV with electron energies of 10–1000eV. Using the recent work
1.4
1.2
1.0
e (100 eV)+ H
2
(300K) B, B΄, C, D
Experiment
Δλ
FWHM
=140 mÅ
Theory
0.8
0.6
0.4
0.2
0.0
946 948
1(4,4)Q D-X
0(3,0)R C-X
1(14,0)R B-X
3(4,4)Q D-X
2(3,0)Q C-X
1(2,3)Q D-X
2(14,0)P B-X
atomic line 1s-5p
3(3,0)Q C-X
3(3,0)P C-X
3(2,3)P D-X
3(19,1)P B-X
3(2,3)Q D-X
2(5,1)Q C-X
1(0,2)R D-X
4(3,0)R C-X
1(0,2)Q D-X
1(13,0)R B-X
3(5,1)P C-X
3(0,2)Q D-X
2(13,0)R B-X
3(0,2)Q D-X
2(13,0)P B-X
5(3,0)P C-X
1(0,2)R D-X
1(1,2)R B-X
3(18,1)P B-X
1(5,1)R C-X
1(3,0)Q C-X
950 952
Wavelength (Å)
Calibrated intensity (arb. units)
954 956 958 960
Figure 28.9 Over-plot of the observed spectra (FWHM = 140 m Å) and model spectra of H
2
for the low
pressure regime of background pressure of 1.2 × 10
−5
Torr. The high-pressure spectrum spans the wavelength
range from 945 to 960 Å. The model uses the transition probabilities of Abgrall et al. (1993a,b,c, 1994, 1997)
with a transmission function for self-absorption at 100 eV electron-impact energy and a gas temperature
of 300 K.
776 Charged Particle and Photon Interactions with Matter
of Glass-Maujean etal. (2007a,b,c,d, 2009) and comparing the synthetic spectra through n = 8 with
the ESL high-resolution spectra, it has been possible to accurately model direct excitation to the
high-lying, ungerade-singlet Rydberg states. The high-lying states contribute signicantly to the
EUV
spectrum below 90
nm.
28.4.2 h
2
–voir
The electron-impact-induced uorescence spectrum of H
2
from 330 to 1000nm at 20 and 100 eV
was reported for the rst time (Aguilar etal., 2008) at high resolution (λ/Δλ = 10,000). The spectrum
contains the gerade Rydberg series of singlet states EF
1
Σ
g
+
, GK
1
Σ
g
+
, H
−
H
1
Σ
g
+
, I
1
Π
g
, J
1
Δ
g
…→ B
1
Σ
u
+
,
C
1
Π and the Rydberg series of triplet states dominated by the d
3
Π
u
, k
3
Π
u
, j
3
Δ
g
→ a
3
Σ
g
+
,C
3
Π
u
. These
VOIR
bands were recently observed by the Cassini Imaging Subsystem (ISS) in the visible/near IR
lters viewing the Saturn aurora (Dyudina etal., 2007). STIS or Cosmic Origins Spectrograph
(COS) might also be able to observe these directly on the outer planets. A model VOIR spectrum
of H
2
from 750 to 1000nm, based on newly calculated transition probabilities and line positions,
including rovibrational coupling for the singlet-gerade states, is in excellent agreement with the
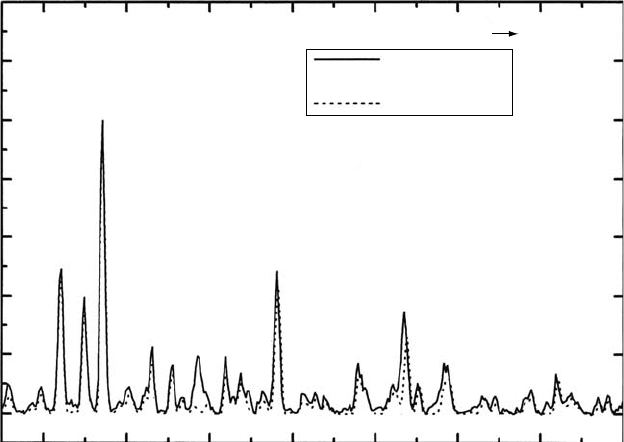
observed intensities. Figure 28.11 shows the experimental data of high-resolution (0.07nm FWHM),
electron-impact-induced UV-VOIR emission spectra from 330 to 1200nm at 100eV.
