Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

THE USE OF ANTISEPTICS IN PERIODONTAL THERAPY • 475
able and no long-term evaluations have been per-
formed.
Amine alcohols
This group of compounds does not truly fit into an
antimicrobial or antiseptic category; indeed they ex-
hibit minimal effects against microbes. Octopenol was
first shown to be effective as an antiplaque agent but
was withdrawn for toxicological reasons. Delmopinol
followed and at 0.1% and 0.2% in mouthrinses was
shown to be effective as a plaque inhibitor and an-
tigingivitis agent in short-term no oral hygiene and
long-term home use studies (Collaert et al. 1992, Mo-
ran et al. 1992b, Claydon et al. 1996). Side effects
include tooth discoloration, transient numbness of the
mucosa, particularly the tongue, and burning sensa-
tions in the mouth. The mode of action of delmopinol
can be debated but, in part, appears to be the inhibition
in formation or disruption of the matrix of early
plaque forming bacteria. No products are available at
present.
Acidified sodium
chlorites (for review see Yates et al.
1997)
This agent does not sit well with any particular group
listed in Table 22-1; however, depending on the acid
chosen and the conditions of the reaction between the
acid and the sodium chlorite, a varied and complex
range of reaction products can ensue. Under ideal
conditions for antimicrobial benefits sodium chlorite
is reacted with a protic acid to produce chlorous acid,
which then liberates a range of higher oxidant species
but contains minimal amounts of chlorine dioxide.
These higher oxidant species have a broad range of
antimicrobial action against bacteria, fungi, yeast and
viruses and products are available in the US within
the veterinary and food industry, both as a preventive
for mastitis in cows and for the preservation of frozen
poultry. Experimental mouthrinses have been tested
in short-term plaque regrowth studies and salivary
bacterial count investigations (Yates et al. 1997). Sur-
prisingly, given that the acid and sodium chlorite are
mixed immediately before rinsing, and that the dura-
tion of the chemical reaction would be limited to the
rinsing time, three experimental formulations were
shown to be as good as chlorhexidine against plaque
regrowth and showed the same substantivity as chlor
-
hexidine. Although not tested in longer-term studies,
side effects, particularly staining and alternation of
taste, would appear unlikely with the acidified so-
dium chlorite mouthrinses. Unfortunately, the low pH
of the formulations would be expected to cause some
dental erosion and this has been proven in studies
in
situ
(Pontefract et al. 2001). Such erosion, which was
found comparable to that of orange juice
in situ,
would
tend to obviate the long-term continuous use of such
agents. However, acidified sodium chlorite mouthrin-
ses could find application in preventive dentistry
similar to those to be described for chlorhexidine (see
the section "Clinical uses of chlorhexidine" later in
this chapter). The erosive effects would not, in short
to medium-term use, reach clinically significant lev-
els. To date no commercial products are available.
Other
antiseptics (for review see Addy 1986)
A number of antiseptics/antimicrobial agents have
been studied for plaque inhibition. Most have been
found to have little or no effect
in vivo;
a few have been
formulated in mouthrinse products including povi-
done iodine and hexetidine. Povidone iodine at 1%
has a substantivity of only 60 minutes (Addy & Wright
1978) and lacks appreciable plaque inhibitory activity
(
Addy et al. 1977) or action in acute infections such as
acute ulcerative gingivitis (Addy & Llewelyn 1978),
for
which it is recommended. Povidone iodine is
largely
without side effects but as a rinse has potential
to
affect thyroid function adversely (Wray et al. 1978).
Hexetidine, a saturated pyrimidine, at 0.1% was
shown to have limited plaque inhibitory action (Ber-
genholtz & Hanstrom 1974) and no evidence for anti-
plaque activity when used as an adjunct for oral hy-
giene (Chadwick et al. 1991). The action of hexetidine
against plaque appears enhanced by zinc salts (Saxer
& Muhlemann 1983) but data are derived only from
short-term studies. Side effects for hexetidine include
tooth staining and mucosal erosion, although both are
uncommon (Bergenholtz & Hanstrom 1974). Never-
theless, mucosal erosion is markedly increased in in-
cidence if the concentration is raised to 0.14% (Bergen
-
holtz & Hanstrom 1974). A mouthrinse product con-
taining 0.1% hexetidine is available in some European
countries.
Conclusions
•
Effective antimicrobial antiplaque agents show pro-
longed persistence of action in the mouth (
substan
tivity). Chlorhexidine is the most effective
anti-
plaque agent to date. Stannous fluoride and
tri
closan oral hygiene products are available with
proven antiplaque activity. The long established
mouthrinse, based on essential oils, has some evi-
dence for adjunctive antiplaque activity.
•
The limited information on natural products, for
example herbal formulations, is not encouraging
and the root extract sanguinarine has been with-
drawn because of the potential to cause precancer-
ous oral lesions.
•
The amine alcohol, delmopinol, appears effective
but products are not available.
•
Acidified sodium chlorite appears as effective as
chlorhexidine but the acidic nature of the rinse may
obviate oral hygiene products ever coming to the
marketplace.
•
Combinations of agents sometimes provide addi-
tive or synergistic action, but with the exception of
triclosan, few products are available.
476 •
CHAPTER 22
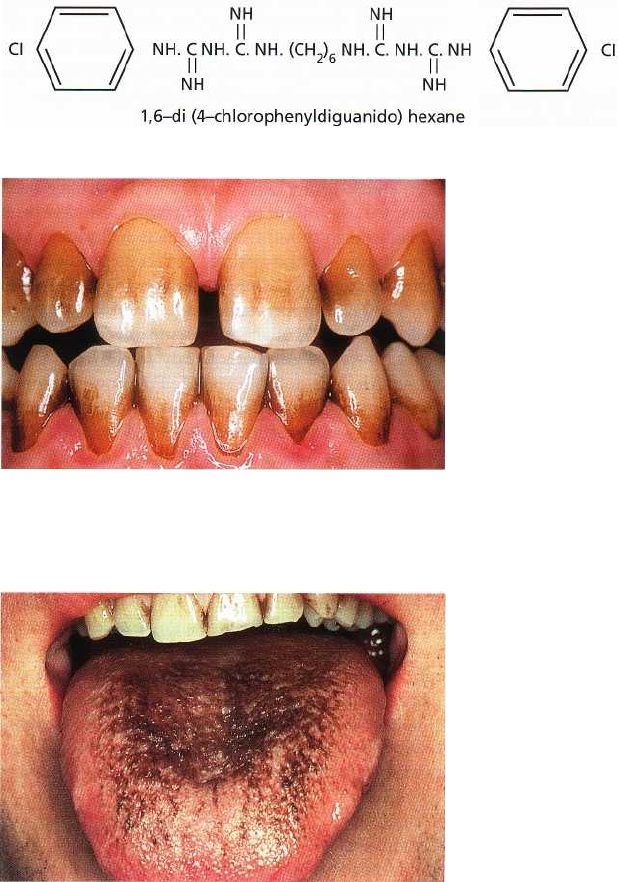
Fig. 22-2. Chlorhexidine molecule.
