Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

740 Charged Particle and Photon Interactions with Matter
Chitin is poly-β-(1-4)-N-acetyl-d-glucosamine, which is the main component of the external
skeleton in Arthropoda and the periostracum in Mollusca. Its deacetylated derivative is chitosan.
Chitin and chitosan are not soluble in water. After carboxymethylation, the resulting carboxymethyl
chitin, CMCht, and carboxymethyl chitosan, CMChts, become soluble in water. Their chemical
structures are shown in Figure 27.3. The chemically modied CMCht and CMChts can be cross-
linked in these paste-like states (Wasikiewicz et al., 2006), though intrinsic chitin and chitosan are
decomposed by high energy irradiation. Figure 27.4 shows the effect of concentration on gel frac-
tion. EB irradiation of 50kGy gave gel fractions of 65% and 40% for CMCht and CMChts, respec-
tively, in the 30%–40% concentration range. At lower and higher concentrations, the gel fraction
went
down.
In
the swelling, the correlations were divided into two categories. In both the cases of CMCht
and CMChts, the hydrogels show similar tendencies. There are linear relationships between the
gel fraction and swelling of CMCht and CMChts. Though the swelling decreased with an increase
of the gel fraction, CMChts hydrogels show lower swelling than CMCht hydrogels at the same gel
fraction.
27.2.1.1 applications
of p
olymer
g
els
Polysaccharides are well-known biodegradable materials. This feature is maintained even after
cross-linking with high energy radiation. The obtained gel can be degraded by microorganisms after
disposal. This characteristic is useful for environment-friendly products such as bedsore prevention
mats
(Chmielewski, 2006), coolants, and water absorbents for livestock excrement treatment.
0 20 40 60 80
0
20
40
60
80
100
(a)
(b)
(c)
Dose (kGy)
Gel fraction (%)
Figure 27.2 Gel fraction of cross-linked CMC having different degrees of substitution (DS) at concentra-
tion of 20%; (a) DS = 2.2, (b) DS = 1.3, and (c) DS = 0.86. (From Fei, B. et al., J. App. Polym. Science, 278,
2000.
With permission.)
O
OR
2
n
O
CH
2
OR
2
CH
2
OR
2
OR
2
NHR
1
NHR
1
O
O
O
Figure 27.3 Chemical structures of carboxymethyl chitin and chitosan; R
1
= H or COCH
3
and R
2
=
CH
2
COONa or H.

Radiation Processing of Polymers and Its Applications 741
Bedsore prevention requires the bedsore prevention mat to be suitably elastic for promoting
blood circulation. CMC hydrogel was applied as bedsore prevention mat, which is used as bed mats
during the surgical operation procedures (Figure 27.5). Before the operation is conducted, the mat is
pre-heated to body temperature (37°C) using an oven heater. Temperature could be maintained dur-
ing operation. Clinical tests proved that CMC mat was very effective in the prevention of bedsores.
The comparison of blood ow with CMC mat, with those of other mats such as board and general
mattress revealed that CMC hydrogel mat reached the highest value. The hydrogel can disperse the
body pressure and improve circulation of blood during operation. Thus, it could prevent bedsores
in
patients.
CMC
hydrogel has also been applied as a coolant. This hydrogel coolant has a high capacity to
retain low temperatures longer than general liquid coolants. Fish and vegetables could be trans-
ported from the production district to distant markets using a hydrogel coolant. This product uses
swollen
CMC dry gel.
CMC
dry gel can absorb water, several hundreds times its own weight. The addition of CMC
dry gel controls the water content in livestock excreta and accelerates its composting process. Field
heaping and open storage of livestock excreta have been legally prohibited in Japan, since the
release of excrement and urine contaminates groundwater and river water. The high water content
of around 90% in excrement retards its fermentation to fertilizer. The addition of 0.2% CMC dry gel
to the excrement as a water absorbent can reduce the water content by up to 70% and can enhance
Concentration (%)
Gel fraction (%)
Degradation
0
20
40
60
80
100
0 20 40 60 80 100
Cross-linking
CM-chitin
CM-
chitosan
Figure 27.4 Effect of concentration on gel fraction in cross-linked CM-chitin and CM-chitosan; CM-chitin
and
CM-chitosan were irradiated with 50
kGy
at 30% aqua paste-like state.