The absolute emission cross section values for excitation to the singlet-gerade states at 100eV
for optically thin, single-scattering condition was measured to be 4.58 ± 1.37 × 10
−18
cm
2
; for
excitation
to the triplet states at 20
eV,
the cross section was 1.38 ± 0.41 × 10
−19
cm
2
(Aguilar etal.,
2008). The singlet-gerade emission cross sections are due to cascading into the UV (including the
Lyman and Werner band systems), and the triplet-state emission cross sections are due to cascade
dissociation of the H
2
(a
3
Σ
g
+
− b
3
Σ
u
+
) continuum that produces fast hydrogen atoms (Ajello and
Shemansky, 1993).
In a complementary type of experiment to the VOIR, a newly devised pulsed spectroscopic
technique demonstrated (Dziczek etal., 2000; Liu etal., 2002) that the gerade series (EF, GK, H
−
H,
I,J…) make a signicant contribution (∼50% at 20eV) to the UV spectrum of H
2
, by virtue of their
cascade to states that correlate to H(1s) and H(2s,2p) (i.e., the upper states of the Lyman and Werner
300
250
100 eV
Werner
Lyman
200
150
100
50
0
0 100 200 300 400 500
Excitation energy (eV)
σ (10
–19
cm
2
)
600 700 800 900 1000 1100 1200
Figure 28.10 Total cross section (in 10
−19
cm
2
) of the Lyman and Werner band systems at 300K. The cross
section of the Lyman band system is represented by dots, while that of the Werner band system is shown by
the
solid line (Liu etal., 1998; Glass-Maujean etal., 2009).
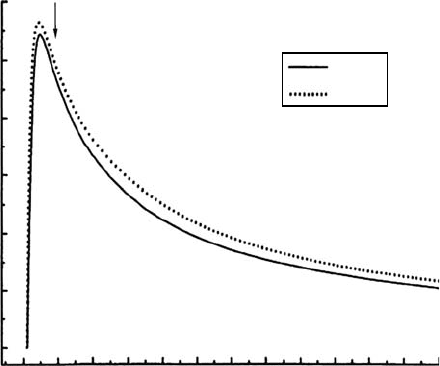
UV Molecular Spectroscopy from Electron Impact for Astrophysics 777
bands, respectively). This large cascade cross section is important because the mean secondary
electron energy in planetary thermosphere and in cosmic-ray-induced ionization in molecular cloud
lies between 20 and 100eV (Gredel etal., 1989; Ajello etal., 2002a, 2005b). The intensity of UV
resonance transitions excited by electron impact is determined by both the direct and cascade pro-
cesses. The lifetime (τ) for decay by spontaneous emission from a dipole-allowed transition is typi-
cally short (<10 ns, see Figure 28.7). To measure the electron-impact cascade spectrum of the
n= 2 H
2
Lyman band system (B
1
Σ
u
+
− X
1
Σ
g
+
), we use the longer lifetimes (>30ns, see Figure 28.7)
for cascade from higher lying states (EF, GK, H
−
H, …
1
Σ
g
+
). The pulsed gun technique takes advan-
tage of the drastic difference in lifetimes (∼1ns vs. ∼100ns) between the ungerade (direct) states and
singlet-gerade (cascade) states, respectively. The rst laboratory studies and modeling of the UV
spectrum of H
2
attributed to cascade used a pulsed gun technique to separate cascade and direct
excitation effects (Dziczek etal., 2000; Liu etal., 2002). Pulsing the electron gun and gating the
photon detector to measure the cascade spectrum after the directly excited population decays also
allows a determination of the cascade cross section because the spectral pattern from direct excita-
tion and cascade are different. Direct excitation produces a large population in the B-state centered
at v′ = 7, whereas cascade populates the lower vibrational levels most strongly, beginning at v′ = 0.