Fig. 22-3. Brown discoloration of the teeth of an individ
-
ual rinsing twice a day for 3 weeks with a 0.2% chlor
-
hexidine mouthrinse.
Fig. 22-4. Brown discoloration of the tongue of an indi
-
vidual rinsing twice a day for 2 weeks with a 0.2%
chlorhexidine mouthrinse.
CHLORHEXIDINE
Chlorhexidine is available in three forms, the diglu-
conate, acetate and hydrochloride salts. Most studies
and most oral formulations and products have used
the digluconate salt, which is manufactured as a 20%
V/V concentrate. Digluconate and acetate salts are
water-soluble but hydrochloride is very sparingly sol-
uble in water. Chlorhexidine was developed in the
1940s by Imperial Chemical Industries, England, and
marketed in 1954 as an antiseptic for skin wounds.
Later, the antiseptic was more widely used in medi-
cine and surgery including obstetrics, gynecology,
urology and presurgical skin preparation for both
patient and surgeon. Use in dentistry was initially for
presurgical disinfection of the mouth and in endodon-
tics. Plaque inhibition by chlorhexidine was first in-
vestigated in 1969 (Schroeder 1969), but the definitive
study was performed by Loe and Schiott (1970). This
study showed that rinsing for 60 seconds twice per
day with 10 ml of a 0.2% (20 mg dose) chlorhexidine
gluconate solution in the absence of normal tooth
cleaning, inhibited plaque regrowth and the develop-
ment of gingivitis. Numerous studies followed, such
that chlorhexidine was one of the most investigated
compounds in dentistry (for review see Jones 1997).
Chlorhexidine is a bisbiguanide antiseptic, being a
symmetrical molecule consisting of four chlorophenyl
rings and two biguanide groups connected by a cen-
tral hexamethylene bridge (Fig. 22-2). The compound
is a strong base and dicationic at pH levels above 3.5,
with two positive charges on either side of a hex-
amethylene bridge (Albert & Sargeant 1962). Indeed,
it is the dicationic nature of chlorhexidine, making it
extremely interactive with anions, which is relevant to
its efficacy, safety, local side effects and difficulties
with formulation in products.
Toxicology, safety and side effects
The cationic nature of chlorhexidine minimizes ab-
sorption through the skin and mucosa, including from
the gastrointestinal tract. Systemic toxicity from topi-
cal application or ingestion is therefore not reported,
nor is there evidence of teratogenicity in the animal
model. Even in intravenous infusion in animals, chlor
-
hexidine is well tolerated and has occurred acciden-
tally in humans without serious consequences. Hy-
persensitivity reactions including anaphylaxis have
been reported in fewer than 10 people in Japan and
resulted from the application of non-proprietary
chlorhexidine products to sites other than the mouth.
There was insufficient information to confirm that the
reactions were actually due to chlorhexidine.
Neurosensory deafness can occur if chlorhexidine is
introduced into the middle ear and the antiseptic
should not be placed in the outer ear in case the
eardrum is perforated. The antiseptic has a broad
antimicrobial action, including a wide range of Gram-
positive and Gram-negative bacteria (Wade & Addy
1989). It is also effective against some fungi and yeasts
including candida, and some viruses including HBV
and HIV. Bacterial resistance has not been reported
with long-term, oral use, or evidence of supra-infec-
tion by fungi, yeasts or viruses. Long-term oral use
resulted in a small shift in the flora towards the less
THE USE OF ANTISEPTICS IN PERIODONTAL THERAPY • 477
sensitive organisms but this was rapidly reversible at
the end of the 2-year study (Schlott et al. 1976).
In oral use as a mouthrinse, chlorhexidine has been
reported to have a number of local side effects (Flotra et
al. 1971). These side effects are:
1.
Brown discoloration of the teeth and some restora-
tive materials and the dorsum of the tongue (to be
discussed further) (Figs. 22-3 and 22-4).
2.
Taste perturbation where the salt taste appears to
be
preferentially affected (Lang et al. 1988) to leave
food and drinks with a rather bland taste.
3.
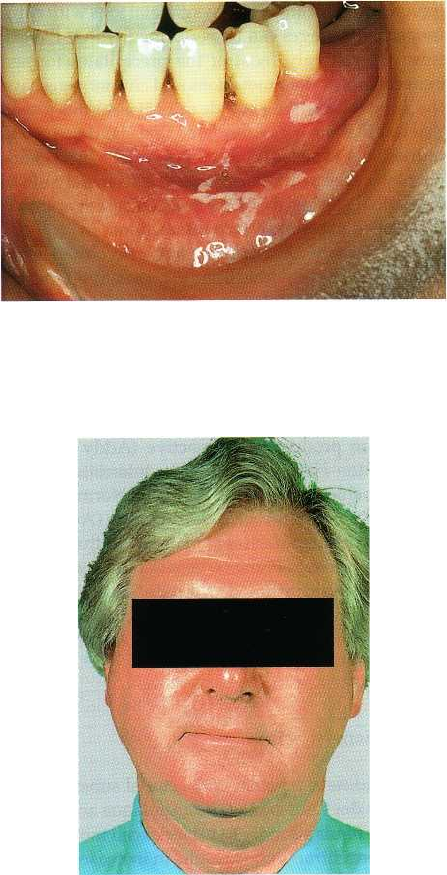
Oral mucosal erosion (Fig. 22-5). This appears to be
an idiosyncratic reaction and concentration de-
pendent. Dilution of the 0.2% formulation to 0.1%,
but rinsing with the whole volume to maintain dose,
usually alleviates the problem. Erosions are
rarely
seen with 0.12% rinse products used at 15 ml
volume.
