Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

THE USE OF ANTIBIOTICS IN PERIODONTAL THERAPY • 495
Fig. 23-1. Conventional periodontal therapy and main
tenance imply repeated treatment of sites with
local
ized unresponsive or recurrent disease,
resulting in
sometimes substantial hard tissue
trauma.
are not completely eradicated, persistence or re-
growth of certain microorganisms in treated sites
should be considered the main cause of unsatisfactory
treatment outcomes.
Can antimicrobial agents, delivered
either locally or systemically, enhance the effect of root
instrumentation, limit its side effects, or could they even be
a substitute in some cases?
ht the late 1930s and early 1940s the appearance of
potent chemotherapeutic agents selectively active
against bacteria, revolutionized the treatment of bac-
terial infections. The discovery of such drugs – sul-
fonamides, penicillin and streptomycin – led many to
believe that bacterial infections were about to vanish.
After six decades of experience with these and hun-
dreds of additionally developed chemotherapeutic
drugs, the potential and limitation of antimicrobial
therapy are better understood. Emerging problems,
resulting from the widespread use of antibiotics have
modified the general perception of the capabilities of
antimicrobial agents. Over the years, bacteria have
developed a remarkable ability to withstand or repel
many antibiotic agents and are increasingly resistant
to formerly potent agents. The use of antibiotics may
disturb the delicate ecologic equilibrium of the body,
allowing the proliferation of resistant bacteria or non-
bacterial organisms. Sometimes this may initiate new
infections that are worse than the ones originally
treated. In addition, no antibacterial drug is absolutely
non-toxic and the use of any antimicrobial agent car-
ries with it accompanying risks.
Before any antimicrobial agent can be recom-
mended for periodontal therapy a number of condi-
tions need to be fulfilled. First, the drug must show
in
vitro
activity against the organisms considered most
important in the etiology of the disease. Next it should
be demonstrated that a dose sufficient to kill the target
organisms can be reached within the subgingival en-
vironment. At this dose the drug should not have
major local or systemic adverse effects. It should be
safe to maintain the required concentration over a
long enough period to significantly affect the micro-
biota. Well-controlled longitudinal studies then need
to be carried out in patients with periodontal disease,
demonstrating a favorable clinical outcome of therapy
with the agent. Finally, one would like to see a practi-
cal advantage over conventional treatment alterna-
tives (better outcome, less adverse effects, simpler to
perform, cheaper, faster).
Specific characteristics of the periodontal
infection
In discussing the potential usefulness of chemo-
therapeutic agents to treat periodontal diseases, one
should be clear that antibiotics and antiseptics can kill
living bacteria, but will neither eliminate calculus nor
remove bacterial debris. The recognition of periodon-
titis as an infection caused by living microorganisms
is thus a fundamental issue for any chemotherapeutic
treatment concept. The term infection refers to the
presence and multiplication of microorganisms in or
on body tissues. The uniqueness of plaque-associated
dental diseases as infections relates to the lack of
massive bacterial invasion of tissues. Although there
is evidence for bacterial penetration in severely dis-
eased periodontal tissues, notably in periodontal ab-
scesses and in acute necrotizing ulcerative lesions
(
Listgarten 1965, Saglie et al. 1982a,b, Allenspach-
Petrzilka & Guggenheim 1983, Carranza et al. 1983),
it has not been generally accepted that true bacterial
invasion (including multiplication of bacteria within
tissues) is crucial for periodontal disease progression.
Bacteria in the subgingival plaque obviously interact
with host tissues even without direct tissue penetra-
tion. Thus, for any antimicrobial agent used in peri-
odontal therapy to have an effect there is the require-
ment that the agent is available at a sufficiently high
concentration not only within, but also in the sub-
gingival environment outside the periodontal tissues
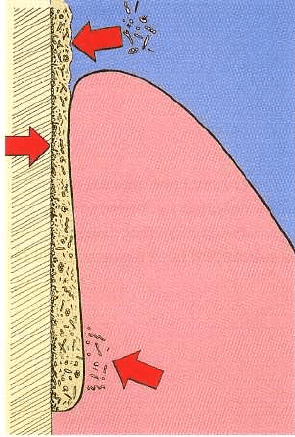
496 • CHAPTER 23
(Fig.
23-2).
Periodontal pockets may actually contain
an enormous amount of bacteria. This may cause
problems for antimicrobial agents to work properly
because they may be inhibited, inactivated or de-
graded by non-target microorganisms.
In addition, the subgingival microbiota accumulate
on the root surface to form an adherent layer of plaque.
Accumulation of bacteria on solid surfaces can be
observed on virtually all surfaces immersed in natural
aqueous environments and is called "biofilm" forma-
tion. Extensive bacterial growth, accompanied by ex-
cretion of copious amounts of extracellular polymers,
is
a typical phenomenon in biofilms. Biofilms effec-
tively protect bacteria from antimicrobial agents (An-
war et al.
1990, 1992). Bacteria involved in adhesion-
mediated infections that develop on permanently or
temporarily implanted materials such as intravascu-
lar catheters, vascular prostheses or heart valves are
notoriously resistant to systemic antimicrobial ther-
apy and tend to persist until the device is removed
(
Gristina
1987,
Marshall
1992).
Several mechanisms
leading to this increased resistance of bacteria in
biofilms have been proposed. Due to limited diffu-
sion, antimicrobial agents may simply not reach
deeper parts of a biofilm at sufficiently high levels
during a given time of exposure. Within biofilms an
unequal distribution of electrical charge may develop.
