Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

700 17. Chemoselective Ligation: Bioorthogonal Reagents
(Camarero et al., 1998). The ligation process also could form cyclic peptides, although the effi -
ciency of the reaction was less than the conjugation of two separate peptides.
So effi cient is this method to link peptide sequences together in a native amide bonded state
that complete proteins of various sizes have been synthetically made (Hackeng et al., 1999),
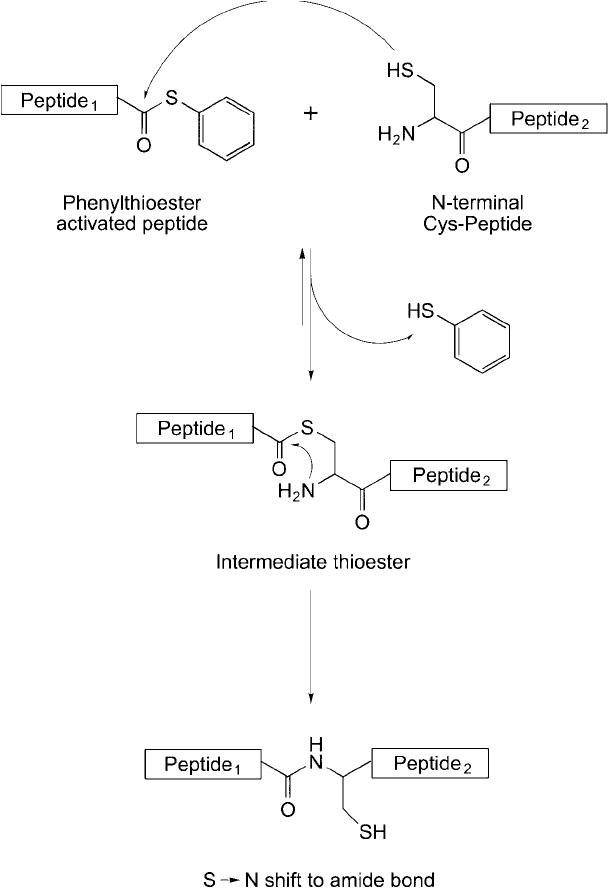
Figure 17.25 The native chemical ligation reaction can be used to form larger peptides from smaller peptides,
if one contains a cysteine residue at its N-terminal and the other one contains a thioester on its C-terminal.
Reaction of the peptide derivatives gives a native peptide (amide) bond.
such as the preparation of a serine protease (Pal et al., 2003), bovine pancreatic trypsin inhibitor
(Lu et al., 1998), cytochrome b562 (Low et al., 2001), and the triple zinc fi nger protein, Zif268
(Beligere and Dawson, 1999). Many other examples of full or partial protein synthesis can be
found in the published literature (for reviews, see Dawson and Kent, 2000; David et al., 2004).
Native chemical ligation also can be extended to the conjugation of peptides or proteins to
other molecules or surfaces. For instance, Reulen et al. (2007) prepared liposomes that con-
tained cysteine–PEG–phospholipid derivatives and then coupled thioester-modifi ed peptides or
proteins to form a protein–liposome conjugate. Using this procedure, approximately 100 mol-
ecules of a collagen binding protein could be coupled to the cysteine-containing liposomes.
In addition, Dose and Seitz (2005) employed native chemical ligation to synthesize peptide
nucleic acids (PNAs) by linking shorter segments of PNAs to make long contiguous strands,
which could not be made through typical oligo synthesis procedures.
6.1. Expressed Protein Ligation and Inteins
Although there are a limited number of reports indicating that N-terminal cysteine-containing
proteins can be expressed recombinantly, the in vivo production of a protein having a C-terminal
thioester is considerably more diffi cult. That ’s why the initial development of native chemical liga-
tion techniques was restricted to the use of peptide synthesis to generate the two peptide derivatives
for ligation. However, the recombinant generation of C-terminal thioesters and N-terminal cysteine
residues in peptides for native chemical ligation now can be done using intein technology. In the
same frame that native chemical ligation reactions were being discovered and explored, intein tech-
nology was being investigated as a new in vivo native protein splicing and ligation mechanism.