By
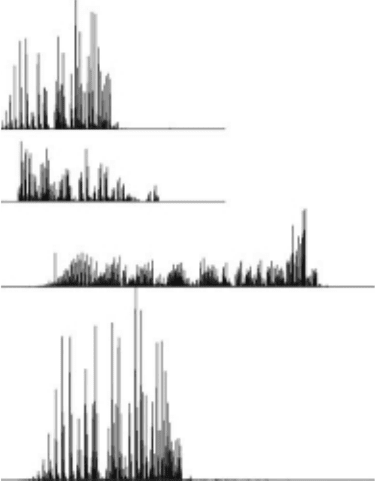
studying Figure 28.12, we clearly see that there are regions in the FUV spectrum dominated by
cascading. The most important two wavelength regions that are exclusively (more than 90%) due
to cascade lie near the 133–135 and 139–142nm. These regions correspond to the rotational lines of
the (0,1) and (0,2) vibrational bands of the B−X Lyman bands, the two strongest bands of the v′ = (0, v″)
progression. The strongest feature in the cascade spectrum occurs at 161nm and involves the super-
position
of rovibronic transitions from v′ = 4, 5, and 6.
The
medium-resolution spectrum at 14eV is shown in Figure 28.13b in both the FUV- and mid-
dle ultraviolet (MUV) portion of the VOIR extending from 100 to 530nm, including the Lyman
bands and the H
2
(a
3
Σ
g
+
→ b
3
Σ
u
+
) continuum (James etal., 1998b). The rst detection of the H
2
a-b
continuum MUV emission in astronomy (Pryor etal., 1998) was made through comparison to our
0.35
0.30
0.25
0.20
0.15
0.10
0.05
0.00
Hβ
Hα
EF-B bands
Grating 2
Grating 3
Theory
–0.05
3000 4000 5000 6000 7000 8000
Wavelength (Å)
Cross section (Mb)
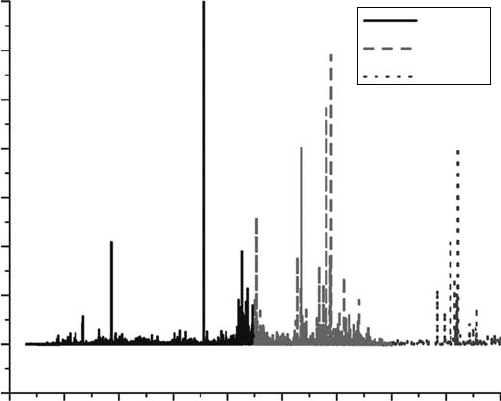
9000 10000 11000 12000
Figure 28.11 High-resolution spectrum with intensity in units of cross section (cm
2
) for the electron-impact-
induced uorescence spectrum from 3,300 to12,000Å of H
2
at 100eV in three wavelength regions: (1)grating-2
(3300–7500Å) in black, grating-3 (7,500–10,000Å) in long-dash light gray, and theory (10,000–12,000 Å) in
short-dash dark gray. The Hα multiplet is off scale (Aguilar etal., 2008).
778 Charged Particle and Photon Interactions with Matter
Relative intensity (arb. units)
20 eV DC spectrum
20 eV pulse spectrum
Model 20 eV spectrum (no cascade)
3.0
2.5
2.0
1.5
1.0
0.5
0.0
90 100 110 120
Wavelength (nm)
130 140 170150 160
Figure 28.12 The 20 eV steady state (cascade + direct) spectrum and linear regression t using the 20 eV
pulsed-gun cascade spectrum and 20eV model direct excitation spectrum. H Lα is included in the model as
a
monochromatic line. The photon gate delay for the pulsed-gun spectrum is 135
ns
(Dziczek etal., 2000).