Intrusion may thus be further complicated in certain
areas of the biofilm depending on the charge of the
penetrating molecule. Because of a limited availability
of nutrients within the biofilm, bacteria may also re-
duce their metabolism, rendering them less suscepti-
ble to killing by agents interfering with protein, DNA
or cell wall synthesis. Most interesting are the results
from recent
in vitro
experiments, indicating that the
attachment of bacteria to surfaces may trigger genes
which activate specific resistance mechanisms. Since
these mechanisms are switched on upon contact, they
may occur already in newly forming, very thin
biofilms (Costerton et al.
1995).
Fig. 23-2. Specific conditions for the use of antimicro
-
bial agents in periodontal therapy. The periodontal
pocket as an open site is subject to recolonization after
therapy (top arrow). The subgingival bacteria are pro
-
tected from antimicrobial agents in a biofilm (middle
arrow). The agent must be available at a sufficiently
high concentration not only within, but also in the sub
-
gingival environment outside, the periodontal tissues
(bottom arrow).
The above described problems suggest that treat-
ment of periodontal diseases by antimicrobial agents
alone will probably not suffice and indicate that me-
chanical instrumentation to disrupt the biofilm and to
remove the bulk of bacterial deposits must precede
antimicrobial therapy.
Infection concepts and treatment goals
Infections may be divided into exogenous and endo-
genous on the basis of the source of the infecting agent.
While
exogenous infections
are caused by organisms
acquired from an external source (primary patho-
gens),
endogenous infections
are caused by organisms
present already in the healthy host (commensal organ
-
isms). Infections caused by endogenous microbes are
called
opportunistic infections
if they occur in the usual
habitat of the organisms. They may be the result of
changing ecologic conditions or may be due to a de-
crease in host resistance. Exogenous infections are
caused by organisms that are not part of the normal
flora. They are transmitted to healthy subjects from
diseased humans or animals, or from carriers that
show no signs of disease. A.
actinomycetemcomitans
and
P.
gingivalis
are regarded as true infectious agents
in
periodontal disease by some researchers. This view
is
based on findings of low prevalence of these micro-
organisms in periodontally healthy individuals in
North America and Europe, evidence for transmis-
sion, such as from parent to child or between spouses,
findings of immune responses towards these bacteria
that markedly exceed those expected for endogenous
infections and on clinical studies showing that A.
actinomycetemcomitans
and
P.
gingivalis
can be elimi-
nated with appropriate mechanical treatment and ad
-
junctive antibiotic therapy (for review see van Winkel-
hoff et al. 1996).
The majority of organisms associated with peri-
odontal disease can, however, also be detected fre-
THE USE OF ANTIBIOTICS IN PERIODONTAL THERAPY • 497
Table 23-1. Comparison of local and systemic antimicrobial therapy
Issue
Systemic administration
Local administration
Drug distribution Wide distribution
Narrow effective range
Drug concentration
Variable levels in different body compartments
High dose at treated site, low levels elsewhere
Therapeutic potential
May reach widely distributed microorganisms May act better locally on biofilm associated
better
bacteria
Problems
Systemic side effects
Reinfection from non-treated sites
Clinical limitations
Requires good patient compliance
Infection limited to the treated site
Diagnostic problems Identification of pathogens, choice of drug
Distribution pattern of lesions and pathogens,
identification of sites to be treated
quently at low numbers in the absence of periodontal
disease, and the view that A.
actinomyceterncomitans
and
P.
gingivalis
cause true infections has been op-
posed on the basis of cross-sectional studies showing
a
high prevalence of the two organisms in certain
populations, particularly those from developing
countries. If
A.
actinomyceterncomitans
and
P.
gingivalis
are truly exogenous pathogens, the elimination of
these organisms should be a primary objective of ther
-
apy. In the therapy of opportunistic infections, how-
ever, elimination is not a realistic goal. Successfully
suppressed putative pathogens may grow back if fa-
vorable ecologic conditions (e.g. deep pockets) persist.
Continuous control of ecologic factors will be neces-
sary after initial treatment. Thus, therapy will not have
an unambiguously defined endpoint.
The periodontal flora never consists of one single
species. Frequently, several potential pathogens can
be identified at the same time, suggesting that inter-
actions between microorganisms play an important
role in the development of periodontal disease. The
pathogenicity of simultaneously present organisms
could be enhanced in an additive or synergistic way.
Antagonistic relationships between microorganisms
could, however, also occur. Based on the concept that
the presence of beneficial bacterial species may sup-
press the pathogenic impact of pathogens, one can
speculate that it may be advantageous to specifically
eliminate target bacteria only and to allow the growth
of potentially beneficial microorganisms. Such con-
templations have been used as an argument to propa
-
gate narrow spectrum antibiotics for periodontal ther-
apy
Drug delivery routes
Antimicrobial agents may be delivered by direct
placement into the periodontal pocket or via the sys-
temic route. Each method of delivery has specific
advantages and disadvantages (Table 23-1). Local
therapy may allow the application of antimicrobial
agents at levels that cannot be reached by the systemic
route. Local therapy may be particularly successful if
the presence of target organisms is confined to the
clinically visible lesions. On the other hand, systemi-
cally administered antibiotics may reach widely dis-
tributed microorganisms. Studies have shown that
periodontal bacteria may be distributed throughout
the whole mouth in some patients (Mombelli et al.
1991a, 1994), including non-dental sites, such as the
dorsum of the tongue or tonsillary crypts (Zambon et
al. 1981, Van Winkelhoff et al. 1988, Muller et al. 1993,
1995, Pavicic et al. 1994). Disadvantages of systemic
antibiotic therapy relate to the fact that the drug is
dissolved by dispersal over the whole body, and only
a small portion of the total dose actually reaches the
subgingival microflora in the periodontal pocket. Ad-
verse drug reactions are a greater concern and more
likely to occur if drugs are distributed via the systemic
route. Even mild forms of unwanted effects may se-
verely decrease patient compliance (Loesche et al.
