Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

690 17. Chemoselective Ligation: Bioorthogonal Reagents
used with surfaces (e.g., NHS esters, EDC conjugation), which suffer from hydrolysis and deg-
radation over time, the alkyne or azide components can be used to activate a surface and stored
indefi nitely until needed. A ligand modifi ed with the opposite reactant then can be spotted on
the array surface in the presence of Cu(I) to initiate covalent attachment through triazole ring
formation.
Another version of the click chemistry azide/alkyne reaction has been developed to eliminate
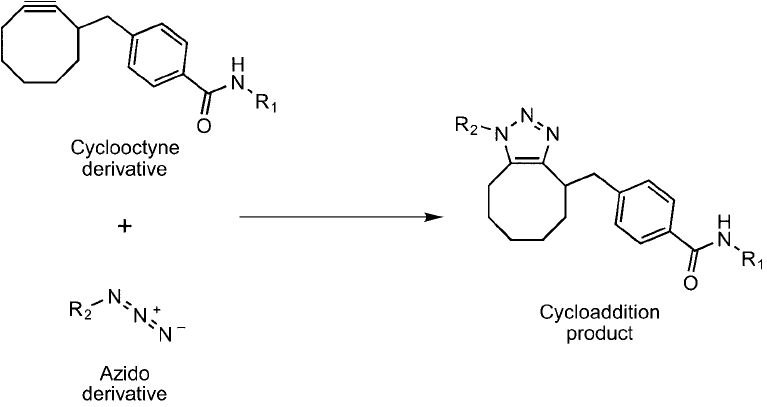
the requirement for Cu(I) to be added to catalyze the triazole ring formation. Agard et al. (2004)
used a cyclooctyne ring to take the place of the typical linear alkynes used as the reactant partner
for azides in the Cu(I) catalyzed reactions. This cyclic triple bond reactant is activated, because of
ring strain, to produce better kinetics in the 3 2 cycloaddition reaction (Prescher and Bertozzi,
2005; Agard et al., 2006). The reaction with an azide lessens the ring strain of the alkyne within
the cyclooctyne structure, and thus drives the reaction without the addition of cytotoxic copper
(Figure 17.16 ). This cycloaddition reaction still is much slower than a copper-catalyzed reaction,
but its usefulness on living cells may give it an advantage in this application.
5. Staudinger Ligation
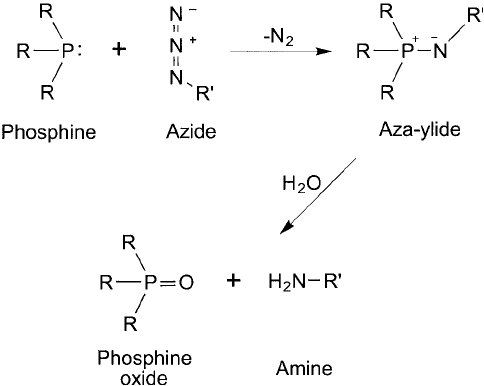
Early in the last century, the Nobel Prize winning chemist Hermann Staudinger discovered
a reaction between phosphines and azides, which became known as the Staudinger reaction
(Staudinger and Meyer, 1919). Triphenylphosphine reacts with azides to form an intermediate
iminophosphorane with the release of nitrogen gas. This intermediate quickly breaks down in
aqueous environments to yield triphenylphosphine oxide and a primary amine ( Figure 17.17 ).
Figure 17.16 Cyclooctyne derivatives can be used as alternative click chemistry reactants, as they are capable
of reacting with an azide group without the presence of Cu
1
to form a cycloaddition product. This reaction
proceeds at a slower rate than the Cu
1
-catalyzed process, but it avoids the cytotoxic effects that copper addi-
tion can have on cells.
This reaction has since been used successfully to synthesize amines in countless numbers of
organic compounds and still remains one of the most common organic reactions performed
today. Often azides are thought of as hidden amines, because the azide is relatively inert to
other reactants until it is revealed through the Staudinger reaction.