Inteins are certain sequences of amino acids in precursor proteins that can catalyze a cleavage of
their own internal peptide structure, resulting in the excision of the intein peptide segment and liga-
tion together of the two peptide segments fl anking the excised region (exteins). The exteins are thus
ligated together with a native peptide (amide) bond. The immediate C-terminal side fl anking the
intein usually contains either a serine/threonine residue or a cysteine amino acid group at the splice
junction. The N-terminal extein side next to the intein also can contain either a serine/threonine
group or a cysteine residue, which are able to form an ester or thioester functionality, respectively.
If a cysteine group is present at the N-terminal splice junction side of the intein, then the thioester
intermediate that forms is very similar to the reaction intermediate of native chemical ligation.
The intein segment is excised through a number of steps that fi rst involves an N ; S shift at
the C-terminal cysteine residue at the extein junction to create a thioester intermediate. Then
nucleophilic attack of either the serine/threonine hydroxyl or the cysteine thiol of the extein on
the N-terminal side of the intein proceeds to cleave the C-terminal extein and ligate it to the
N-terminal extein thiol (or hydroxyl) through another (thio)ester bond. Therefore, the cleavage
and ligation reaction proceeds through a second ester or thioester intermediate that involves
either an O ;N shift or an S ;N shift, which cleaves off the intein segment entirely. The com-
plete thioester reaction route is shown in Figure 17.26 , which can be seen to be very similar to
that of a native chemical ligation reaction, illustrated previously.
Muir et al. (1998) realized that the intein reaction could be used to facilitate a native chemi-
cal ligation with a synthetic N-terminal cysteine-containing peptide or cysteine-containing
molecule. With the discovery of a mutant intein that could form an intermediate thioester
but not go on to complete the splice and ligation reaction (Xu and Perler, 1996; Chong et al .,
6. Native Chemical Ligation 701
702 17. Chemoselective Ligation: Bioorthogonal Reagents
1997), this expression technology now could be combined with the native chemical ligation
reaction to facilitate a new Expressed Protein Ligation (EPL) method.
Fusion vectors are available that combine a recombinant protein with a mutant mini intein
segment (not containing an endonuclease domain) and followed by a chitin binding domain
(CBD; Zhang et al., 2001). These mutants typically also have an alanine substitution that
replaces the cysteine or serine/threonine usually found on the C-extein splice junction. Alanine
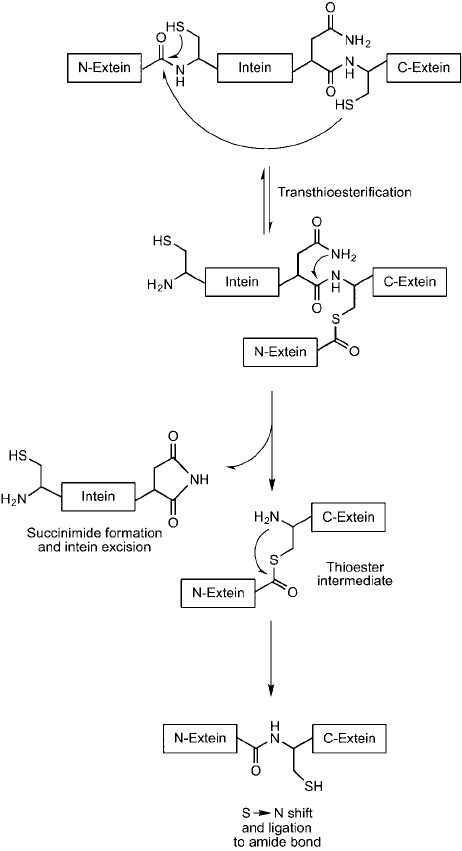
Figure 17.26 The native process leading to intein excision and ligation of extein fragments involves a sequence
of reactions involving transthioesterifi cation, cleavage of the intein fragment, and a S ; N shift, which ligates
the two extein peptides together via an amide bond.
cannot facilitate attack on the thioester on the N-extein side and thus the intein is not cleaved.