1993). Local delivery is independent of patient com-
pliance.
Local drug delivery systems are means of drug
application to confined areas. For the treatment of
periodontal disease, local delivery of antimicrobial
drugs ranges from simple pocket irrigation, over the
placement of drug-containing ointments and gels, to
sophisticated devices for sustained release of antibac
-
terial agents. In order to be effective, the drug should
not only reach the entire area affected by the disease,
including the base of the pocket, but should also be
maintained at a sufficiently high local concentration
for some time. With a mouthrinse or supragingival
irrigation it is not possible to predictably deliver an
agent to the deeper parts of a periodontal defect (
Pitcher et al. 1980, Eakle et al. 1986). Agents brought
into periodontal pockets by subgingival irrigation are
washed out rapidly by the gingival fluid. Based on an
assumed pocket volume of 0.5 ml and a gingival fluid
flow rate of 20 µl/h, Goodson (1989) estimated that
the half-time of a non-binding drug placed into a
pocket is about 1 minute. Even a highly concentrated,
highly potent agent would thus be diluted below a
minimal inhibitory concentration (MIC) for oral mi-
croorganisms within minutes. If an agent can bind to
surfaces and be released in active form, a prolonged
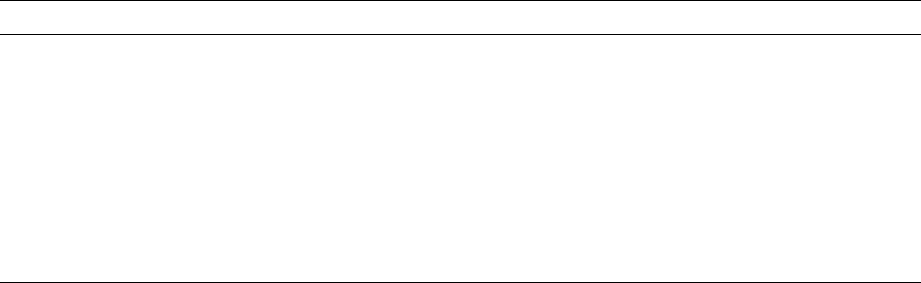
498 • CHAPTER
23
Fig. 23-3. An antimicrobial gel is applied with a syringe inserted into a residual pocket (a). For retention of the
agent in the site, the viscosity of the carrier should change immediately. A large portion of the product may
other
-wise be expelled from the pocket quickly (b).
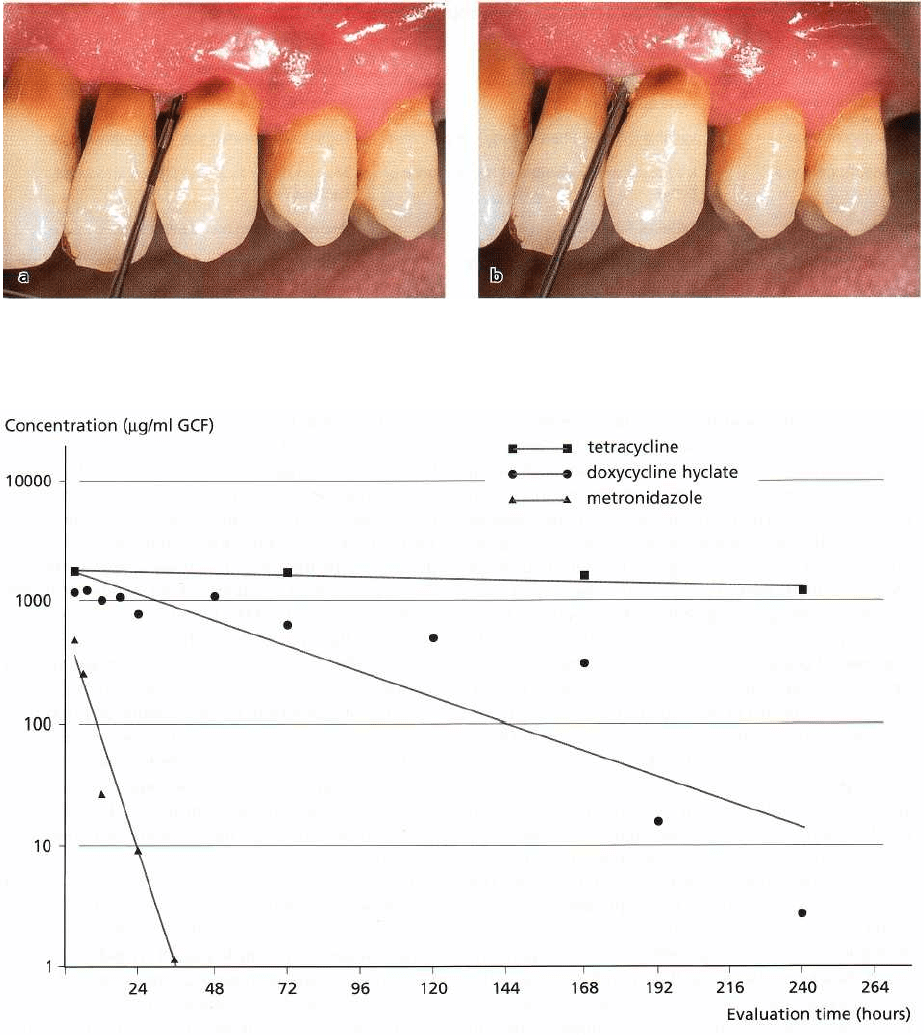
Fig. 23-4. Mean concentration of tetracycline in gingival crevicular fluid (GCF) during tetracycline fiber treatment
(Tonetti et al. 1990), of doxycycline hyclate after application in a biodegradable polymer (Stoller et al. 1998), and of
metronidazole after application of 25% metronidazole dental gel (Stoltze 1992).