Hydrogel
Figure 27.5 Bedsore prevention mat composed of hydrogel obtained by cross-linking of CMC.
742 Charged Particle and Photon Interactions with Matter
the fermentation. Previously, saw dust was used for this purpose. Usage of CMC dry gel minimized
the consumption of saw dust to one-sixth. The advantages of this novel technology are to reduce
storage
area for saw dust, heavy work, and odor diffusion to environment.
CMCht
and CMChts are considered to be the most abundant natural amino polysaccharide and
have versatile applications in cosmetics, food, and medical materials (Kumar, 2000). They were
used as metal-ion adsorbents as they carry an amine group that has afnity toward metal ions
such as copper, zinc, and mercury (Benavente, 2008). Such commercialized, adsorbent resins were
generally synthesized by copolymerizing the precursor monomer, chloromethylstyrene, and cross-
linker, divinylbenzene. The resulting cross-linked resin has the convertible site of –CH
2
Cl in the
chloromethylstyrene moiety to the functional group having afnity toward metal ions. This precur-
sor resin can be chemically modied to a metal-ion adsorbent having –SO
3
, –COOH, and –NH
2
groups. However, these resins are derived from petroleum products. To secure petroleum resources
and decrease environmental burdens, the naturally occurring polymers such as CMCht and CMChts
should
be used as raw materials for synthesizing metal-ion adsorbents.
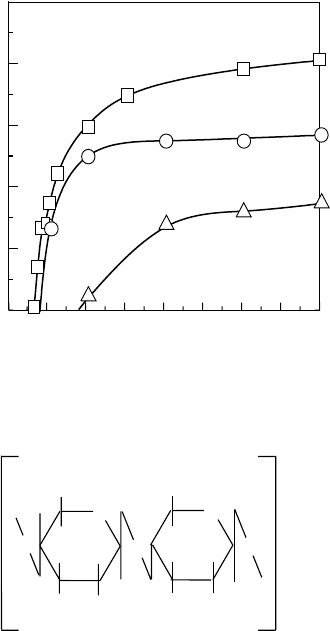
The
metal adsorption of CMCht and CMChts hydrogels shows the adsorption of various metal
ions as shown in Figure 27.6 (Wasikiewicz et al., 2005a). The CMCht hydrogel adsorbed Pd, Au,
Cd, V, and Pt in 60min. In the case of CMChts, Au and Pt were preferentially adsorbed in 120min.
Adsorbed metals on CMCht and CMChts hydrogels can be eluted by dilute hydrochloric acid solu-
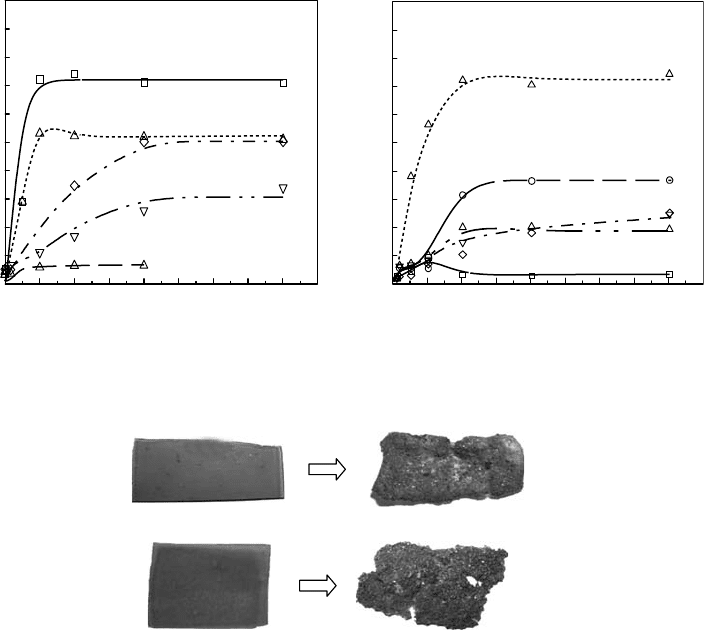
tion. After disposal of CMCht and CMChts, the size and thickness of the lms in soil become
smaller. Figure 27.7 shows CMCht and CMChts hydrogel lms, 1mm thick, before and after keeping
0
40
60
100
Adsorption (%)
Adsorption time (min)
Pd
Au
Cd
V
Pt
20
80
0 120 240 360 480
0 120 240 360 480
Adsorption time (min)
Au
Pt
Cd
V
Pd
0
40
60
100
20
80
Adsorption (%)
(b)(a)
Figure 27.6 Metal adsorption of CM-chitin and chitosan gels at pH 3.9; (a) CM-chitin hydrogel and
(b)CM-chitosan hydrogel. (Modied from Wasikiewicz, J.M. et al., Nucl. Instrum. Methods Phys. Res.Sect.B,
236,
617, 2005a.)