Using the CBD portion, the entire expressed fusion protein can be purifi ed on a chitin column
through specifi c affi nity binding and then the N-extein segment induced to cleave using DTT,
which results in release of the desired recombinant protein having a C-terminal thioester.
However, if the expressed protein is treated on the affi nity support using thiophenol, this
also will release the protein and result in a phenylthioester at its C-terminal, which is the reac-
tive intermediate imminently suitable for native chemical ligation. Treatment of this activated
thioester protein with a N-terminal cysteine peptide induces the native chemical ligation reac-
tion and couples the peptide to the expressed protein through an amide bond (Severinov and
Muir, 1998) ( Figure 17.27 ).
EPL extends the applicability of native chemical ligation to recombinantly produced proteins
using the mutant mini intein vector system. Proteins being expressed using this method will
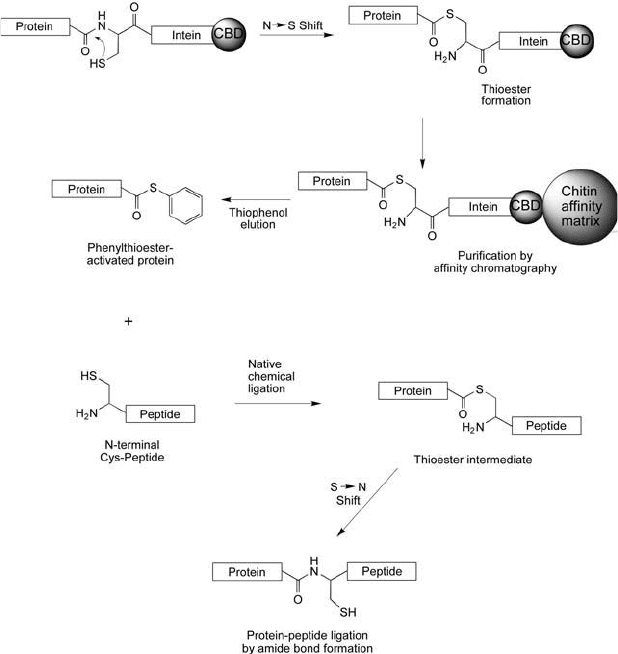
Figure 17.27 The EPL process involves a fusion protein containing an intein tag plus a CBD. The fusion protein is
captured on an immobilized chitin resin and after removal of contaminating proteins, it is eluted using thiophenol,
which cleaves at the thioester bond between the intein and the desired expressed protein. This releases a phenylth-
ioester-activated protein that can be used in the native chemical ligation reaction with another peptide containing
an N-terminal cysteine residue. Conjugation results in a native amide (peptide) bond formed between them.
6. Native Chemical Ligation 703
704 17. Chemoselective Ligation: Bioorthogonal Reagents
contain a C-terminal thioester and therefore can be conjugated to any reagent or probe molecule
containing a cysteine group with an available -amine and thiol group. Expressed proteins also
can be immobilized onto solid surfaces by coupling them solely at their C-terminal ends.
Girish et al. (2005) coupled proteins onto surfaces using this approach, but in this case
expressing a protein containing an N-terminal Cys residue and then reacting it with a thioester
group on a glass slide ( Figure 17.28 ). A similar strategy also has been used with success to label
specifi cally expressed proteins in live cells using thioester-containing probes (Nilsson et al .,
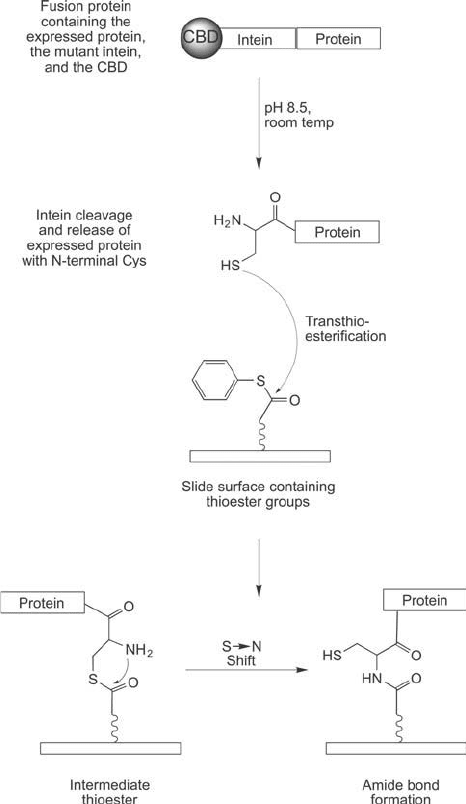
Figure 17.28 EPL reactions can be used to couple a fusion protein to a surface containing a thioester derivative.