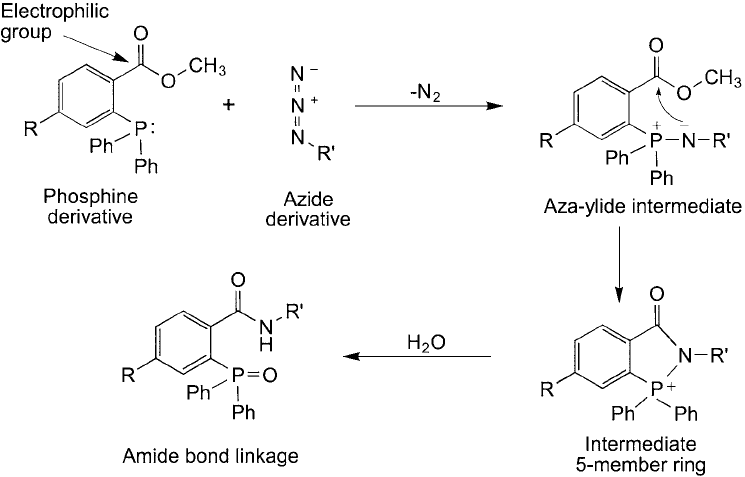
A signifi cant modifi cation to the Staudinger reaction was developed by Saxon and Bertozzi
(2000) that effectively turns it into a covalent coupling reaction with bioconjugation potential
(see also Saxon and Bertozzi, 2003, 2006). Termed “Staudinger ligation ”, this reaction is done
using a triphenylphosphine derivative that contains an electrophilic group next to the phospho-
rus core. Ortho positioning of an electrophilic benzyl methyl ester group on one of the phenyl
rings provides a reactive site for the nucleophilic nitrogen from an azide group that temporar-
ily forms an aza-ylide interaction with the phosphorus atom core. Nucleophilic attack of the
nitrogen on the carbonyl “electrophilic trap ” releases methanol and forms a stable amide bond
(Figure 17.18 ).
Thus, using the Staudinger ligation process, a triphenylphosphine derivative containing a ben-
zyl methyl ester group can be covalently conjugated to an azide derivative through the formation
of an amide linkage. The two derivatives can have attached to them virtually any other mole-
cules, such as proteins, carbohydrates, other biological molecules, fl uorescent tags, biotin groups,
or other organic compounds, to create a conjugate between them.
The Staudinger ligation reactants also are extremely bioorthogonal. As discussed previously
in the section on click chemistry, the (aliphatic) azide component is extremely stable in aqueous
or biological solution and won ’t cross-react with other functional groups. The azide derivative
is not appreciably reduced even given the reducing potential inside cells. In addition, the aryl
derivative of triphenylphosphine used as a partner in this reaction also has been shown to be
very stable in complex solutions, even to the point of not being an effective reducing agent for
disulfi des, as are many other phosphine compounds, including triphenylphosphine (Saxon and
Bertozzi, 2000).
Figure 17.17 The Staudinger reaction involves the reduction of an azide to a primary amine with loss of N
2
and the concomitant oxidation of a phosphine derivative to a phosphine oxide.
5. Staudinger Ligation 691
692 17. Chemoselective Ligation: Bioorthogonal Reagents
There now are available a number of alkyl azide compounds that may be used in click
chemistry reactions and the Staudinger ligation processes. It is not recommended, however, to
use aryl azide compounds, as these are light sensitive and photoreactive as well as highly sus-
ceptible to reduction in the presence of thiols. Unfortunately, at the time of this writing there
are fewer choices in aryl phosphine compounds to participate in this reaction, as commercial
sources of labeling reagents are limited.
The reaction between an alkyl azide and the aryl triphenylphosphine occurs without the
requirement for other catalysts or activators, such as the need for Cu(I) in the click reaction.
This provides an advantage for working with biological samples, since there is no possibil-
ity for toxicity or side reactions with added components being present. For this reason, the
Staudinger ligation reaction is very amenable to being done with living cells without affecting
cell viability.
Additionally, the azide group can be incorporated into amino acids, sugars, and lipids to
label cellular molecules in vivo prior to reacting with a phosphine probe (see the previous
Figures 17.11 and 17.12 ). Kiick et al. (2002) demonstrated that both azido and alkynyl amino
acid derivatives could be used as methionine surrogates and get integrated into proteins with
nearly the same effi ciency as normal methionine. Growing cells in the presence of one of these
azido monomers results in their incorporation into biopolymers at specifi c sites. In this manner,
biomolecules can be purposely tagged using Staudinger ligation with a detectable phosphine
probe, which is all done within living cells to track or locate them within their native environ-
ment. For instance, Prescher et al. (2005) used azido-sugar derivatives to modify cell-surface
Figure 17.18 The Staudinger ligation reaction uses a modifi ed phosphine derivative containing an electrophilic
group that acts as a trap for the nucleophilic nitrogen in the intermediate aza-ylide. The resultant shift yields an
amide bond derivative between the phosphine-containing molecule and the azide-containing molecule.