4.
Unilateral or bilateral parotid swelling (Fig. 22-6).
This is an extremely rare occurrence and an expla-
nation is not available.
5.
Enhanced supragingival calculus formation. This
effect may be due to the precipitation of salivary
proteins on to the tooth surface, thereby increasing
pellicle thickness and/or precipitation of inorganic
salts on to the pellicle layer. Certainly pellicle form-
ing under the influence of chlorhexidine shows an
early and highly calcified structure (Leach 1977).
Chlorhexidine also has a bitter taste, which is difficult
to mask completely.
Chlorhexidine staining
The mechanisms proposed for chlorhexidine staining
can be debated (Eriksen et al. 1985, Addy & Moran
1995, Watts & Addy 2001) but have been proposed as:
1.
Degradation of the chlorhexidine molecule to re-
lease parachloraniline
2.
Catalysis of Maillard reactions
3.
Protein denaturation with metal sulfide formation
4.
Precipitation of anionic dietary chromogens.
Degradation of chlorhexidine to release parachlora-
niline appears not to occur on storage or as a result of
metabolic processes. Also, alexidine, a related bis-
biguanide, does not have parachloraniline groups, yet
causes staining identical to that of chlorhexidine
(Addy
& Roberts 1981). Non-enzymatic browning re-
actions (
Maillard reactions) catalysed by chlorhexidine are a
theoretical possibility (Nordbo 1979);
however,
evidence is indirect, circumstantial or inconclusive (
Eriksen et al. 1985). The theory does not con
sider the
fact that other antiseptics and metals such as
tin, iron
and copper also produce dental staining.
Protein
denaturation produced by chlorhexidine with the
interaction of exposed sulfide radicals with metal
Fig. 22-5. Mucosal erosion occurring following a few
days of rinsing twice a day with a 0.2% chlorhexidine
mouthrinse.
Fig. 22-6. Bilateral parotid swelling following a few
days of rinsing with a 0.2% chlorhexidine mouthrinse.
ions is also theoretically possible (Ellingsen et al. 1982,
Nordbo et al. 1982) but there is no direct evidence to
support this concept. Again, the theory does not take
into account similar staining by other antiseptics and
metal ions. Laboratory and clinical studies also could
not reproduce this process (Addy et al. 1985, Addy &
Moran 1985). Precipitation of anionic dietary chromo-
gens by cationic antiseptics, including chlorhexidine
and polyvalent metal ions as an explanation for the
phenomenon of staining by these substances, is sup-
ported by a number of well-controlled laboratory and
clinical studies (for reviews see Addy & Moran 1995,
Watts & Addy 2001). Thus, the locally bound antisep-
tics or metal ions on mucosa or teeth can react with
polyphenols in dietary substances to produce stain-
ing. Beverages such as tea, coffee and red wine are
particularly chromogenic, but other foods and bever-
ages will interact to produce various colored stains.
These reactions between chlorhexidine and other cat-
478 • CHAPTER 22
ionic antiseptics and polyvalent metal ions with chro-
mogenic beverages can be performed within the test
tube. Interestingly, most of the precipitates formed
between polyvalent metal ions and chromogens have
the same color as their sulfide salts. It is for this reason
that original theories considered that staining, seen in
individuals exposed to these polyvalent metal ions,
usually in the workplace, was due to metal sulfide
formation. Again, laboratory and clinical experiments
have failed to produce such interactions.
It is perhaps the staining side effect that limits
long-term use of chlorhexidine in preventive dentistry
(
Flotra et al. 1971) and occurs with all correctly formu
-
lated products including gels, toothpastes and sprays.
Indeed, the staining side effect can be used to assess
patient compliance in the use and activity of formula-
tions. In the latter case laboratory and clinical studies
on staining have revealed a proprietary chlorhexidine
mouthrinse product to be inactive (Addy & Wade
1995, Renton-Harper et al. 1995). Interestingly, this
particular chlorhexidine product was reformulated in
the UK to produce an active formulation (Addy et al.
1991), but the manufacturers maintained the original
formulation within France when both laboratory and
clinical studies confirmed markedly reduced poten-
tial of the product to cause staining in the laboratory,
and plaque inhibition in the clinic (Addy & Wade
1995, Renton-Harper et al. 1995).
Mechanism of action
Chlorhexidine is a potent antibacterial substance but
this alone does not explain its antiplaque action. The
antiseptic binds strongly to bacterial cell membranes.
At low concentration this results in increased perme-
ability with leakage of intracellular components in-
cluding potassium (Hugo & Longworth 1964, 1965).
At high concentration, chlorhexidine causes precipi-
tation of bacterial cytoplasm and cell death (Hugo &
Longworth 1966). In the mouth chlorhexidine readily
adsorbs to surfaces including pellicle-coated teeth.
Once adsorbed, and unlike some other antiseptics,
chlorhexidine shows a persistent bacteriostatic action
lasting in excess of 12 hours (Schiott et al. 1970). Ra-
dio-labelled chlorhexidine studies suggest a slow re-
lease of the antiseptic from surfaces (Bonesvoll et al.
1974a,b) and this was suggested to produce a pro-
longed antibacterial milieu in the mouth (Gjermo et al.
1974). However, the methods could not determine the
activity of the chlorhexidine, which was almost cer-
tainly attached to the salivary proteins and desquam-
ating epithelial cells and therefore unavailable for
action. Consistent with the original work and conclu-
sions (Davies et al. 1970), a more recent study and
review suggested that plaque inhibition is derived
only from the chlorhexidine adsorbed to the tooth
surface (Jenkins et al. 1988). It is possible that the
molecule attaches to pellicle by one cation leaving the
other free to interact with bacteria attempting to colo-
nize the tooth surface. This mechanism would, there-
fore, be similar to that associated with tooth staining.