(b)
(a)
Figure 27.7 Degradation of CM-chitin and chitosan gels kept in soil for 10 weeks; (a) CM-chitin hydrogel and
(b) CM-chitosan hydrogel. (From Wasikiewick, J.M. et al., J. Appl. Polym. Sci., 102, 758, 2006. With permission.)
Radiation Processing of Polymers and Its Applications 743
in soil for 10 weeks from October to December in Japan. In the case of CMChts, a number of small
holes are noticeable. It can be concluded that prolonged storing of these lms in the ground would
cause their complete disintegration as a result of bacterial activity. Natural polymers including chi-
tin and chitosan are renewable resources and are spontaneously degraded by naturally occurring
microorganisms. Such polymers do not increase the burden to the natural environment since they
do not produce any toxic waste products during the degradation process.
27.2.2 biodegradable plaSticS
Poly(l-lactic acid), PLA, is a transparent and hard plastic and is produced by condensation polymer-
ization of lactic acid obtained by fermentation of starch (Reddy et al., 2008). In this regard, PLA is a
typical renewable plastic. In the near future, nonbiodegradable engineering plastics will be replaced
by PLA on the viewpoint of environmental preservation. One of the promising applications of PLA
is in thermally molded products such as those used in food packaging, bottles, medical, and phar-
maceutical products, which require high thermal stability. However, PLA is thermally deformed at
temperatures higher than its glass transition temperature of 60°C, though it has a high melting point
of
175°C.
PLA
is not cross-linked by irradiation without a certain cross-linker, since PLA is degraded
by ionizing radiation. It was found that the addition of polyfunctional monomers (PFM) as cross-
linkers to PLA could induce cross-linking (Charlesby, 1981). PFM such as triallyl isocyanurate
(TAIC), trimethallyl isocyanurate (TMAIC), trimethylolpropane triacrylate (TMPTA), trimethy-
lolpropane trimethacrylate (TMPTMA), 1,6-hexanediol diacrylate (HDDA), and ethylene glycol
bis[pentakis(glycidyl allyl ether)] ether (EG) have been widely used as cross-linkers for polyolens,
owing
to their high reactivity against polymer chains.
After
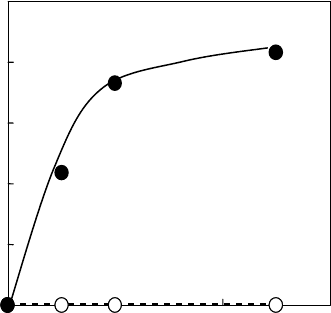
screening of various PFM, it was found that TAIC is a suitable cross-linker for PLA. Figure
27.8 shows the effect of dose on the gel fraction of irradiated PLA with 1% and 3% TAIC. The gel
fraction of the cross-linked PLA was estimated by the weight of its insoluble part after immersion
in chloroform for 48h. The gel fraction reached 83.3% for 3% of TAIC after irradiation of 50kGy.