time of antibacterial activity could be expected. Such
an effect has in fact been noted for salivary concentra-
tions of chlorhexidine after use of chlorhexidine
mouthrinse (Bonesvoll & Gjermo 1978). Although
there
are indications that this may also occur to a
certain
extent within the periodontal pocket, for in-
stance after
prolonged subgingival irrigation with tet
racycline (
Tonetti et al. 1990), the potential to create a
drug
reservoir of significant size on the small surface area
available in a periodontal pocket is limited. To
maintain a high concentration over a prolonged pe
riod
of time, the flushing action of the crevicular fluid
flow has to be counteracted by a steady release of the
drug from a larger reservoir. Considering the small
volume of a periodontal pocket and the pressure ex-
erted by the tonus of the periodontal tissues on any-
thing inserted, it appears unlikely that this task can be
completed by a carrier that does not maintain its
physical stability for some time and that cannot be
secured against premature loss. Gels, for instance,
rapidly disappear after instillation into periodontal
pockets (Fig. 23-3a,b), unless they change their viscos-
ity immediately after placement (Oostervaal et al.
1990,
Stoltze 1995). Viscous and/or biodegradable de-
THE USE OF ANTIBIOTICS IN PERIODONTAL THERAPY •
499
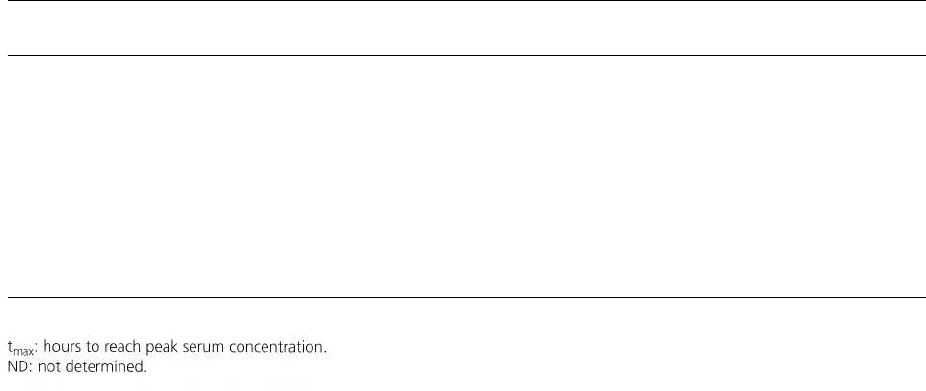
Table 23-2. Characteristics of antimicrobial agents used in the treatment of periodontal disease
(adapted from Lorian 1986, Slots & Rams 1990)
Antimicrobial agent
Dose
c Serum (pg/ml)c Crevicular fluid
tmax Serum (h)
Half-life (h)
(mg) (pg/ml)
Penicillin
500
3
ND
1
0.5
Amoxicillin
500 8
3-4
1.5-2 0.8-2
Doxycycline
200
2-3 2-8
2
12-22
Tetracycline
500
3-4
5-12
2-3 2-3
Clindamycin
150
2-3 1-2
1
2-4
Metronidazole
500
6-12 8-10
1-2
6-12
Ciprofloxacin
500
1.9-2.9
ND
1-2 3-6
c
concentration.
vices show an exponential decrease of their concentra
-
tion in gingival fluid. Depending on the physical and
chemical nature of the carrier, the drug reservoir in the
periodontal pocket will be depleted within hours to
days after placement. Controlled delivery of an an-
timicrobial agent over several days has been shown
for tetracycline released from non-degradable mono-
lithic ethylene vinyl acetate fibers (Fig. 23-4).
EVALUATION OF
ANTIMICROBIAL AGENTS FOR
PERIODONTAL THERAPY
In the large range of antimicrobial agents, a limited
number have been tested thoroughly for use in peri-
odontal therapy. The drugs more extensively investi-
gated for systemic use include tetracycline, minocy-
cline and doxycycline, erythromycin, clindamycin,
ampicillin, amoxicillin, and the nitro-imidazole com-
pounds metronidazole and ornidazole. The drugs in-
vestigated for local application include tetracycline,
minocycline, doxycycline, metronidazole and chlor-
hexidine.
The first antibiotics used in periodontal therapy
were mainly systemically administered penicillins.
The choice was initially based on empirical evidence
exclusively. Penicillins and cephalosporins act by in-
hibition of cell wall synthesis. They are narrow-spec-
trum and bactericidal. Among the penicillins,
amoxicillin has been favored for treatment of peri-
odontal disease because of its considerable activity
against several periodontal pathogens at levels avail-
able in gingival fluid. The molecular structure of peni-
cillins includes a f3-lactam ring that may be cleaved by
bacterial enzymes. Some bacterial f3-lactamases have
a high affinity for clavulanic acid, a 13-lactam molecule
without antimicrobial activity. To inhibit bacterial [3-
lactamase activity, clavulanic acid has been added
successfully to amoxicillin. This combination (Aug-
mentin
®
) has been tested for periodontal therapy in
clinical studies. Tetracycline-HC1 became popular in
the 1970s due to its broad spectrum antimicrobial
activity and low toxicity. The tetracyclines, clindamy-
cin and erythromycin are inhibitors of protein synthe
-
sis. They have a broad-spectrum of activity and are
bacteriostatic. In addition to their antimicrobial effect,
tetracyclines are capable of inhibiting collagenase
(
Golub et al. 1985). This inhibition may interfere with
tissue breakdown in periodontal disease. Further-
more they bind to tooth surfaces, from where they may
be released slowly over time (Stabholz et al. 1993). The
nitro-imidazoles (metronidazole and ornidazole) and
the quinolone antibiotics (e.g. ciprofloxacin) act by
inhibiting DNA synthesis. Metronidazole is known to
convert into several short-lived intermediates after
diffusion into an anaerobic organism. These products
react with the DNA and other bacterial macromole-
cules, resulting in cell death. The process involves
reductive pathways characteristic of strictly anaerobic
bacteria and protozoa, but not aerobic or microaero-
philic organisms. Thus, metronidazole affects specifi-
cally the obligately anaerobic part of the oral flora,
including P.
gingivalis
and other black-pigmenting
Gram-negative organisms, but not A.
actinomycetem-
comitans,
a facultative anaerobe.