glycans with azide groups at sialic acid residues, which are typically the outer-most sugars on
glycoproteins. It was found that cells most effectively took up these compounds if the hydroxyl
groups were acetylated, presumably due to the easy transport of such hydrophobic derivatives
through lipid membranes. The acetyl groups are removed within the cells by esterases, thereby
providing the azido-sugar to the cell machinery for synthesizing glycoconjugates. The azido-
glycan modifi cations produced on the cell surface using this process then could be labeled by
the Staudinger ligation reaction with a phosphine-Flag-tag derivative and subsequently detected
using fl uorescently labeled anti-Flag tag antibody and fl ow cytometry.
Due to the bioorthogonal nature of the reactants used for Staudinger ligation as well as
the ability to incorporate azido analogs in biopolymers in vivo and the mild effect the rea-
gents have on living cells, the process has been termed “a gift to chemical biology ” (Köhn and
Breinbauer, 2004). For the fi rst time, effi cient labeling of biomolecules within cells can be done
with very small modifi cations to the biopolymers in vivo. Staudinger ligation permits discrete
chemical tagging with detectable probes or affi nity handles that facilitate purifi cation.
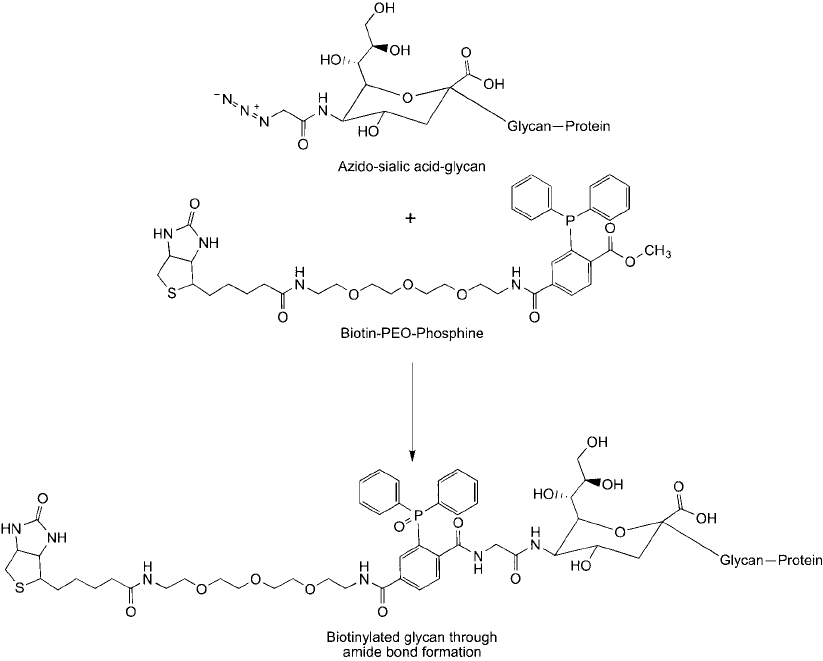
The methods used for in vivo incorporation of azido-monomers and performing a labeling
reaction with live cells are relatively simple. The following protocol is based on the methods of
Saxon and Bertozzi (2000), which uses acetylated azidoacetylmannosamine as the azido-monomer
source and a biotin–PEG–phosphine compound to biotinylate cell surface glycoproteins at the
specifi c azide-sialic acid incorporation sites ( Figure 17.19 ).
Protocol
1. Grow cells ( 1 10
5
cells/ml) for 3 days in appropriate media containing a 20 M con-
centration of acetylated azidoacetylmannosamine.
2. Wash the cells at least twice with 0.1 percent fetal bovine serum in 10 mM sodium phos-
phate, 0.15 M NaCl, pH 7.4 (PBS) to remove excess azido-sugar.
3. Suspend the washed cells in 0.25 ml of PBS, pH 7.4.
4. Add to the washed cells 60 l of a 5 mM concentration of the phosphine derivative to
couple to the azido-sugar groups on the cell surface (e.g., biotin–PEG–phosphine).