400
300
200
100
Counts
0
–100
–200
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
100 150
F-PMT E-PMT
x5
H
2
(a–b)
H
2
14 eV
hv
a–b
a
3
Σ
g
+
b
3
Σ
u
+
x
1
Σ
g
+
H+H (e–3 eV)
200 250 300
Wavelength (nm)
(b)
Relative intensity (arb. units)
350 400 450 500
2000 2500
H
2
(a – b)
(First observation in astronomy)
Dark side –Scaled day side
Lab 14 eV e
–
on H
2
spectrum
Wavelength (Å)
(a)
Laboratory
Galileo
H
2
MUV spectrum
3000
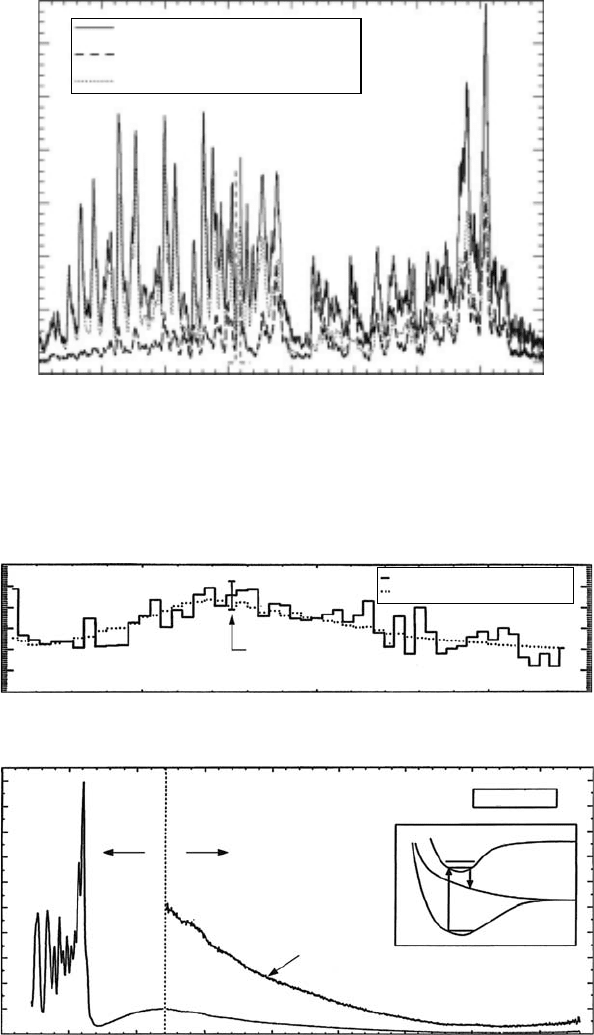
Figure 28.13 (a) The rst observation in astronomy of the H
2
a-b continuum. Jupiter darkside aurora spec-
trum overplotted with a 14eV laboratory spectrum (Proyor etal., 1998). (b) The combined FUV spectrum,
measured with an EMR F-photomultiplier (CeTe photocathode), and the MUV spectrum, measured with an
EMR –E-photomultiplier, of H
2
corrected for instrument sensitivity produced by electron impact at 14 eV
measured at 1.7nm FWHM at 300 K and 2 × 10
−4
Torr background gas pressure in the crossed-beams mode
(James
etal., 1998a,b).
UV Molecular Spectroscopy from Electron Impact for Astrophysics 779
laboratory spectrum (James etal., 1998b). We show the comparison of the Galileo observation of
the
Jupiter dark side aurora with the laboratory spectrum in Figure 28.13a.