It
would also explain why anionic substances such as
sodium lauryl sulfate based toothpastes reduce the
plaque inhibition of chlorhexidine if used shortly after
rinses with the antiseptic (Barkvoll et al. 1989). Indeed,
a more recent study has demonstrated that plaque
inhibition by chlorhexidine mouthrinses is reduced if
toothpaste is used immediately before or immediately
after the rinse (Owens et al. 1997). These inhibitory
effects on chlorhexidine activity by substances such as
toothpastes can be modeled using the chlorhexidine
tea staining method, which shows reduced staining
activity by the chlorhexidine solutions resulting from
an interaction with toothpaste (Sheen et al. 2001).
Plaque inhibition by chlorhexidine mouthrinses
appears to be dose related (Cancro et al. 1973, 1974,
Jenkins et al. 1994) such that similar effects to that seen
with the more usual 10 ml, 0.2% solution (20 mg) can
be achieved with high volumes of low concentration
solutions (Cumming & Loe 1973, Lang & Ramseier-
Grossman 1981). It is worth noting, however, that not
inconsiderable plaque inhibition is obtained with
doses as low as 1—5 mg twice daily (Jenkins et al. 1994).
Also, and relevant to the probable mechanism of ac-
tion, topically applying 0.2% solutions of chlor-
hexidine only to the tooth surface, including by the
use of sprays, produces the same level of plaque inhi-
bition as rinsing with the full 20 mg dose (Addy &
Moran 1983, Francis et al. 1987a, Jenkins et al. 1988,
Kalaga et al. 1989a).
Chlorhexidine products
Chlorhexidine has been formulated into a number of
products.
Mouthrinses
Aqueous alcohol solutions of 0.2% chlorhexidine were
first made available for mouthrinse products for twice
daily use in Europe in the 1970s. A 0.1% mouthrinse
product also became available; however questions
were raised over the activity of the 0.1% product and
in some countries the efficacy of this product is less
than would be expected from a 0.1% solution (Jenkins
et al. 1989). Later, in the US, a 0.12% mouthrinse was
manufactured but to maintain the almost optimum 20
mg doses derived from 10 ml of 0.2% rinses, the prod
-
uct was recommended as a 15 ml rinse (18 mg dose).
The studies revealed equal efficacy for 0.2% and 0.12%
rinses when used at appropriate similar doses
(
Segreto et al. 1986).
Gel
A 1% chlorhexidine gel product is available and can
be delivered on a toothbrush or in trays. The distribu
-
tion of the gel by toothbrush around the mouth ap-
pears to be poor and preparations must be delivered
to all tooth surfaces to be effective (Saxen et al. 1976).
THE USE OF ANTISEPTICS IN PERIODONTAL THERAPY • 479
In trays the chlorhexidine gel was found to be particu
-
larly effective against plaque and gingivitis in handi-
capped individuals (Francis et al. 1987a). The accept-
ability of this tray delivery system to the recipients
and the carers was found to be poor (Francis et al.
198Th). More recently, 0.2% and 0.12% chlorhexidine
gels have become available.
Sprays
0.1% and 0.2% chlorhexidine in sprays are commer-
cially available in some countries. Studies with the 0.
2% spray have revealed that small doses of approxi
-
mately 1-2 mg delivered to all tooth surfaces produces
similar plaque inhibition to a rinse with 0.2% mouth-
rinses (Kalaga et al. 1989a). Sprays appear particularly
useful for the physically and mentally handicapped
groups, being well received by individuals and their
carers (Francis et al 1987a,b, Kalaga et al. 1989b).
Toothpaste
Chlorhexidine is difficult to formulate into toothpaste
for reasons already given and early studies produced
variable outcomes for benefits to plaque and gingivitis
(
Gjermo & Rolla 1970, 1971, Johansen et al. 1972,1975).
More recently, a 1% chlorhexidine toothpaste with and
without fluoride was found to be superior to the con-
trol product for the prevention of plaque and gingivi-
tis in a 6-month home use study (Yates et al. 1993).
However, stain scores were markedly increased as
was supragingival calculus formation, and the manu-
facturer did not produce a commercial product. For a
short time a commercial product was available, hav-
ing been shown to be efficacious for both plaque and
gingivitis (Sanz et al. 1994). Although effective, chlor-
hexidine products based on toothpaste and sprays
produce similar tooth staining to mouthrinses and
gels; taste disturbance, mucosal erosion and parotid
swellings tend to be less or have never been reported.
Varnishes
Chlorhexidine varnishes have been used mainly for
prophylaxis against root caries rather than an anti-
plaque depot for chlorhexidine in the mouth.
Conclusions
•
Chlorhexidine to date is the proven most effective
antiplaque agent for which commercial products
are available to the public.
•
Chlorhexidine is free from systemic toxicity in oral
use, and microbial resistance and supra-infection
do not occur.
•
Local side effects are reported which are mainly
cosmetic problems.
•
The antiplaque action of chlorhexidine appears de-
pendent on prolonged persistence of antimicrobial
action in the mouth (substantivity).
•
A number of vehicles for delivering chlorhexidine
are available, but mouthrinses are most commonly
recommended.
•
Extrinsic dental staining and perturbation of taste
are variably the two side effects of chlorhexidine
mouthrinse usage which limit acceptability to users
and the long-term employment of this antiseptic in
preventive dentistry.
CLINICAL
USES OF
CHLORHEXIDINE
Despite the excellent plaque inhibitory properties of
chlorhexidine, widespread and prolonged use of the
agent is limited by local side effects. Moreover, be-
cause of the cationic nature of the chlorhexidine and
therefore its poor penetrability, the antiseptic is of
limited value in the therapy of established oral condi-
tions including gingivitis, and is much more valuable
in the preventive mode. A number of clinical uses,
some well researched, have been recommended for
chlorhexidine (for reviews see Gjermo 1974, Addy
1986, Addy & Renton-Harper 1996a, Addy & Moran
1997).