5. Incubate for 1 hour at room temperature with gentle mixing.
6. Wash the cells with PBS, pH 7.4, to remove excess biotinylation reagent.
The biotinylated glycans on the cell surfaces subsequently may be probed with (strept)avidin
reagents to detect the azido-sialic acid modifi cations. Alternatively, the cells may be lysed and
the glycoproteins isolated using an immobilized (strept)avidin or monomeric avidin affi nity
resin.
The same approach to in vivo labeling may be done using azide derivatives of sugars admin-
istered intraperitoneally in mice (once per day for 7 days) (Prescher et al., 2005). Tissue sam-
ples then can be taken of particular organs and reacted ex vivo with a phosphine derivative to
undergo Staudinger ligation. This process can facilitate probing of glycans on the cell surfaces
of organs or cells within an animal. Various treatment procedures can be done, such as admin-
istering drug candidates to test animals, to determine the effect on glycosylation of proteins
in vivo .
Staudinger ligation techniques also can be used to detect post-translational modifi cation
of proteins in vivo. Hang et al. (2007) developed a method to monitor fatty acid acylation
of proteins using azido-fatty acids fed to cells. The two major types of fatty acid acylation,
5. Staudinger Ligation 693
694 17. Chemoselective Ligation: Bioorthogonal Reagents
Figure 17.19 An azido-sialic acid derivative that gets incorporated into glycans in cells can be labeled spe-
cifi cally with a biotin-phosphine tag using the Staudinger ligation process. The result is an amide bond linkage
with the glycan.
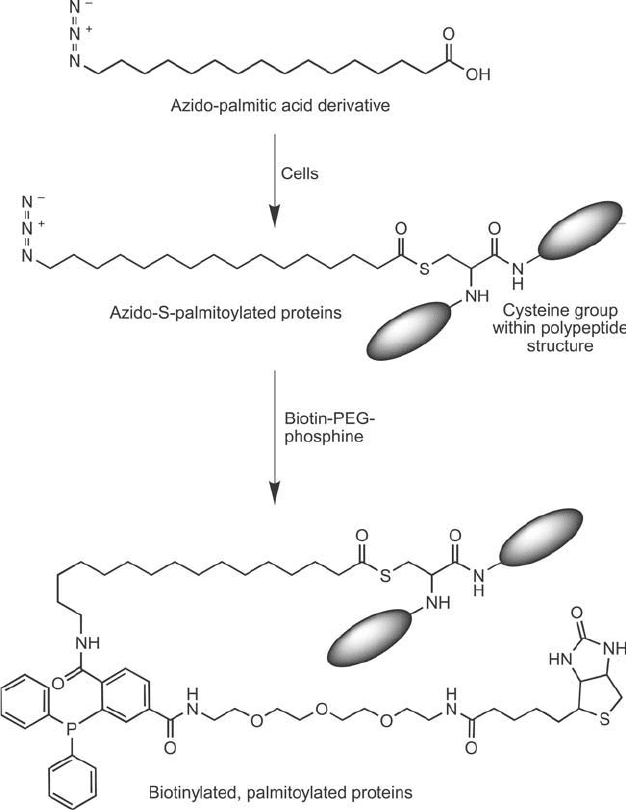
N-myristoylation and S-palmitoylation, could be detected with terminal ( -)azido labeled myr-
istic acid or palmitic acid. Mammalian cells grown in the presence of these fatty acid azide
derivatives resulted in certain proteins being modifi ed to contain -azido-fatty acid modifi ca-
tions. Subsequent Staudinger ligation with a biotin–PEG–phosphine reagent resulted in covalent
attachment to any post-translationally modifi ed protein containing these groups ( Figure 17.20 ).
The biotin group then could be used for detection or purifi cation of N-myristoylated or S -
palmitoylated proteins using (strept)avidin reagents.