After cells are grown and the fusion protein expressed, a pH and temperature shift causes intein cleavage with
release of the expressed protein with an N-terminal cysteine residue. Reaction with the thioester surface results
in a native chemical ligation reaction that forms an amide bond linkage with the expressed protein.
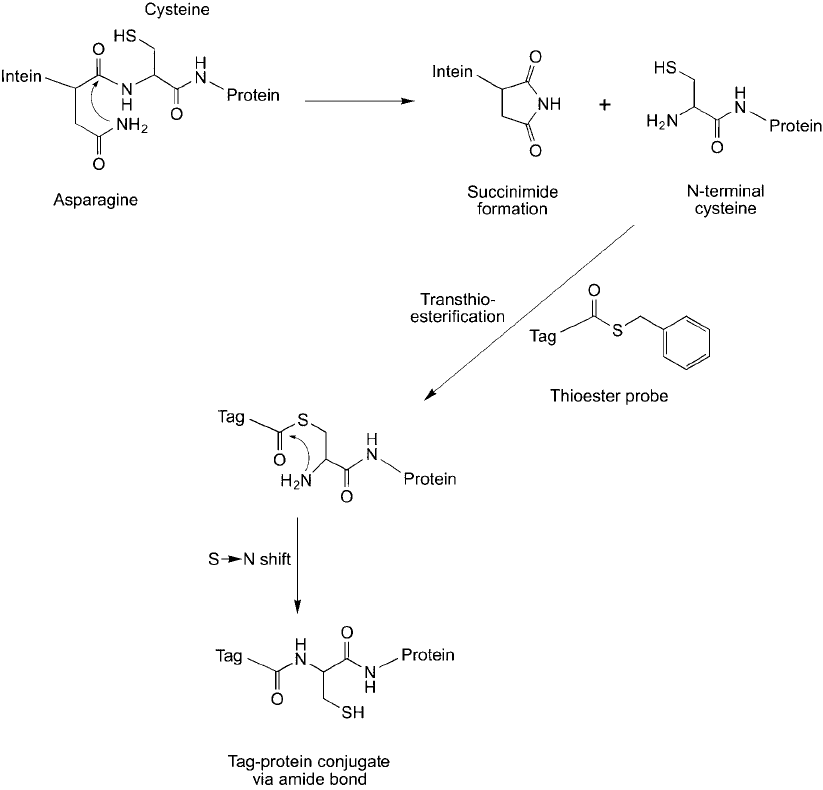
2003). Using the pTWIN vector (New England Biolabs), a recombinant system was developed to
express proteins having an N-terminal Cys using the Ssp DnaB mini intein segment (Yeo et al .,
2003). This intein contains a C-terminal asparagine residue that undergoes a self-cleaving reac-
tion after a shift in pH and temperature. Growing cells in physiological pH conditions does
not cleave the intein. However, after cell lysis, an increase to pH 8.5 under room temperature
conditions will initiate the cleavage reaction. Thioester-containing probes, including thioester-
fl uorescent molecules or thioester–biotin, can be added directly to live cells to label these
expressed proteins via native chemical ligation ( Figure 17.29 ).
Figure 17.29 An expressed protein containing a mutant intein segment can undergo self cleavage to form an
N-terminal cysteine residue, which then can be reacted with a thioester probe to label specifi cally the protein via
an amide bond.