The
set of triplet states above the b
1
Σ
u
+
repulsive state leads to continuous emission with
high-velocity H-atoms (∼3 eV per atom) formed in the spontaneous dissociation. This process
of electron excitation is shown in the inset to Figure 28.13b. The b
3
Σ
u
+
state, which is the lowest
lying repulsive state (1sσ) (2pσ), can be excited to the continuum by direct excitation from the
ground X
1
Σ
g
+
state or via cascade from the a
3
Σ
g
+
− b
3
Σ
u
+
continuum. The a
3
Σ
g
+
state produces the
strongest triplet-state emission from the process H
2
(a
3
Σ
u
+
→ b
3
Σ
g
+
), leading to the famous a-b
continuum, for electron energies below 30 eV (Ajello and Shemansky, 1993). The a
3
Σ
u
+
state is
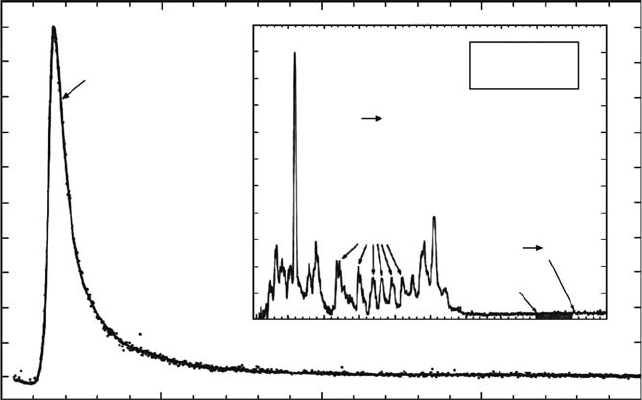
strongly populated by cascade from the c-, d-, and e-states. The excitation function for the a-b
continuum is shown in Figure 28.14. The gure (see inset) also shows a 20 eV spectrum indi-
cating relative importance and wavelength region for strong cascade and a-b continuum in the
FUV. The triplet states are the major source of dissociation of the hydrogen molecule by electron
impact.
Optical excitation functions of the triplet states have also been measured by few experi-
menters in the VOIR. Dieke (Crosswhite, 1972) has shown the presence of many triplet band
systems in his discharge emission experiments with the strongest and most extensive to be the
Fulcher-α band system (3pπ d
3
Π
u
→ 2sσ a
3
Σ
g
+
). The emission cross sections of the Fulcher-α
diagonal bands (Δv = 0) have been studied by Möhlmann and de Heer (1976). Those processes
leading to triplet emissions arise rst from singlet–triplet excitation. The excitation occurs by
electron exchange, which is characterized by a fast rise and decrease in the emission cross
section within a few eV of threshold (see Figure 28.14). Tawara etal. (1990) have reviewed
the excitation cross sections of the X → 2sσ a
3
Σ
g
+
, 2pσ b
3
Σ
u
+
, 2pπ c
3
Π
u
, and 3pσ e
3
Σ
u
+
states
by electron-energy-loss experimental techniques. Emission from the e
3
Σ
u
+
triplet state e → a
transition was observed by Dieke (1958) and Dieke and Cunnigham (1965) as well as by Dieke
in a couple of early publications as referenced by Huber and Herzberg (1979) and Crosswhite
(1972). The c-state, whose v′ = 0 level lies below the a-state, is forbidden for transitions to the
b- and X-state. Thus, there are two Rydberg series terminating in the two bound triplet states,
1.0
1.0
0
0
0 50 100
Energy (eV)
Relative intensity (arb. units)
150 200
210200190180170160
Wavelength (nm)
150140130120110
H
2
(a–b, 190 nm)
Δλ=10 nm
H
2
+e (20 eV)
Δλ=0.5 nm
Direct
+
cascade
Q =190 nm
B
1
Σ
+
u
X
1
Σ
+
g
Lyman bands
L
α
121.57 nm
a
3
Σ
+
g
b
3
Σ
+
u
Relative cross section (arb. units)
Figure 28.14 The H
2
(a
3
Σ
g
+
→ b
3
Σ
u
+
) excitation function from 0 to 200eV (Ajello and Shemansky, 1993).
The inset is a combined FUV and MUV spectrum of H
2
(a
3
Σ
g
+
→ b
3
Σ
u
+
) continuum at 20eV. The cross-hatched
area
shows the band pass of the spectrometer for the excitation function measurement.