The concentrations following systemic administra-
tion of the most common antimicrobial agents used in
the treatment of periodontal disease are listed in Table
23-2. The
in vitro
susceptibility of A.
actinomycetem-
comitans
to selected antimicrobial agents is given in
Table 23-3 and the susceptibility of P.
gingivalis
is listed
in Table 23-4. The data given in these tables may serve
as a base for the choice of an appropriate agent. How-
ever, it is important to remember that
in vitro
tests do
not reflect the true conditions found in periodontal
pockets. In particular, they do not account for the
biofilm effect. One should add that MIC values de-
500 • CHAPTER
23
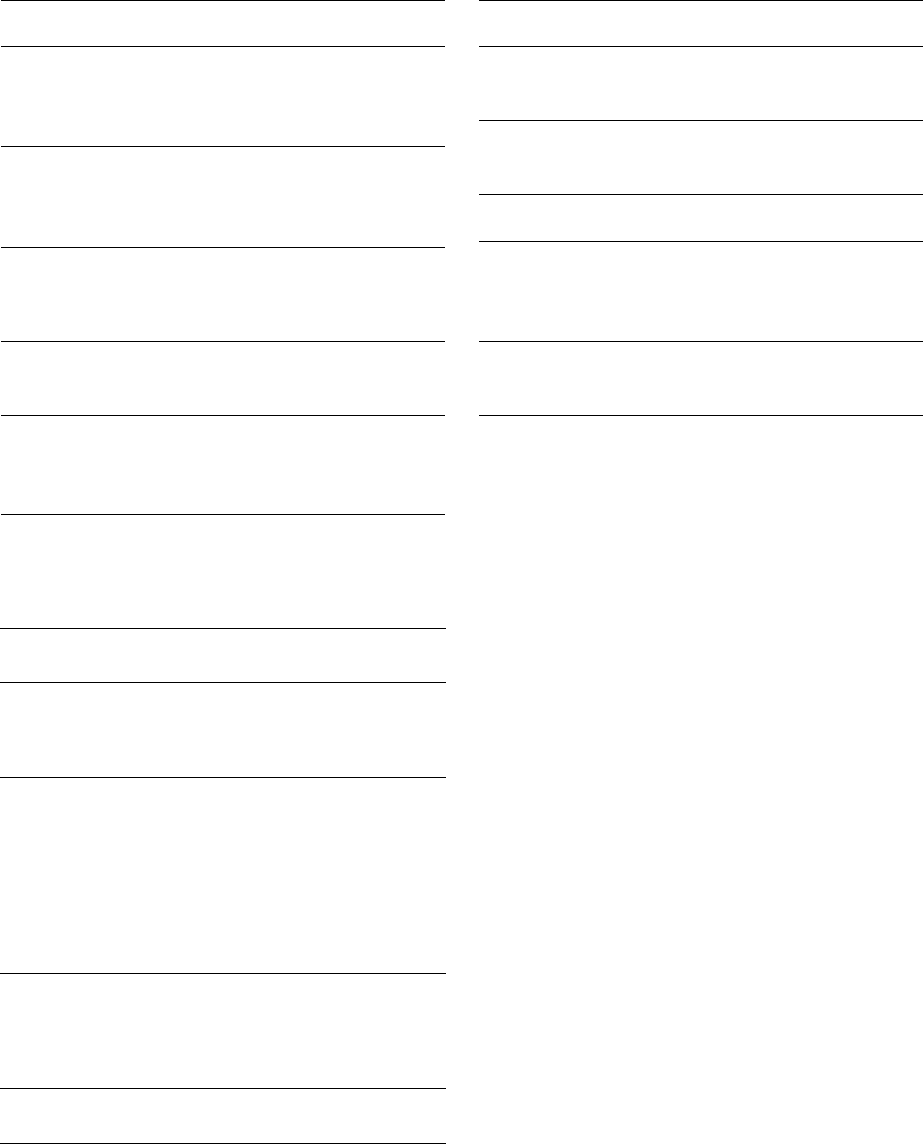
Table 23-3. Susceptibility of A.