6. Native Chemical Ligation 705
706 17. Chemoselective Ligation: Bioorthogonal Reagents
The following protocol for EPL, including purifi cation using a CBD fusion tag followed by
native chemical ligation, is based on the methods of Muir et al. (1998), Chong et al. (1997,
1998), Evans et al. (1998), Severinov and Muir (1998), and the NEB instruction manual for
the IMPACT-TWIN system. The recombinant protein is recovered from the affi nity column as
the thioester derivative ready for reaction with a N-terminal Cys peptide or another tag con-
taining a Cys residue.
Protocol
1. A gene encoding a recombinant protein of interest is cloned into a cleavable mutant intein-
CBD plasmid vector and transfected into cells (e.g., using the IMPACT-TWIN system from
New England Biolabs). The cells are grown in LB media with 200 g/ml ampicillin at 37°C
to an OD
600
nm
of about 0.5–0.8, induced to express the fusion protein complex by the
addition of 1 mM IPTG, and incubated overnight.
2. Prepare a chitin affi nity column by washing with at least 10 bed volumes of 25 mM
HEPES, 250 mM NaCl, 1 mM EDTA, 0.1 percent Triton X-100, pH 7.0 (wash buffer).
3. Recover the cells by centrifugation, lyse them in wash buffer containing protease inhibi-
tors (e.g., 20 M PMSF), and clarify the supernatant by centrifugation.
4. Apply the lysate onto the affi nity column and wash with at least 10 column volumes of
wash buffer to remove not-bound protein. Monitor the eluate by absorbance at 280 nm
to assure that baseline has been reached.
5. Wash the column with 2–3 column volumes of elution buffer (25 mM HEPES or Tris-
HCl containing 500 mM NaCl, 1 mM EDTA, and 2 percent (v/v) thiophenol (or 50 mM
2-mercaptoethansulfonic acid), pH 8.5. Next, add 2 column volumes of elution buffer
containing 1–2 mM of an N-terminal Cys peptide or Cys-containing tag. Stop the fl ow
and incubate the column at room temperature for 24 hours. The thiophenol will cleave
the recombinant protein at the intein splice junction, while the cysteine-containing peptide
will react with the intermediate ester through transthioesterifi cation and an S ; N shift to
ligate the molecules via an amide bond.
6. Elute the conjugated molecules using wash buffer and purify using dialysis or gel fi ltration.

707
18
Poly(ethylene glycol) (PEG) has been used for many years as a modifi cation and conjuga-
tion reagent for biological molecules (Roberts et al., 2002). Part of the rationale for using PEG
polymers in bioconjugation applications includes a dramatic increase in the water solubility
of modifi ed molecules, a decrease in immunogenicity due to the shielding of modifi ed mol-
ecules from the immune system, protection of modifi ed protein and peptides from digestion
by proteol ytic enzymes, and effectively increasing their hydrodynamic volume and decreasing
clearance rates by renal fi ltration, all of which results in an increase in the serum half-life of
modifi ed molecules in vivo. Thus, hydrophobic drugs modifi ed with PEG polymers become
much more water–soluble, foreign proteins and other immunogenic molecules are hidden from
the circu lating antibodies and cells of the immune system, labile peptides or proteins can ’t be
degraded by enzymes, and the reticuloendothelial system and kidneys can ’t remove labeled
drugs as quickly as their unlabeled counterparts. For these reasons, many drug candidates
using PEG modifi cation currently are in clinical trials and several are already on the market.
The majority of PEG applications involve the use of long-chain linear or branched PEG poly-
mers having a molecular mass of at least 2.5 kDa to over 50 kDa (see Chapter 25 for selected
applications of these long PEG polymers). PEG typically is made from ethylene oxide by an
anionic ring-opening reaction, which results in long polymer molecules consisting of the gen-
eral structure HO (CH
2
CH
2
O)
n
H. The number of repeat units ( n) in standard commercial
PEG polymers created through this process can be anything from less than 50 to over 1,100.