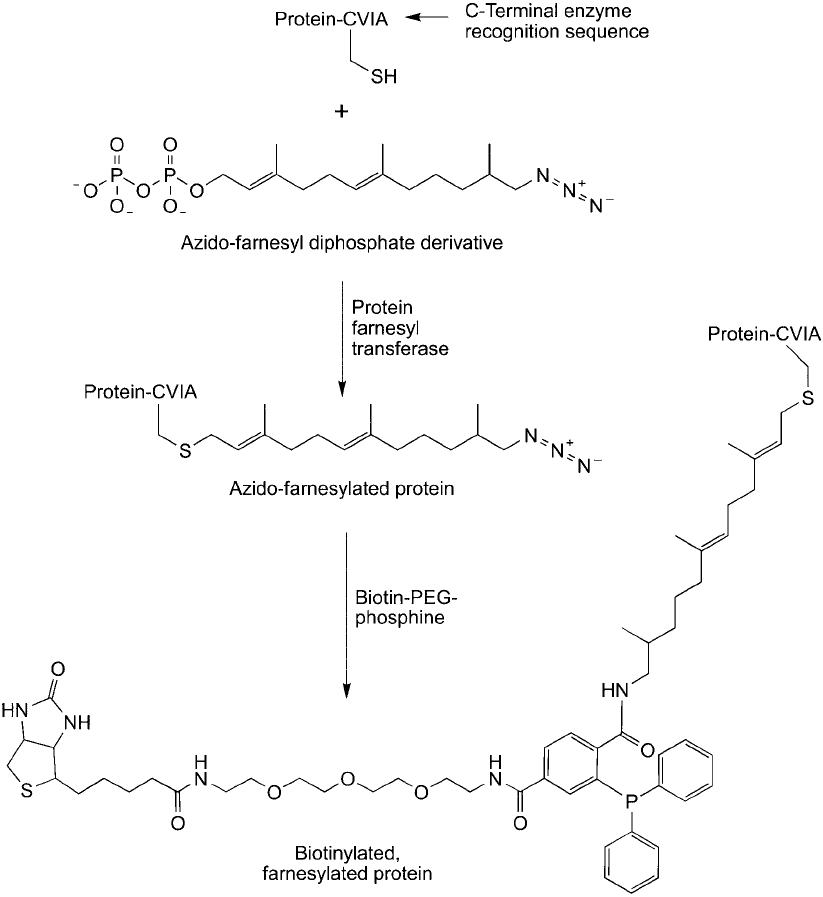
In a similar application, Kho et al. (2004) were able to detect post-translationally modifi ed
proteins using an azido-farnesyl analog. Cells grown in the presence of this derivative enzymat-
ically incorporated the azido group into farnesylated proteins through the action of farnesyl
transferase. These modifi cations then could be targeted through Staudinger ligation using the
same biotin–PEG–phosphine reagent ( Figure 17.21 ).
Protein farnesyl transferase also can be used to add a geranylazide derivative to a synthetic
peptide by incorporating the enzyme recognition sequence “CAAX” at the C-terminal of any
peptide. This enzyme uses a farnesyl diphosphate derivative to transfer covalently the lipid to
the cysteine residue via a thioether bond. Xu et al. (2006) found that the use of 6,7-dihydroger-
anylazide diphosphate resulted in appending the terminal azido derivative onto such peptides,
yielding an azido functionality for subsequent conjugation using Staudinger ligation. This proc-
ess enabled targeted coupling through the C-terminal of any peptide or protein containing the
CAAX box sequence.
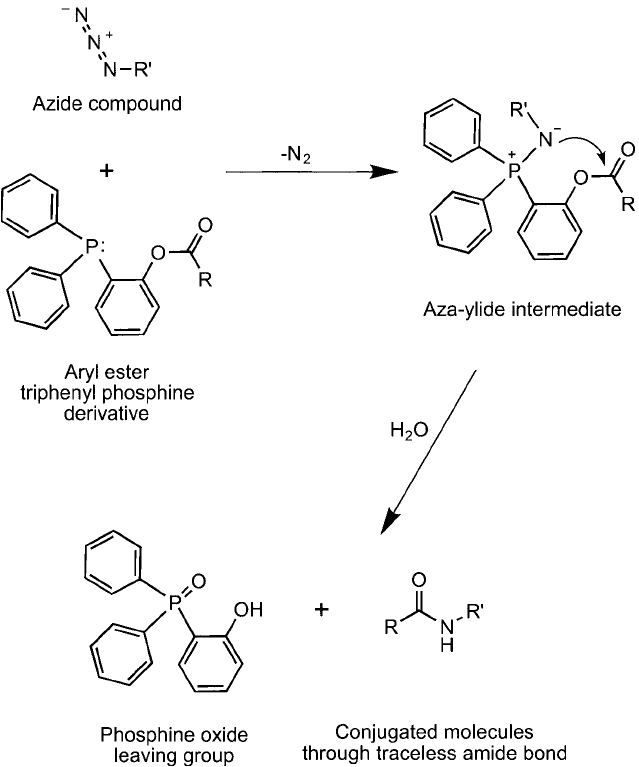
Another important variation of the Staudinger ligation reaction described above involves
the use of cleavable aryl groups on the triphenylphosphine component, which allows for
Figure 17.20 An azido-palmitic acid derivative can be added to cells to obtain palmitoylated proteins that
contain an azide group able to participate in the Staudinger ligation reaction. Biotinylation of these post-
translationally modifi ed sites then can be done in vivo using a biotin-phosphine reagent.