actinomycetemcomi-
tans
to selected antimicrobial agents. MIC90: mini-
mal inhibitory concentration for 90% of the strains
(
adapted from Mombelli & van Winkelhoff 1997)
Antimicrobial
MIC90
Reference
agent
pg/ml
Penicillin
4.0
Pajukanta et al. (1993b)
1.0
Walker et al. (1985)
6.25
Hoffler et al. (1980)
Amoxicillin
1.0
Pajukanta et al. (1993b)
2.0
Walker et al. (1985)
1.6
Hoffler et al. (1980)
Tetracycline
0.5
Pajukanta et al. (1993b)
8.0
Walker et al. (1985), Walker
(1992)
Doxycycline
1.0
Pajukanta et al. (1993b)
3.1
Hoffler et al. (1980)
Metronidazole
32
Pajukanta et al. (1993b)
32
Jousimies-Somer et al. (1988)
12.5
HOffler et al. (1980)
Table 23-5. Adverse effects of antibiotics used in the
treatment of periodontal diseases
Antimicrobial
agent
Frequent effects
Infrequent effects
Penicillin
hypersensitivity
(mainly rashes),
nausea, diarrhea
hematological toxicity,
encephalopathy,
pseudomembranous
colitis (ampicillin)
Tetracycline
gastrointestinal
intolerance,
Candidiasis, dental
staining and
hypoplasia in
childhood, nausea,
diarrhea, interaction
with oral
contraceptives
photosensitivity,
nephrotoxicity,
intracranial
hypertension
Metronidazole
gastrointestinal
intolerance, nausea,
antabus effect,
diarrhea, unpleasant
metallic taste
peripheral neuropathy,
furred tongue
Clindamycin
rashes, nausea,
diarrhea
pseudomembranous
colitis, hepatitis
pend on technical details that may vary between labo
-
ratories. As a consequence, demonstration of
in vitro
susceptibility is no proof that an agent will work in
treatment of periodontal disease.
Since the subgingival microbiota in periodontitis
often harbors several putative periodontopathic spe-
Table 23-4. Susceptibility of
P.
gingivalis
to selected
antimicrobial agents (adapted from Mombelli & van
Winkelhoff 1997)
Antimicrobial
MIC90
Reference
agent
pg/ml
Penicillin
0.016
Pajukanta et al. (1993a)
0.29
Baker et al. (1983)
Amoxicillin
0.023
Pajukanta et al. (1993a)
< 1.0
Walker (1992)
Doxycycline
0.047
Pajukanta et al. (1993a)
Metronidazole
0.023
Pajukanta et al. (1993a)
2.1
Baker et al. (1983)
2.0
Walker (1992)
Clindamycin
0.016
Pajukanta et al. (1993a)
< 1.0
Walker (1992)
cies with different antimicrobial susceptibility, combi-
nation drug therapy may be useful. A combination of
antimicrobial drugs may have a wider spectrum of
activity than a single agent. Overlaps of the antimicro
-
bial spectrum may reduce the possible development
of bacterial resistance. For some combinations of
drugs there may be synergy in action against target
organisms, allowing a lower dose of the single agents.
A synergistic effect against A.
actinomyceternconhitans
has been noted
in vitro
between metronidazole and its
hydroxy metabolite (Jousimies-Sourer et al. 1988,
Pavicic et al. 1991) and between these two compounds
and amoxicillin (Pavicic et at. 1992). With some drug
combinations there may, however, also be antagonistic
drug interaction. For instance, bacteriostatic agents
such as tetracyclines, which suppress cell division,
decrease the antimicrobial effect of bactericidal antibi
-
otics such as (3-lactam drugs or metronidazole, which
act during bacterial cell division. Combination drug
therapy may also lead to increased adverse reactions.
Table 23-5 lists common adverse reactions to sys-
temic antibiotic therapy (for a detailed overview the
reader is referred to Walker 1996). The penicillins are
among the least toxic antibiotics. Hypersensitivity re-
actions are by far the most important and most com-
mon adverse effects of these drugs. Most reactions are
mild and limited to a rash or skin lesion in the head or
neck region. More severe reactions may induce swel-
ling and tenderness of joints. In highly sensitized
patients a life-threatening anaphylactic reaction may
develop. The systemic use of tetracyclines may lead to
epigastric pain, vomiting or diarrhea. Tetracyclines
can induce changes in the intestinal flora, and super-
infections with non-bacterial microorganisms (i.e.
Candida albicans)
may emerge. Tetracyclines are depos
-
ited in calcifying areas of teeth and bones where they
THE USE OF ANTIBIOTICS IN PERIODONTAL THERAPY • 501
cause yellow discoloration. Systemic administration
of clindamycin may be accompanied by gastrointesti
-
nal disturbances, leading to diarrhea or cramps and
may cause mild skin rashes. The suppression of the
normal intestinal flora increases the risk for coloniza
-
tion of
Clostridium difficile,
which may cause a severe
colon infection. Although not related to C.
difficile,
gastrointestinal problems are also the most frequent
adverse event of systemic metronidazole therapy.
Nausea, headache, anorexia, vomiting may be experi
-
enced. Symptoms may be more pronounced with al-
cohol consumption, because imidazoles affect the ac
-
tivity of liver enzymes. Because some cases have de-
veloped permanent peripheral neuropathies (numb-
ness or paresthesia), patients should be advised to
immediately stop therapy if such symptoms occur.
Systemic antimicrobial therapy in clinical
trials
As mentioned before, the ultimate evidence for the
efficacy of systemic antibiotics must be obtained from
treatment studies in humans with periodontitis. Be-
cause compromises are often necessary to conduct an
investigation under given clinical, technical or finan-
cial circumstances, a large number of studies reported
in the literature can be criticized using the basic study
quality criteria for evidence-based medicine. Studies
may be difficult to interpret and compare due to an
unclear status of patients at baseline (treatment his-
tory, disease activity, composition of subgingival mi-
crobiota), insufficient or non-standardized mainte-
nance after therapy, short observation periods, or lack
of randomization and controls. Studies not only vary
with regard to the treatment provided, but also in the
selection of subjects, sample size, range of study pa-
rameters, outcome variables, the duration of the study,
and the controls to which the test procedure is com-
pared. In most studies, systemic antibiotics have been
used as an adjunct to scaling and root planing. Typi-
cally, the effect of mechanical therapy plus the antimi
crobial agent has been compared to mechanical treat
-
ment alone. In studies evaluating the effect of antimi
-
crobial therapy in patients with refractory periodon-
titis or with recurrent abscess formation, placebo con
-
trol is often lacking for ethical reasons.