However, in these conventional PEG polymers of any given size, there actually exists a distribu-
tion of chain lengths, as is typical for any polymer-based substance. The range of chain lengths
for a given PEG size is approximately Gaussian in distribution, which means that most PEG
reagents prepared by standard polymerization processes are fairly disperse. The level of polydis-
persity is usually indicated by Mw/Mn, which is called the polydispersity index (PDI) and is
equal to the weight average molecular weight (Mw) divided by the number average molecular
weight (Mn). If the PDI is equal to 1, the polymer is said to be monodisperse. For PEG polymers,
the PDI is typically less than 1.2, which is quite good for polymeric materials, and this value
probably refl ects the concern over the use of polydisperse PEG for therapeutic applications.
However, even at this low level of PDI, the chain distribution often is quite high. Kenworthy
et al. (1995) reported that the mean number of repeat units and variance for PEG 2000 aver-
ages 53 units with a variance of 11, while a PEG 5000 polymer has an average 130 units with a
Discrete PEG Reagents
708 18. Discrete PEG Reagents
variance of 20. Shorter chain PEG polymers have a tendency to have greater polydispersity than
the large polymers. For example, the commercially available PEG 1500 can have between 19
and 48 repeat units in a typical preparation, which correspond to a molecular weight distribu-
tion of 800–2,100 Da (Davis and Crapps, 2006). Thus, most commercial sources of crude PEG
polymers are highly disperse and they probably should be avoided entirely for critical bioconju-
gation work, unless they have been carefully purifi ed to isolate a single chain length.
However, true monodisperse PEG reagents have become available now, which are made not by
polymerizing small monomers, but by linking discrete PEG segments together to create pure poly-
mers of known structure and purity. These discrete PEG molecules can be made in chain lengths
from as little as 2 to over 24 repeating ethylene oxide units, and theoretically, virtually any chain
length can be produced by building up from smaller precursors (Davis and Crapps, 2006).
Using a convergent synthesis process, a short PEG segment containing a hydroxyl protecting
group on one side and a free hydroxyl on the other end is reacted with another PEG segment
containing a reactive group on one end and a protecting group on the other end. The reactive
group also must be a good leaving group, so that once it reacts with the hydroxyl on the other
PEG unit, conjugation reaction occurs to form an ether bond. This results in the covalent link-
ing of the PEG molecules together to form a longer PEG compound equal to the combined
length of the reactants. Deprotection of the ends then can be done to add additional function-
ality, such as a reactive group, functional group, or to form a hydroxyl or methoxy end.
Prior to this synthetic method being developed, small PEG-containing compounds were lim-
ited to very short ethylene oxide segments, such as the commonly used reagents ethylene glycol
and tetraethylene glycol. Now using new discrete PEG reagents, the advantages that PEG com-
pounds have provided for use in the modifi cation and crosslinking of biomolecules can be incor-
porated at known polymer lengths into any bioconjugation reagent to enhance its properties.
Discrete PEG reagents have been reported that incorporate reactive groups, fl uorescent
probes, metal chelates, drug molecules, affi nity ligands, biotin, and a host of other constituents.
For instance, Wei et al. (2006) developed a PEG-functionalized texaphyrin derivative, which
was shown to have enhanced solubility and anti-cancer activity in vivo. Four mPEG
4
chains dec-
orating the central gadolinium(III) texaphyrin were found to convey dramatic anti-proliferative
effects compared to the parent chelate without PEGs present.
In another application of a PEG
4
spacer, Clevenger et al. (2004) prepared a biotinylated
derivative of the antibiotic geldanamycin (GDA) to use as an inhibitor of the 90 kDa heat
shock protein Hsp90. Use of the PEG linker in building such an organic drug complex has the
advantage of adding a hydrophilic arm to an otherwise very hydrophobic probe. The biotin–
PEG
4
–GDA conjugate could be used to bind the active site of Hsp90 proteins and then affi nity
purify them on a (strept)avidin-containing resin.
Similarly, Kruszynski et al. (2005) used the reagent NHS–PEG
4
–biotin to make biotinylated
analogs of human MCP-1. This compound, described later in this section, provides a long-chain
biotin handle that has better solubility properties than the corresponding aliphatic reagent
NHS–LC–biotin, which has been used in many applications (Chapter 11, Section 1). Kornilova
et al. (2005) used the same PEG reagent to biotinylate various -secretase peptide inhibitors to
create probes of this multi-protein complex.