As an adjunct to oral hygiene and professional
prophylaxis
Oral hygiene instruction is a key factor in the treat-
ment plan for patients with periodontal disease and
as part of the maintenance program following treat-
ment. Adequate plaque control by periodontal pa-
tients is therefore essential to successful treatment and
the prevention of re-occurrence of the disease. Chlor-
hexidine should therefore increase the improvement
in gingival health through plaque control, particularly
following a professional prophylaxis to remove exist-
ing supra and immediately subgingival plaque. There
is, however, a potential disadvantage of using such an
effective chemical plaque control agent at this stage of
the periodontal treatment plan. Thus, following oral
hygiene instruction, it is normal, usually by the use of
indices, to quantify the improvement in plaque con-
trol by patients so instructed and, in particular, the
improvement at specific sites which previously had
been missed by individual patients. By virtue of the
excellent plaque control effects of chlorhexidine, the
response to oral hygiene instruction cannot be accu-
rately assessed since the antiseptic will overshadow
any deficiencies in mechanical cleaning. Indeed, as the
original research demonstrated, patients could main-
tain close to zero levels of plaque following a profes-
sional prophylaxis without using any form of me-
chanical oral hygiene (Loe & Schiott 1970).
Postoral surgery including periodontal surgery or
root planing
Chlorhexidine may be used postoperatively since it
offers the advantage of reducing the bacterial load in
the oral cavity and preventing plaque formation at a
time when mechanical cleaning may be difficult be-
cause of discomfort. In periodontal surgery, periodon-
tal dressings have largely been replaced by the use of
480 • CHAPTER 22
chlorhexidine preparations, in particular mouthrin-
ses, since healing is improved and discomfort reduced
(
Newman & Addy 1978, 1982). Regimens vary but
chlorhexidine should be used immediately post treat-
ment and for periods of time until the patient can
re-
institute normal oral hygiene. Depending on the
appointment schedule, chlorhexidine could be used
throughout the treatment phase and for periods of
weeks after completion of the treatment plan. If dress
-
ings are used, chlorhexidine is of limited value to the
postoperative site since it does not penetrate beneath
the periodontal dressings (Pluss et al. 1975). The idea
of full mouth disinfection using chlorhexidine both
supra and subgingivally has recently been assessed by
one group of researchers (Quirynen et al. 1995). In the
event, few adjunctive benefits could be shown and it
appeared that the more dominant factor was the time
over which the non-surgical treatment plan was com-
pleted. Thus, root planing performed totally within 24
hours was more effective than root planing completed
over more conventional periods of several weeks (for
review see Quirynen et al. 2001).
For patients with jaw fixation
Oral hygiene is particularly difficult when jaws are
immobilized by such methods as intermaxillary fixa-
tion. Chlorhexidine has been shown to reduce mark-
edly the bacterial load, which tends to increase during
jaw immobilization, and improve plaque control
(Nash
& Addy 1979).
For oral hygiene and gingival health benefits in the
mentally and physically handicapped
Chlorhexidine has been found particularly useful in
institutionalized mentally and physically handi-
capped groups, improving both oral hygiene and gin-
gival health (Storhaug 1977). Spray delivery of 0.2%
solutions was found particularly useful and accept-
able to patients and care workers (Francis et al.
1987a,b, Kalaga et al. 1989b).
Medically compromised individuals predisposed
to
oral infections
A number of medical conditions predispose individu-
als to oral infections, notably candidiasis. Chlor-
hexidine is effective as an anticandidal agent but is
most useful when combined with specific anticandi-
dal drugs, such as nystatin or amphoteracin B (Simon
etti et al. 1988). Indications for chlorhexidine use com
-
bined with anticandidal drugs have been for the pre-
vention of oral and systemic infections in the immu-
nocompromised, including those with blood dy-
scrasias, those receiving chemotherapy and/or radio-
therapy and notably bone marrow transplant patients
(
Ferretti et al. 1987, 1988, Toth et al. 1990). The value
of chlorhexidine appears greatest when initiated be-
fore oral or systemic complications arise. A chlor-
hexidine spray was also found to produce sympto-
matic/psychological oral care benefits in the termi-
nally ill (Dobbins et al. 1992).
High-risk caries
patients
Chlorhexidine rinses or gels can reduce considerably
the streptococcus mutans counts in individuals who
are caries prone. Additionally, and interestingly, chlor
hexidine appears synergistic with fluoride and com-
bining chlorhexidine and fluoride rinses appears
beneficial to such at risk individuals (Dolles & Gjermo
1980, Lindquist et al. 1989).
Recurrent oral ulceration
Several studies have shown that chlorhexidine
mouthrinses and chlorhexidine gels reduce the inci-
dence, duration and severity of recurrent minor
aphthous ulceration (Addy et al. 1974, 1976, Hunter &
Addy 1987). The mechanism of action is unclear but
may relate to a reduction in contamination of ulcers
by oral bacteria, thereby reducing the natural history
of the ulceration. Regimens have included three times
daily use of chlorhexidine products for several weeks.
Interestingly, one study showed that triclosan rinses
reduce the incidence of recurrent mouth ulcers (Skaare
et al. 1996). There have been no controlled studies of
chlorhexidine in the management of major aphthous
ulceration or other oral erosive or ulcerative condi-
tions, although anecdotally chlorhexidine appears in
-
effective. Again, this may reflect the low therapeutic
potential of this and other antiseptics.
Removal and
fixed orthodontic appliance wearers
Plaque control in the early stages of orthodontic ap-
pliance therapy may be compromised and chlor-
hexidine can be prescribed for the first 4-8 weeks.
Additionally, chlorhexidine has been shown to reduce
the number and severity of traumatic ulcers during
the first 4 weeks of fixed orthodontic therapy (Shaw
et al. 1984).
In denture stomatitis
Chlorhexidine has been recommended in the treat-
ment of candidal associated infections; however, in
practice even applying chlorhexidine gel to the fitting
surfaces of denture produces, in many cases, slow and
incomplete resolution of the condition. Again, chlor-
hexidine is less effective in the therapeutic mode and
it is more advantageous to treat denture stomatitis
with specific anticandidal drugs and then employ
chlorhexidine to prevent recurrence. The denture itself
can be usefully sterilized from candida by soaking in
chlorhexidine solutions (Olsen et al. 1975a,b).
Immediate preoperative chlorhexidine rinsing and
irrigation
This technique can be used immediately prior to op-
erative treatment, particularly when ultrasonic pol-
ishing or high-speed instruments are to be used. Such
preoperative rinsing markedly reduces the bacterial
load and contamination of the operative area and
operator and staff (Worral et al. 1987). Additionally, in
susceptible patients, irrigation of chlorhexidine
around the gingival margin reduces the incidence of
THE USE OF ANTISEPTICS IN PERIODONTAL THERAPY • 481
bacteremia (MacFarlane et al. 1984). However, this
should be seen only as an adjunct to appropriate
systemic antimicrobial prophylaxis.