TMAIC gave a slightly lower gel fraction. In the cases of TMAPT, TMPTMA, HDDA, and EG,
the gel fractions did not reach 30%. TAIC was the most effective cross-linker for PLA since it has
three functional groups of C=C and an isocyanuric ring that achieves a greater three-dimensional
network
by irradiation than that achieved by acrylate-type PFM.
0
20
40
60
80
100
0 20 40 60
Dose (kGy)
Gel fraction (%)
Figure 27.8 Effect of dose on cross-linking of PLA with TAIC; (○) 1% TAIC and (●) 3% TAIC. (Modied
from
Nagasawa, N. et al., Nucl. Instrum. Methods Phys. Res. Sect. B, 236, 611, 2005.)

744 Charged Particle and Photon Interactions with Matter
Figure 27.9 shows the thermal deformation of cross-linked PLA with 3% TAIC and PLA without
cross-linking (Nagasawa et al., 2005). The thermal deformation was evaluated on PLA lms heated
from room temperature up to 200°C in a nitrogen atmosphere under a constant load of 0.5g at a
heating rate of 10°C/min. Under thermal stretching, the PLA started to elongate at the glass transi-
tion
temperature of 60°C, and then thermal stretching reached 60% at 100°C. The cross-linked PLA
showed no elongation at glass transition temperature and has low elongation of thermal stretching
less than 10% even at 200°C. This result revealed that radiation-induced cross-linking induced the
thermal resistance of PLA, high enough for versatile applications. Enzymatic degradation of PLA was
evaluated by degradation using proteinase K enzyme from Tritirachium album. The weight loss of
PLA was 60% after 140h incubation, whereas the cross-linked PLA with TAIC were degraded 30%
in weight losses. After cross-linking, the diffusion of the enzyme is considered to be disturbed by the
network structure of PLA. However, enzyme degradability was still maintained at half of intrinsic
PLA. This result implies that PLA is an environmentally acceptable material, even after cross-linking.
Cross-linked PLA can be applied to tableware such as cups and plates. PLA containing 3% TAIC
was molded to cups and plates by an extruder and then irradiated with 50kGy to induce cross-
linking. The obtained tableware were transparent since PLA is an intrinsically colorless polymer.
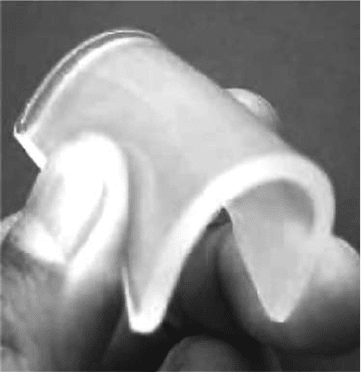
When boiling water was poured into cups with and without cross-linked PLA, the cup without PLA
cross-linking deformed and changed to white as shown in Figure 27.10. But the cross-linked PLA
Temperature (°C)
0 50 100 150 200
0
40
20
60
ermal stretching (%)
Figure 27.9 Thermal deformation of PLA and cross-linked PLA; PLA was kneaded with 3% of TAIC
and then irradiated with 50kGy; broken line is for PLA and dotted line cross-linked PLA. (Modied from
Nagasawa,
N. et al., Nucl. Instrum. Methods Phys. Res. Sect. B, 236, 611, 2005.)
(a)
(b)
Figure 27.10 Effect of cross-linking on thermal property of PLA; (a) PLA and (b) cross-linked PLA.
(Modied
from Nagasawa, N. et al., Nucl. Instrum. Methods Phys. Res. Sect. B, 236, 611, 2005.)
Radiation Processing of Polymers and Its Applications 745
cup maintained its original transparency and shape. The color change from transparent to milky
was caused by the crystallization of PLA. After cross-linking, the movement of PLA molecules was
restricted by the cross-linking point. Due to this reason, the cross-linked PLA cup could maintain
transparency
when it was heated close to 100°C.