5. Staudinger Ligation 695
696 17. Chemoselective Ligation: Bioorthogonal Reagents
a “traceless” ligation reaction to occur. This strategy results in a zero-length amide bond
between the phosphine derivative and the azide derivative, thus removing the triphenylphosphine
component from the fi nal conjugate and linking the two molecules together directly (Nilsson
et al., 2000, 2001; Saxon et al., 2000; Soellner et al., 2002; Saxon and Bertozzi, 2003, 2006).
Figure 17.21 An azido-farnesyl diphosphate derivative can be added to cells to obtain farnesylated proteins
containing terminal azide groups that can be targeted in a Staudinger ligation reaction. Biotinylation of these
post-translationally modifi ed proteins can be done in vivo using a biotin-phosphine derivative.
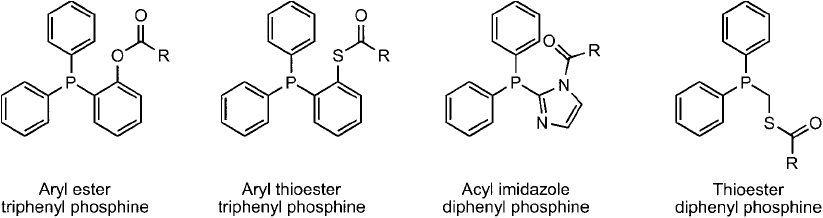
The phosphanes useful in this process are built from acyl derivatives of compounds such as
those shown in Figure 17.22 . During the Staudinger ligation process, once the azide reactant
forms the aza-ylide with the phosphine, electrophilic attraction induces the nitrogen to attack
the electron defi cient carbonyl, which in turn causes release of the phosphonium group and
forms the amide bond ( Figure 17.23 ).
The most useful phosphane derivatives in a traceless Staudinger reaction include the acyl
modifi ed 2-diphenylphosphanylphenol and the acyl modifi ed diphenylphosphanylmethanethiol.
These two core structures provide the best rate of reaction and effi ciently form the amide bond
conjugate (Köhn and Breinbauer, 2004). The traceless Staudinger ligation method no doubt will
become a popular choice to avoid retention of the bulky phosphane species in bioconjugates.
The reaction can be used with success to produce long polypeptide chains by linking together
azido-peptides with phosphine-peptides to form the appropriate biological peptide bond between
them (Nilsson et al., 2003). This makes it possible to create synthetically polypeptides that are
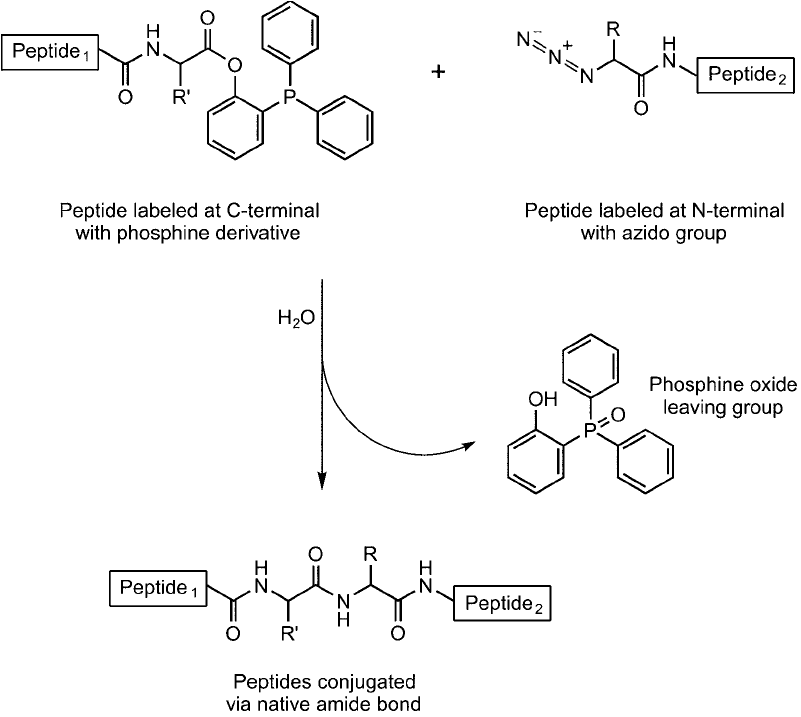
too long to create using standard solid phase peptide synthesis procedures ( Figure 17.24 ). In
addition, the traceless Staudinger ligation reaction can be used to link biomolecules containing
azide groups to surfaces containing the traceless phosphane derivative (Soellner et al., 2003).