Subgingival irrigation
Numerous antimicrobial agents have been used as
subgingival irrigants in the management and treat-
ment of periodontal diseases (for reviews see
Wennstrom 1992, 1997). Alone, irrigation with antimi
-
crobial agents produces effects little different from
using saline and of short duration, suggesting that the
action is a washing-out effect. Irrigation combined
with root planing appears to provide no adjunctive
benefits.
Conclusions
•
There are a significant number of indications for
the use of chlorhexidine in preventive dentistry,
most of
which rely on the antimicrobial properties
of the
antiseptic and its duration of action.
•
The most valuable chemical plaque control uses of
chlorhexidine are in the short to medium term when
mechanical tooth cleaning is not possible, difficult
or inadequate and during which time local side
effects are likely to be minimized.
•
Chlorhexidine is more effective as a preventive
rather than a therapeutic agent and therefore must
be of questionable value as a subgingival adjunct in
the treatment of periodontitis (see next chapter).
EVALUATION OF CHEMICAL
AGENTS AND PRODUCTS
(for reviews see Addy et al. 1992, Addy 1995, Moss et
al. 1995, Addy & Moran 1997)
The number and use of oral hygiene products has
grown enormously in recent years and, as an example,
hundreds of millions of pounds per year are spent on
oral hygiene products in the UK. There can be no
doubt that the oral hygiene industries, through their
collaboration and research with the dental profession
and their promotion of their products have, in no
small way, contributed to the improvement in dental
health seen in many countries. However, claims for
efficacy of oral hygiene products are frequently made
and it is essential that these are supported by scientific
evidence. Without such evidence the profession and
the public may be confused or misled. The dental
profession is, however, faced with a large number of
oral hygiene products supported by huge quantities
of varied promotional literature and media advertis-
ing, which makes impossible, in many cases, any valid
judgement or assessment of the efficacy or value of
individual products to specific patient groups or the
public as a whole. Even those with specialized inter-
est, and research experience in specific aspects of oral
hygiene product evaluation, must find validation
based on published literature a daunting task. This is
made all the more difficult since what constitutes
proof of efficacy is not generally agreed even amongst
so-called experts. Few countries of the world have
central control over what evidence is required before
efficacy clearance can he made and there are very few
guidelines suggesting requirements for proof of effi-
cacy for oral hygiene products.
The scientific evaluation of dental products, and for
that matter preventive and therapeutic agents in
medicine as a whole, is a relatively modern concept
but today must be the backbone on which to base
claims of efficacy. Anecdotal and case reports, uncon-
trolled studies and data listed as "held on file" by
manufacturers, whilst interesting, should not be used
as the basis for efficacy claims. Blind, randomized,
controlled clinical and laboratory studies must be the
methods used today to obtain data on the activity of
agents, formulations and products. Terminology and
phraseology in product claims also needs to be care-
fully reviewed and assessed. Perhaps the greatest area
for criticism must be the implied claim by the manu-
facturer and/or the inferences left to be drawn from
promotional material by the dental profession or pub-
lic. A classic scenario for which there is precedence can
be stated as follows: A is the cause of B, C reduces A,
leaving the inference to be drawn that C can control B.
Perhaps nowhere is this more apparent than in the use
of agents which are known to control plaque, and
therefore it can be implied, without evidence, they
must control gingivitis. The now familiar claim would
be "this product reduces plaque, the major cause of
gum disease". Similarly, creative arithmetic is not in-
frequently used to give inflated impressions of effi-
cacy Proportional differences, rather than actual dif-
ferences, are not infrequently quoted, as are percent-
ages of percentages giving hundreds of percent im-
provements over another product or control, yet the
actual benefit is a fraction of the scoring index used.
Finally, "piggy back" claims are not uncommon, when
a
known active ingredient is formulated into a new
product and equivalent efficacy to established prod-
ucts is assumed. In respect of terminology for oral
hygiene products, the European Workshop on Perio-
dontology in 1996 defined certain terms (Lang & New
-
man 1997):
• Antimicrobial agents:
Chemicals that have a bacte-
riostatic or bacteriocidal effect
in vitro
that alone
cannot be extrapolated to a proven efficacy
in vivo
against plaque.
• Plaque reducing/inhibitory agents:
Chemicals that
have
only been shown to reduce the quantity
and/or
affect quality of plaque, which may or may
not be
sufficient to influence gingivitis and/or car
ies.
• Antiplaque agents:
Chemicals that have an effect on
plaque sufficient to benefit gingivitis and/or caries.
• Antigingivitis agents:
Chemicals which reduce
gingival inflammation without necessarily influ-
482 • CHAPTER 22
encing bacterial plaque (includes anti-inflamma-
tory agents).
Thus the fact that antimicrobial agents such as anti-
septics kill or inhibit the growth of bacteria does not
necessarily mean they will be effective plaque inhibi-
tors (Gjermo et al. 1970). Also, the mere incorporation
of a known antiplaque agent into a formulation is not
a guarantee of efficacy because inactivation by other
ingredients may occur.
This section looks at methods that have been used
to test oral hygiene products both in the laboratory
and the clinic. No one protocol can provide all the
answers, and research and development of agents into
products is a step-by-step process, hopefully culmi-
nating in a body of evidence proving efficacy, beyond
doubt, of a final product. Methods
in vitro
and
in vivo
will be summarized but animal testing will not be
discussed except to acknowledge that the use of ani-
mals is still necessary in drug development, in under-
standing the mode of action of drugs and, particularly,
in evaluating safety from a toxicological point of view.
However, the evaluation of oral hygiene products on
animals, particularly for efficacy, must be questioned
on a number of scientific and moral grounds.
Most laboratory and clinical methods have been
developed to test antimicrobial agents but methodolo-
gies are available, or present ones could be modified,
to study potential antiadhesive and plaque removal
chemicals. (For reviews see Addy et al. 1992, 1995a,b,
Addy & Moran, 1997.)
Studies in vitro
Bacterial tests
Antimicrobial tests including minimum inhibitory
concentration (MIC), minimum bactericidal concen-
tration (MBC) and kill curves can be determined.