Cross-linked
PLA can be used in heat-shrinkable tubes. The resulting cross-linked PLA tube is
expanded diametrically to double its size at 200°C and then holds the expanded shape when it cools
down to room temperature. This is a heat-shrinkable tube that thermally shrinks to unexpanded
tube size. PLA without cross-linking cannot be expanded at the temperature over its melting point
of 160°C. Such heat-shrinkable polyethylene tube have already been commercialized. However,
PLA heat-shrinkable tubes have several advantages such as biodegradability, high heat resistance,
and transparency.
If the elastic property can be imparted into PLA, new applications such as renewable lapping
lms and impact absorbing liners can be realized. Plasticizers are used to induce the elastic property
into hard plastics like PLA. When PLA is simply mixed with plasticizer, PLA temporarily becomes
elastic. However, the plasticizer is gradually expelled from the PLA. In the case of PLA without
cross-linking, the plasticizer is not maintained in the PLA for 30min at 100°C. This is because the
PLA crystallizes at this temperate and simultaneously the plasticizer is expelled from PLA. After
cross-linking, PLA can contain the plasticizer up to the concentration of 35% and obtains stable
elasticity
as shown in Figure 27.11.
27.3 new material produCtion using radiation-induCed graFting
27.3.1 general application
The membranes prepared by radiation-induced graft polymerization (radiation-grafted membranes)
have been widely applied to separation processes (pervaporation, reverse osmosis, and electrodi-
alysis), electrochemical processes (bottom battery and fuel cells), and adsorbents for metal ions
(Figure 27.12) (Saito and Sugo, 2001; Gupta et al., 2004; Nasef and Hegazy, 2004). These mem-
branes, in general, consist of similar components, hydrophilic grafted polymers (grafts) attached
into hydrophobic trunk polymers (substrates), namely, the so-called anion-exchange membrane
structures. The membranes containing the grafts with acids such as carboxylic, phosphoric, and
sulfonic acids and those with bases such as amino groups and ammonium salts are called “cation-
and anion-exchange membranes,” respectively. Hydrophobic polymer substrates can be classied as
Figure 27.11 Elasticity appeared by holding plasticizer in cross-linked PLA. (From Nagasawa, N. et al.,
High Functionalization and Recycling Technology for Bioplastic NTS Inc.pp. 162–169, 2008. With permission.)
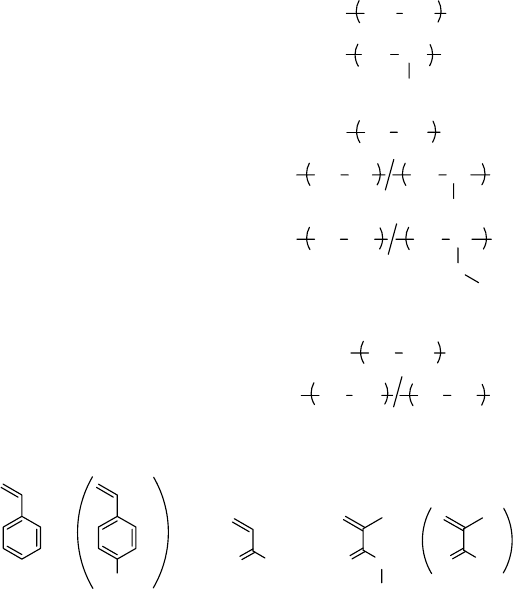
746 Charged Particle and Photon Interactions with Matter
conventional polyolen lms (polyethylene (PE) and polypropylene (PP)), peruoro- and partially
uorinated-polymer lms (poly(tetrauoroethylene)) (PTFE), poly(tetrauoroethylene-co-hexau-
oropropylene) (FEP), poly(tetrauoroethylene-co-peruorovinyl ether) (PFA), poly(vinylidene uo-
ride) (PVDF) and poly(ethylene-co-tetrauoroethylene) (ETFE), and natural polymer resins (wool,
cotton, and paper), as shown in Figure 27.13. To the above substrates, acrylic acid (AAc), methyl
methacrylate (MMA), styrene derivatives, vinyl pyridine, and the corresponding salts are intro-
duced as hydrophilic graft polymers (grafts), and subsequent chemical transformation results in
radiation-grafted
membranes.