Chronic and
aggressive
periodontitis in
the adult
patient
Several case reports claimed clinical success with
tet-
racycline
therapy; however, in placebo controlled tri-
als, only slight differences were noted in the change of
mean probing depths and attachment levels between
patients receiving tetracycline or placebo as an ad-
junct to mechanical therapy (Listgarten et al. 1978,
Hellden et al. 1979, Slots et al. 1979, Scopp et al. 1980,
Lindhe et al. 1983).
Systemic
metronidazole
in conjunction with scaling
and root planing yielded slightly better results than
scaling alone (Clark et al. 1983, Lekovic et al. 1983,
Lindhe et al. 1983, Joyston-Bechal et al. 1984, 1986,
Loesche et al. 1984, Soder et al. 1990). No improvement
in clinical effects of therapy was found in patients
receiving metronidazole in conjunction with peri-
odontal surgery over surgery alone (Sterry et al. 1985,
Mahmood & Dolby 1987). In rapidly progressive pe-
riodontitis, metronidazole seemed to improve clinical
conditions for up to 6 months compared to periodon-
tal scaling alone (Soder et al. 1989, 1990).
A limited number of patients with advanced adult
periodontitis were treated with
clindamycin
without
concurrent mechanical therapy, except oral hygiene
instructions. Despite this limited treatment regimen,
clinical and microbiologic parameters improved over
the following 6 months.
P.
gingivalis
appeared to be
particularly susceptible to clindamycin therapy; this
species was no longer detected at 6 months. Other
organisms, however, were resistant to clindamycin
and were found at slightly higher levels after 6 months
(Ohta et al. 1986).
Haffajee et al. (1995) examined the effects of sys-
temic amoxicillin plus clavulanic acid, tetracycline
HC1, or placebo therapy, given for 30 days in conjunc
-
tion with subgingival debridement and modified
Widman flap surgery, in adults with progressive peri-
odontitis. Significantly greater mean gains in clinical
periodontal attachment and decreased probing
depths
were measured at 10 months post-treatment in
subjects receiving adjunctive systemic amoxicillin
plus clavulanic acid or tetracycline therapy as com-
pared to the placebo regimen. Although decreases in
numbers of subgingival putative periodontal patho-
gens paralleled the marked clinical improvements, no
pathogen was eliminated from the subgingival micro-
biota.
Metronidazole plus amoxicillin, Augmentin or
ciprofloxacin have been used successfully in the treat
-
ment of advanced A.
actinomycetemcomitans
associated
periodontitis (Christersson et al. 1989, Kornman et al.
1989, van Winkelhoff et al. 1989, 1992, Goene et al.
1990, Pavicic et al. 1992, 1994, Rams et al. 1992). These
combinations markedly suppressed or even elimi-
nated A.
actinomycetemcomitans
and other subgingival
organisms from periodontitis lesions and other oral
sites.
Serial drug regimens studied to date in periodon-
tics include systemic
doxycycline
administered in-
itially and followed by either
amoxicillin
plus clavu-
lanic acid or
metronidazole
(Aitken et al. 1992, Matisko
& Bissada 1992). The sequential use of drugs over-
comes the potential risk of antagonism between bac-
teriostatic and bactericidal antibiotics. The value of
serial antibiotic therapy in the management of ad-
vanced periodontitis merits further investigation.
Conclusion
In general, adult periodontitis can and should be
treated without systemic antibiotics. The adjunctive
use of antibiotics in adult periodontitis should be
502 • CHAPTER 23
limited to cases with advanced or progressive disease.
A. actinornycetemcomitans
associated periodontitis
may
be treated with adjunctive metronidazole plus
amoxicillin.
"Refractory" periodontitis
A beneficial effect of
tetracycline
therapy was reported
in several studies in adult patients with "refractory"
periodontitis. Double blind studies showed signifi-
cant reductions in probing pocket depth with systemic
tetracycline (Rams & Keyes 1983, McCulloch et al.
1989, 1990).
McCulloch et al. (1990) selected patients on the
basis of periodontal disease activity; active disease
was defined as loss of clinical attachment or abscess
formation. In this study,
doxycycline,
administered for
3 weeks, reduced probing pocket depths significantly
more than placebo and resulted in more gain of clinical
attachment, however, only at sites with evidence of
recent disease activity. The doxycycline regimen re-
duced the relative risk for subsequent periodontal
breakdown over a 7-month period by 43%. Even
though adjunctive doxycycline therapy was effective
in some active periodontitis patients, it failed to pre-
vent periodontal breakdown in 13 of 29 individuals.
Recurrent disease activity after systemic tetracycline
therapy was also reported by other authors (van
Winkelhoff et al. 1989, Goene et al. 1990, Aitken et al.
1992, Walker et al. 1993). Disease progression may
have been caused by subgingival organisms not suffi-
ciently suppressed by the doxycycline therapy. A.
act-
inomycetemcomitans
and E.
corroders
were not mark-
edly suppressed in the doxycycline treated patients
(
McCulloch et al. 1990) and the difference in the com-
position of the subgingival microbiota between the
placebo and doxycycline patients was not significant
after 7 months (McCulloch et al. 1990, Kulkarni et al.
1991). Other studies have also pointed to a possible
failure of systemic tetracycline in the suppression of
subgingival A.
actinornycetemcomitans
(Muller et al.
1989, 1993, van Winkelhoff et al. 1989). In some in-
stances an increase of this organism was even noted
(
Muller et al. 1990). Furthermore, systemic tetracy-
cline therapy may lead to colonization of superinfect-
ing and opportunistic pathogens (Bragd et al. 1985,
Haffajee et al. 1988, Hull et al. 1989). It may thus be
speculated that non-antimicrobial effects, such as col
-
lagenase inhibition, may have been more important
for the observed outcome than the antibiotic action
(
Golub et al. 1983, 1985).