These tests indicate the antibacterial activity and an-
timicrobial spectrum of agents and formulations
against a range of oral bacteria. Continuous culture
techniques can also be used but they may not provide
more meaningful data. It is likely that, with techno-
logical advances, laboratory models to accurately rep-
licate the plaque biofilm will become available to test
chemical plaque control agents. At present, antimicro
-
bial tests
in vitro
primarily only indicate activity, or
lack of it, and they are very poor predictors per se
of
plaque action in
vivo.
This is because, so far, methods
do not provide particularly reliable information on the
substantivity of the antimicrobial agent. Nevertheless,
antimicrobial tests are valuable for a variety of rea-
sons. With few exceptions, agents without activity
in
vitro
will not provide activity
in vivo.
The additive or
negative effects of ingredient mixtures can be deter-
mined. The availability of active ingredients incorpo-
rated in the product can be assessed. The adverse
influence of the oral environment can be modeled; for
example, the influence of saliva or proteins on the
antibacterial activity of agents can be tested.
Uptake measurements
One aspect of substantivity is adsorption of antimicro
bials and other potential plaque inhibitory agents on
to surfaces. This can be quantified using a variety of
substrates such as hydroxyapatite, dentine, enamel,
acrylic and other polymers. The influence of other
factors or agents on the uptake of a particular agent
can also be assessed. Such data are of interest but must
be interpreted with caution since they only measure
uptake, not activity once adsorbed. Nevertheless,
desor
p
tion of an agent from such surfaces can be
measured by a variety of analytical techniques thereby
giving some indication of both the adsorption profile
and the subsequent substantivity of the agent to the
substrate surface.
Other methods
Activity or availability of an ingredient in a formula-
tion can be measured or assessed. Methods include
chemical analyses; however, some methods chemi-
cally extract the agent from the formulation in its
entirety and therefore do not necessarily demonstrate
that it is freely available and active within the formu-
lation. For the cationic antiseptics and polyvalent met
-
al salts, their potential to bind dietary chromogens
from beverages such as tea can be used to assess the
possibility that they may cause staining
in vivo.
More
usefully, the test method can be employed to deter-
mine and compare the availability of the same ingre-
dient in different formulations. Such methods have
shown considerable differences in availability of
chlorhexidine and cetylpyridinium chloride in appar-
ently similar mouthrinses (Addy et al. 1995b). More-
over, how other oral hygiene products might interfere
with the activity of chemical plaque control agents,
such as toothpaste with chlorhexidine and cetylpyrid
-
inium chloride, has given surprisingly accurate pre-
dictions of clinical outcome (Owens et al. 1997, Sheen
et al. 2001, 2002). Again, these methods give little
indication of substantivity and therefore the staining
method
in
vitro
cannot be used to compare different
agents for propensities to cause staining
in vivo.
For
example, a 0.05% cetylpyridinium chloride mouth-
rinse produces comparable tea staining on a substrate
surface to a 0.2% chlorhexidine mouthrinse, yet clini
-
cally the amount of staining reported for chlor-
hexidine is considerably greater than that for ce-
tylpyridinium chloride and this can be explained by
the fact that the substantivity of the former is greater
than the latter.
Study methods
in vivo
A considerable number of protocols have been devel-
oped to evaluate potential antiplaque agents and
products. Ideally, because of the number of ingredi-
ents and more particularly formulations, a step-by-
step pyramid approach is taken. Thus initially, study
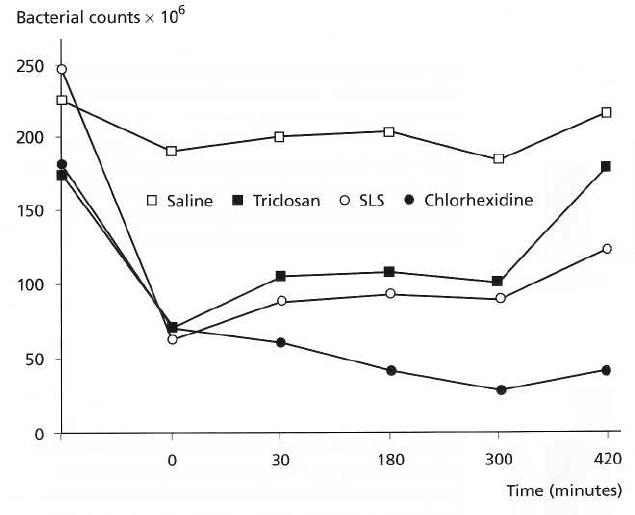
THE USE OF ANTISEPTICS IN PERIODONTAL THERAPY • 483
Fig. 22-7. Salivary bacterial counts
over time following mouthrinsing
with chlorhexidine, saline, so-
dium lauryl sulfate and triclosan.
Following a single rinse with
chlorhexidine, sodium lauryl sul-
fate and triclosan there is an im-
mediate large reduction in bacte-
rial counts. This continues and
persists to the 420-minute end-
point of the study for chlor-
hexidine (positive control) with a
tendency for counts to revert to-
wards baseline for triclosan and
sodium lauryl sulfate. With saline
(placebo control) there is little
change in counts over time.
designs are used which permit, if necessary, the
screening of relatively large numbers of agents and
formulations and on relatively small numbers of sub-
jects.
Depot studies
Retention of agents in the mouth may be measured by
determining the amount expectorated versus the
known dose (the buccal retention test) or by measur-
ing plaque and saliva levels of the agent over time.
Such retention assessments can be misleading because
retention is only one aspect of substantivity and the
measurement techniques do not provide information
on the activity of the retained agents. Moreover, the
buccal retention test does not distinguish drug ab-
sorption from adsorption nor determine how much is
swallowed. Thus, for example, studies using radio-
labeled chlorhexidine purported to demonstrate slow
release from oral surfaces and this occurred over a
protracted period of time. However, saliva derived
from subjects following rinsing with chlorhexidine
only provided antimicrobial activity for up to 3 hours
following rinsing (Addy & Wright 1978). This is
clearly
markedly less than the known substantivity or
persistence of action of chlorhexidine in the mouth of
at
least 12 hours (Schiott et al. 1970). It is likely, there
-
fore, that the initial desorption studies using a radio-
label were merely detecting chlorhexidine adsorbed
to
desquamating oral surfaces, particularly the mu
cosa.