The
grafting of poly(copper acrylate), poly(vinylpyridinium salts), and phosphonium salts gave
antibacterial natural polymers. The grafted membranes, consisting of poly(AAc) incorporated into
Hydrocarbon polymers
Perfluorocarbon polymers
Partially fluorinated carbon polymers
Polyethylene
Polypropylene
Poly(tetrafluoroethylene)
Poly(tetrafluoroethylene-
co-hexafluoroethylene)
Poly(tetrafluoroethylene-
co-perfluorovinyl ether)
Poly(vinylidene fluoride)
Poly(ethylene-
co-tetrafluoroethylene)
CH
2
CH
2
CH
2
CH
CH
3
CF
2
CF
2
CF
3
CF
3
CF
2
CF
2
CF
2
CF
2
CF
2
CH
2
CF
2
CF
2
CF
CF
O
CF
2
CF
2
CH
2
CH
2
Monomers
Styrene
SO
3
H
O OH
O O
O OH
Acrylic acid
(AAc)
Methyl methacrylate
(MMA)
PE
PP
PTFE
FEP
PFA
PVDF
ETFE
Figure 27.13 Structures of polymer substrates and monomers constituting grafted membranes.
Separation process
• Pervaporation
(alcohol, organic chemicals from water)
•
Reverse
osmosis
•
Metal adsorbents (hazardous metal separation or precious metal correction)
Electrochemical
processes
•
Electrodialysis
(production of water, NaCl)
•
Chloroalkali
(production of NaOH, Cl
2
)
Battery/energy
system
•
Bottom
battery
•
Polymer
electrolytes for fuel cell
Figure 27.12 Application elds of radiation-grafted membranes.
Radiation Processing of Polymers and Its Applications 747
PE, can be applied to pervaporation membranes to remove only water from aqueous alcohol solutions
owing to higher afnity of the poly(AAc) grafts to water than alcohols (Gupta et al., 2004). The mem-
branes having carboxylic, sulfonic, and phosphoric acids in the grafts into PE can act as metal-ion
adsorbents owing to the high afnity of the grafts to the metal ions in aqueous solutions (Saito and
Sugo, 2001). Furthermore, the radiation-grafted membranes, possessing poly(AAc) grafts into PE can
be commercialized as a battery separator of bottom cells because only hydroxide ions, but not metal
ions (Ag(OH)
2
−
) can pass through the membranes (Ishigaki et al., 1982a,b; Hsiue and Huang, 1985).
After the successful utilization of radiation-grafted membranes in battery cells, the grafted
membranes consisting of uorinated-polymer substrates, having higher mechanical and thermal
strengths, and the grafts, having more acidic sulfonic acid groups, have been developed for polymer
electrolyte membranes (PEM) for fuel cells, which require higher conductivity and durability at
higher temperatures. Sections 27.3.2 and 27.3.3 focus on recent advancements of radiation-grafted
membranes as metal adsorbents to recover precious metal ions and in battery applications such as
a
bottom cells and fuel cells.
27.3.2 fibrouS Metal-ion adSorbentS
To synthesize the metal adsorbent, the functional groups having strong afnity toward metal ions
should be imparted into these trunk polymers by grafting, as shown in Figure 27.14. Other func-
tional groups were reviewed by Smith and Alexangratos (2000). When a monomer has a chelating
group in its side chain, the metal adsorbent is directly synthesized only by grafting. In the case of a
monomer having a precursor for coordination, chemical modication is necessary after its grafting
(Seko et al., 2005). Table 27.1 lists the representative functional group for metal adsorption, cor-
responding
grafting monomer, and chemical reagent (Basuki et al., 2003).
GMA
is a useful monomer for the precursor of metal adsorbents. The grafting of GMA generally
is carried out using organic solvents such as methanol (Kavalla et al., 2004) and dimethyl sulfoxide
(Aoki et al., 2001). It was found that grafting yield was dramatically enhanced when GMA was
emulsied by surfactants in water instead of organic solvents (Seko et al., 2007). This aqueous
GMA emulsion grafting on PE bers gave a degree of grafting of 130% in preirradiation conditions
of
10
kGy,
grafting temperature of 40°C, and grafting time of 2
h.