6. Native Chemical Ligation
Native chemical ligation is an important alternative chemoselective peptide conjugation tech-
nique to the previously discussed non-native methods (Dawson et al., 1994). This system can
provide discrete coupling of the N-terminal of one peptide to the C-terminal of another pep-
tide using a unique reaction process, essentially extending a peptide chain while maintaining
native sequence and bonding characteristics. The reactant partners are prepared through typi-
cal peptide synthetic procedures, wherein one peptide is made to contain an -thioester on its
C-terminal carboxylate and the other peptide is synthesized to contain a cysteine amino acid resi-
due at its N-terminal. The reaction of these two derivatives proceeds through nucleophilic attack
of the cysteine thiol of one peptide onto the carbonyl group of the -thioester at the C-terminal
of a second peptide. The result forms an intermediate thioester by transthioesterifi cation, which
then spontaneously rearranges by an S ; N acyl transfer to form a native amide bond between
the peptides with no foreign organic structure remaining ( Figure 17.25 ). The reaction proceeds
Figure 17.22 Certain unique phosphine derivatives can be used in the design of modifi cation or conjugation
reagents to create a traceless Staudinger ligation process, wherein the phosphine group is lost and an amide
bond between an azide-containing molecule and the phosphine-containing molecule results.
6. Native Chemical Ligation 697
698 17. Chemoselective Ligation: Bioorthogonal Reagents
at physiological pH using fully unprotected (i.e., unblocked amino acid functional groups),
which is unusual in peptide synthetic strategies.
Peptides typically are prepared for this ligation process using -alkyl thioesters, because they are
simple to make at the time of peptide synthesis. However, due to the relatively slow reaction kinet-
ics of alkyl thioesters, most native chemical ligation processes have been catalyzed through the use
of thiol compound additives, such as benzyl mercaptan or thiophenol (Dawson et al., 1997). These
compounds react with the initial -alkyl thioester to form another intermediate, an aryl thioester,
which is more reactive toward the N-terminal cysteine on the other peptide to be coupled. A study
Figure 17.23 A traceless Staudinger ligation process involves the formation of an intermediate aza-ylide with
subsequent attack of the nucleophilic nitrogen atom on the neighboring electrophilic group. The formation of
an amide bond then occurs concomitant with the loss of the phosphine component, thus forming a zero-length
crosslink between the two molecules.
of the rate of reaction for different thiol catalysts indicated that (4-carboxymethyl)thiophenol per-
formed the best. The addition of this compound to a native chemical ligation reaction resulted in
at least a 10-fold improvement in reaction rates (Johnson and Kent, 2006).
Another advantage to the use of a thiol additive is that the abundance of free thiol groups in
the reaction environment will prevent the oxidation of the cysteine thiol at the N-terminal of
the other peptide. Without added thiol transesterifi cation catalysts, disulfi de formation result-
ing in dimerization of the Cys-peptide would be a dominant side reaction in aqueous, oxygen-
ated buffer conditions.
Native chemical ligation has been used successfully to couple two unprotected peptides
together during solid phase synthesis, wherein one of the peptides is attached to the resin using
a thioester linkage and the other peptide is introduced containing a cysteine at its N-terminal
Figure 17.24 The traceless Staudinger reaction can be used to form larger peptides from smaller peptides, if one
contains an azido group at the N-terminal and the other one contains a phosphine ester at its C-terminal. The
reaction gives a native peptide (amide) bond with loss of the phosphine group.
6. Native Chemical Ligation 699