Hydrophilic short biotin–PEG tags also have found their way into the design of multifunc-
tional crosslinkers to study protein structures by mass spec. Fujii et al. (2004) developed a
homobifunctional NHS ester crosslinker that in addition has a PEG–biotin handle ( Figure 18.1 ).
The reagent actually is a trifunctional compound similar to the biotinylated PIR compound
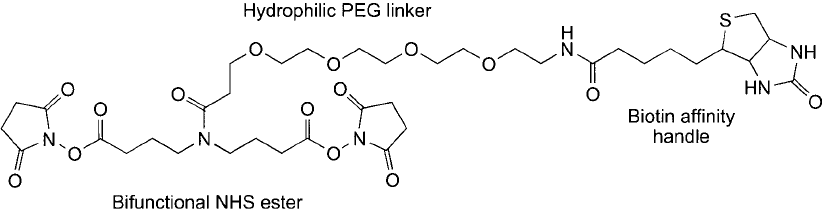
described in Chapter 28, Section 1.4. The NHS ester arms are of identical length to provide link-
ages of the same molecular distance in each direction, while the PEG group on the biotin arm
avoids the hydrophobic collapse of alkyl chain spacers by providing an extremely hydrophilic
linker with high freedom of motion in aqueous environments. Due to the increased water solu-
bility provided by the PEG linker arm, the sensitivity of mass spec analysis was enhanced over
the use of more traditional aliphatic spacers. In particular, this discrete PEG compound was
found to be optimal for performing mass spec analysis in three-dimensions (MS
3
).
Coupling of affi nity molecules to surfaces also can be enhanced by the use of discrete PEG
linkers. Nishimura et al. (2005) modifi ed an amino surface with a NHS–PEG
12
–maleimide cross-
linker to create a hydrophilic self-assembled monolayer (SAM) surface that was thiol reactive for
the conjugation of sulfhydryl-modifi ed RNAs. This array then was used to investigate the binding
specifi city of synthetic kanamycins with selected RNA sequences to prove the specifi c interaction
of ribosomal RNA with this molecule. The PEG linkers on surfaces provide lower nonspecifi c
binding character than alkyl linkers, when preparing SAM surfaces for affi nity interactions.
The low nonspecifi city of PEG layers also was used to eliminate biomolecule binding to cer-
tain areas of an array. Kidambi et al. (2004) patterned an mPEG-carboxylate molecule onto
polyelectrolyte multilayers to mask portions of the surface. The extremely low binding char-
acter of PEG provides advantages for creating patterned surfaces that other modifi ers using
aliphatic alkyl linkers do not provide.
SAM surfaces on metals, such as gold particles or planar arrays, have been enhanced in
their performance and properties through the use of PEG-containing modifi cations. Prime and
Whitesides (1991) used an mPEG–thiol combined with a slightly longer thiol–PEG-carboxylate
compound to create monolayers on gold through dative bonding with the thiol groups. The
thiol–PEG-carboxylate compound typically is used at about 10 percent of the concentration
of the thiol–mPEG compound to form a lawn of low-binding mPEG molecules, which is inter-
spersed with enough carboxylates to provide sites for covalent attachment of affi nity ligands
or antibodies. Spangler et al. (2004) improved upon this strategy by creating a dendritic struc-
ture branching off from a central phenyl ring core and containing two thiol arms and a single
PEG arm containing either an mPEG group or a PEG-carboxylate ( Figure 18.2 ). The presence
of two thiol groups increases the strength of the linkage with the gold surface, thus providing
resistance to oxidative cleavage of the SAM surface.
In another application involving a study of biotin compounds for potential use in viv o,
it was found that the preferred structures included molecules that contained PEG spacers to
18. Discrete PEG Reagents 709
Figure 18.1 A trifunctional reagent for studying protein interactions by mass spec. The bis-NHS ester arms
crosslink interacting proteins, while the discrete PEG-containing biotin arm can be used to isolate or detect the
conjugates using (strept)avidin reagents.