Antimicrobial tests
For antimicrobial agents only, salivary bacterial count
assessments are much more indicative of substantiv-
ity and are predictive of antiplaque action for the same
agents. The method involves measuring salivary bac-
terial counts before and at time points after a single
rinse with the agent (Fig. 22-7) and was first described
for chlorhexidine (Schiott et al. 1970). In the case of
toothpaste, the product can be either brushed or
rinsed as an aqueous slurry (Addy et al, 1983, Jenkins
et al. 1990). Agents and products produce variable
reductions in counts ranging from none, as with water,
to greater than 90% as with chlorhexidine. More im-
portantly, the duration of reduction from baseline var
-
ies from minutes to hours. Thus, povidone iodine only
reduces counts for approximately 1 hour, cetylpyrid-
inium chloride for 3 hours (Roberts & Addy 1981),
whereas chlorhexidine produces such effects for over
12 hours (Schiott et al. 1970). Toothpastes generally
show reductions in counts between 3 and 5 hours,
probably largely due to contained detergents and/or
specific ingredients such as triclosan (Addy et al.
1989).
Experimental plaque studies
Short-term plaque regrowth studies are perhaps the
most commonly used clinical experiments to screen
chemical oral hygiene products. They have the advan
-
tage of assessing the chemical action of the formula-
tion divorced from the indeterminate variable of
toothbrushing. Typically, plaque regrowth from a zero
baseline and the influence of the test agent is recorded.
Originally used for mouthrinses, the method has been
modified for toothpaste by delivering the formulation
in a tray applied to the teeth (Gjermo & Rolla 1970,
1971, Etemadzadeh et al. 1985) or as a slurry rinse
(
Addy et al.1983). Studies are usually cross-over, al-
lowing many formulations to be evaluated against
suitable controls. Study periods range from 24 hours
to several days, usually 4-5 (Harrap 1974, Addy et al.
1983). A negative control such as water and a positive
484 • CHAPTER 22
control such as chlorhexidine may be used (Fig. 22-8).
These help to position the activity of the test formula-
tions between the extremes. Also, because the results
from these controls can be predicted, their use tends
to
confirm or otherwise the conduct of these blind,
randomized study designs.
Experimental gingivitis studies
Experimental gingivitis studies (Loe & Schiott, 1970)
are based on the original experimental gingivitis in
man
protocol first used to demonstrate the direct eti
ological
relationship between plaque and gingivitis
(Loe et al.
1965). This latter original study did not
return subjects
to zero baseline plaque scores or gin
gival health,
whereas most subsequent methods, to evaluate oral
hygiene products, have taken this approach with
baseline parameters. Study periods usu
ally range
from 12, but more particularly 19–28 days. In the
absence of normal tooth cleaning, the development of
plaque and gingivitis are recorded under the
influence
of test and control formulations. Studies
may be either
cross-over or parallel.
Home use studies
For chemical oral hygiene products and usually tooth-
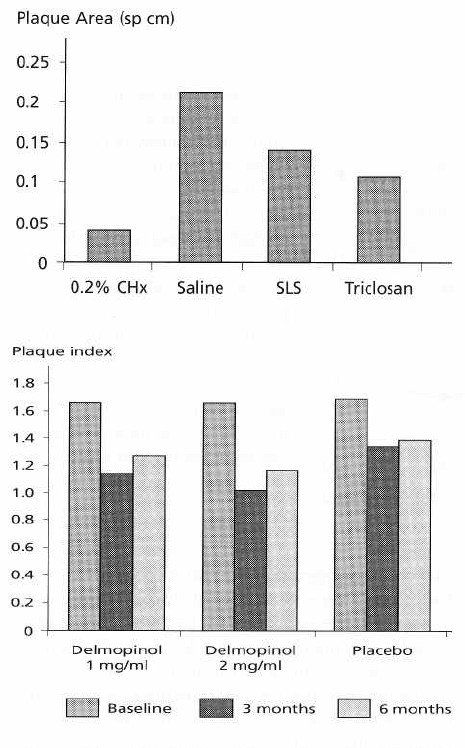
Fig. 22-8. Plaque area following the use of chlor-
hexidine, saline, sodium lauryl sulfate and triclosan
mouthrinses after 4-day periods. Considerable plaque
inhibition was afforded by chlorhexidine (positive con-
trol) when toothcleaning was suspended. Both sodium
lauryl sulfate and triclosan show significant plaque
inhibitory action compared to saline (placebo control)
albeit significantly less than chlorhexidine.
Fig. 22-9. A 6-month study of delmopinol rinses as ad
-
juncts to oral hygiene. Significant improvements in
plaque scores are seen in the delmopinol mouthrinse
groups at 3 and 6 months compared to placebo. A Haw
thorne effect of improved toothcleaning irrespective of
the treatment is apparent in the placebo group, particu
larly at 3 months, although this is still present at 6
months.
pastes and mouthrinses, the final evaluation requires
that they are shown to be effective against plaque and,
more particularly, gingivitis when used along with
normal mechanical tooth cleaning. Studies can be over
days or weeks but usually, in accordance with guide-
lines such as those for the American Dental Associa-
tion (Council of Dental Therapeutics 1985) they need
to
be 6 months or longer, particularly since safety needs
to be assessed (Fig. 22-9). Most studies are par
allel in
design. Protocols have used two approaches.
One is
perhaps more therapeutic in concept whereby
subjects
have to exhibit a certain level of plaque
and/or
gingivitis before entry (Johansen et al. 1975).
The
other is more preventive in concept and there is a
prestudy period in which subjects with gingivitis re-
ceive prophylaxis and instruction to improve their
gingival health. Those satisfactorily responding are
entered, and change in gingival health is monitored in
the test and control groups (Stephen et al. 1990). Sev-
eral factors tend to confound home use studies of oral
hygiene products and may mask a proven chemical
antiplaque action determined from short-term plaque
and experimental gingivitis studies. Most important
is
the so-called Hawthorne effect where subjects
knowingly involved in oral hygiene studies improve
their tooth cleaning (Fig. 22-9). Secondly, baseline pro-
phylaxes are commonly given and the influence of this
on the subsequent gingivitis levels is not known. In
mouthrinse studies used as adjuncts to tooth cleaning,