Nonwoven fabric made of polyethylene is used as a trunk polymer. This is because the resulting
fabric adsorbent causes swift adsorption of metal ions and ensures easy handling in the adsorption
process. For example, the metal adsorbed in the fabric adsorbent can be picked up from the solution by
forceps after it is dipped into the metal solution for a few minutes. Additionally, a conventional adsor-
bent resin is used in a volume-normalized ow rate, namely space velocity, around 10h
−1
. At ow rates
far more than 10h
−1
, the adsorption capacity dramatically decreases in a column mode adsorption.
However, the adsorbent fabric can be used in the space velocity more than 1000h
−1
(Jyo et al., 2003).
As applications of metal adsorbents, toxic and rare metals were attempted to be collected for envi-
ronmental preservation and metal resource security, respectively. During scallop processing, the mid-
gut gland is discarded since this part contains 20–40ppm of cadmium. The discarded mid-gut gland
Graft chains
Graft polymerization
Monomer
Addition of
monomer
Radicals
Creation of
active species
Irradiation
(EB and γ-ray)
Trunk
polymer
Figure 27.14 Schematic diagram of radiation-induced graft polymerization.

748 Charged Particle and Photon Interactions with Matter
is incinerated even though it contains a large amount of nutrients such as straight-chain fatty acids
and fat. The mid-gut gland is treated with amidoxime adsorbent (Shiraishi et al., 2003). The bench
scale plants for removal of cadmium from mid-gut glands was operated using iminodiacetic acid
adsorbent and cadmium-free mid-gut gland could be used as fertilizer and animal feed. Iminodiacetic
acid adsorbent was used in the bench scale plant because the pH of the eluent was3. In this pH range,
the iminodiacetic acid adsorbent has a higher selectivity to cadmium than the amidoxime adsorbent.
Uranium collection from seawater has been researched from the viewpoint of resource security
of atomic power generation. Amidoxime-type adsorbents synthesized by graft polymerization can
adsorb uranium occurring in an extremely low concentration, 3 ppb, in seawater with the coexis-
tence
of sodium and magnesium ions.
The
performance of uranium adsorbent fabrics was evaluated using 350kg adsorbent stacks
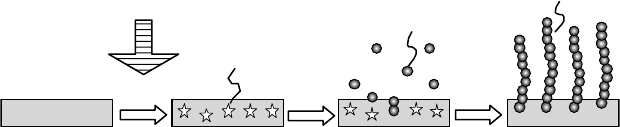
by soaking in the ocean in the ofng of Mutsu-Sekine, Aomori, Japan, as shown in Figure 27.15.
Figure 27.15 Collection process of uranium adsorbent stacks at marine experiment. (From Seko, N. et al.,
Nucl. Technol.,
144, 274, 2003. With permission.)
table 27.1
Functional
g
roups
i
mparted
by g
raft
p
olymerization,
g
rafting
m
onomer
and
Chemical r
eagents
for s
ynthesis
of m
etal
a
dsorbents
Functional group grafting monomer Chemical reagent references
Amidoxime Acrylonitrile Hydoxylamine Seko
et al. (2005)
Amines Glycidyl
methacrylate Diethylamine Kabay et al. (1993)
N-Vinyl
formamide Sodium hydroxide Abrol et al. (2007)
Iminodiethanol Glycidyl
methacrylate
2,2′-Iminodiethanol
Awual et al. (2008)
Iminodiacetic
acid Glycidyl methacrylate Sodium iminodiacetate Ozawa et al. (2000)
Glucamine Glycidyl methacrylate N-Methyl glucamine Choi and Nho (1999)
Sulfonic acid Styrene Sulfuric acid Hoshina et al. (2007)
Glycidyl methacrylate Sodium sulte Vahdat et al. (2007)
Phosphoric acid Glycidyl methacrylate Phosphoric acid Kim and Saito (2000)
2-Hydroxyethyl
methacrylate
phosphoric
acid
Lee et al. (2002)
Source:
Tamada,
M. K., Kobunshi (High Polymers), 58, 397, 2009.
Radiation Processing of Polymers and Its Applications 749
One kilogram of uranium was successfully collected in 3 years’ marine experiment in the form of
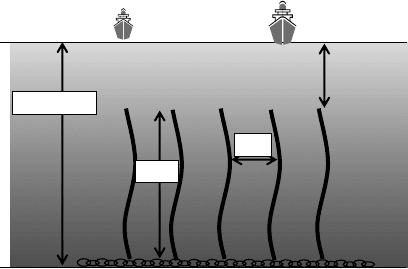
a yellow cake (Seko et al., 2003). To make uranium collection from seawater cost effective, a braid-
type adsorbent was developed. The mooring system for the braid-type adsorbent does not need the
oat on the sea surface and a heavy adsorbent bed. The braid-type adsorbent is fabricated by braid-
ing the amidoxime adsorbent bers. The recovery cost of uranium from seawater was evaluated
on the basis of the recovery system of the braid-type adsorbent. Figure 27.16 shows the assembly
of braid-type adsorbent for a supposed annual collection of 1200 t uranium. When a performance
of 4g of uranium/kg of adsorbent, which can be reused 18 or more times is achieved, the uranium
cost reduces to 25,000 ¥/kg of uranium (96 $/lb-U
3
O
8
) (Tamada et al., 2006). The 4g uranium/kg of
adsorbent
is the most promising performance of uranium adsorbent in marine experiments.
27.3.3 polyMer electrolyte MeMbraneS for battery and fuel cellS
27.3.3.1 aac-grafted pe: bottom battery
The separator membranes in compact-bottom-type silver oxide cells were previously made of regen-
erated cellulose and porous PP lms. However, the conventional silver oxide cells had drawbacks, so
that silver hydroxide ion (Ag(OH)
2
−
) generated as a by-product on anode passes through the separa-
tor and was reduced to precipitated metal Ag, resulting in self-discharge and power deterioration.
To solve the above problems, radiation grafting was applied to the separation membranes; here,
poly(acrylic acid) (poly(AAc)) was grafted onto PE, which is very cheap, good lm forming, and
chemically
stable (Ishigaki et al., 1982a,b; Hsiue and Huang, 1985).
Hsiue
et al. reported that the specic resistivity of the lms rapidly decreased with increasing
grafting degrees up to 20% and reached 50 Ω cm (σ = 0.02 S/cm) (Hsiue and Huang, 1985). In
real production processes, PE roll lms are irradiated with an EB accelerator to generate radicals
and subsequently immersed in an aqueous AAc monomer solution to introduce poly(AAc) grafts
homogeneously through the PE lms (Figure 27.17). The obtained membranes act as high perfor-
mance battery separators because only hydroxide ions (OH
−
) but not by-product Ag(OH)
2
−
can pass
through the grafted membranes, which suppress self-discharge, resulting in the higher shelf life.
To develop the radiation-grafting technique, the bottom-type battery cells prepared using radiation
grafting can be used for a long time and in a stable manner, and the above problem can be solved.
Thus,
the AAc-grafted PE achieved 100% of market share in Japan.
27.3.3.2 Fluoropolymers for Fuel Cell pem
As a result of the successful application of the poly(AAc)-grafted PE to bottom cells, radiation
grafting has been applied to the preparation of PEM for hydrogen type (polymer electrolyte fuel
cell (PEFC)) and direct methanol type fuel cell (DMFC) in the last 10–15 years (Table 27.2).
Sea
surface
Bottom
Braid type
adsorbent
More than
40 m
100–200 m
60 m
Chain
8 m
Figure 27.16 Mooringstateof braid adsorbent forpractical systemof uranium collection. (FromTamada,M.
et
al., Trans. At. Energy Soc. Jpn., 5, 358, 2006. With permission.)