Systemic
clindamycin
and scaling yielded a signifi-
cant improvement in patients treated for active peri-
odontal disease despite previous conventional peri-
odontal treatment and systemic tetracycline therapy
(
Gordon et al. 1985). The clinical effects were associ-
ated with significant reductions of spirochetes, motile
rods and Gram-negative anaerobic rods, including P.
gingivalis
and
P. intermedia,
and an increase of Gram-
positive rods and cocci over 1-2 years (Walker & Gor-
don 1990). However, clindamycin did not perma
nently suppress subgingival
P. gingivalis,
which may
explain the recurrence of disease activity in some pa-
tients. It should also be mentioned that one study
patient developed pseudomembraneous colitis, a gas
-
trointestinal superinfection by
Clostridium difficile
which may be life-threatening.
In one study, refractory adult periodontitis patients,
who in the past had been subjected to periodontal
surgery, systemic tetracycline administration and sup
portive periodontal therapy, were retreated with
scal
ing and root planing in conjunction with either
clin
damycin or amoxicillin plus clavulanic acid (
Magnus
-
son et al. 1994). The two systemic
antimicrobial therapies were prescribed on the basis
of the susceptibility
of the whole subgingival
microbiota (Walker et al.
1983). Over a 2-year
evaluation period, the difference
in the proportions of
sites losing attachment following
either clindamycin
or amoxicillin plus clavulanic acid
therapy was not
significant.
Statistically significant improvements in clinical
parameters have also been observed after mechanical
debridement combined with systemic metronidazole
and ornidazole in patients with recurrent periodontal
disease (Gusberti et al. 1988, Mombelli et al. 1989).
Conclusion
Various antibiotic regimens have been tested for the
treatment of patients not responding to conventional
periodontal therapy. Favorable short term effects have
been reported; however, a great variability in treat-
ment response among patients has been noted. Re-
emergence of putative pathogens has been observed
and has been considered the reason for recurrence of
disease.
Aggressive
localized periodontitis
Reduced gingival inflammation, gain of clinical at-
tachment and alveolar bone were reported following
tetracycline therapy in juvenile patients with aggres-
sive localized periodontitis (Slots et al. 1979, Genco et
al. 1981, Lindhe 1982, Slots & Rosling 1983). However,
as many as 25% of juvenile periodontitis patients
treated with tetracycline may experience a reactiva-
tion of disease, even if their dentition is professionally
cleaned every 3 months after therapy (Lindhe 1982).
Further destruction of periodontal attachment was
noted in sites with high post-treatment levels of A.
actinomyceternconzitans
(Slots & Rosling 1983, Korn-
man & Robertson 1985, Mandell & Socransky 1988,
Asikainen et al. 1990, Saxen & Asikainen 1993). Sup
-
pression of A.
actinornycetemcomitans
does not seem
possible in all aggressive localized periodontitis le-
sions with scaling and root planing and adjunctive
systemic tetracycline.
Suppression of A.
actinornycetemcomitans
has been
reported for up to 18 months in juvenile patients with
aggressive localized periodontitis after mechanical
debridement plus metronidazole (200 mg TID, 10
days). In comparison, systemic tetracycline therapy or
mechanical treatment alone eliminated A.
actinomy-
THE USE OF ANTIBIOTICS IN PERIODONTAL THERAPY • 503
cetemcomitans
in only 44% and 67% of the juvenile
periodontitis patients, respectively (Saxen & Asi-
kainen 1993). Considering the limited effect of
metronidazole on facultative organisms, documented
in
in vitro
susceptibility tests (Walker et al. 1985), these
results are quite surprising. The hydroxy metabolite
of metronidazole may be responsible for the suppres-
sion of subgingival
A. actinomycetemcomitans
(Jousim-
ies-Somer et al. 1988, Pavicic et al. 1992). The success
ful use of metronidazole plus amoxicillin in the
treat
ment of various cases with advanced A.
actinomy-
cetemcomitans
associated periodontitis suggests the
adjunctive use of this combination may also be a good
choice for aggressive localized periodontitis in juve-
nile patients (Christersson et al. 1989, Kornman et al.
1989, van Winkelhoff et al. 1989, 1992, Goene et al.
1990, Pavicic et al. 1994).
Conclusion
Several regimens including the adjunctive admini-
stration of tetracyclines or metronidazole have been
tested for the treatment of localized juvenile periodon
-
titis. Again, re-emergence of putative pathogens, in
this case of A.
actinonnycetenrcomitans,
has been ob-
served and has been considered the reason for recur
-
rence of disease. Metronidazole in combination with
amoxicillin may suppress A.
actinoniycetemcomitans
more efficiently than single antibiotic regimens.
Implications for clinical
practice
Overall, it can be stated that systemic antibiotic ther
-
apy may improve the microbiologic and clinical con-
ditions of periodontal patients under certain circum-
stances. Monotherapy with systemic antibiotics as an
adjunct to mechanical periodontal treatment can sup
-
press the total subgingival bacterial load and may
induce a significant change in the composition of the
subgingival microbiota. However, antibiotic therapy
with single antimicrobial agents cannot predictably
eliminate periodontal organisms such as A.
actinomy-
cetemcomitans.
To
reach this goal, combination therapy,
i.e. metronidazole plus amoxicillin, seems to be more
appropriate. There is evidence to support the use of
systemic antibiotic therapy in cases of
P.
gingivalis
and/or A.
actinann/cetemcomitans
associated early on-
set forms of periodontitis. Systemic antibiotic therapy
is also indicated in generalized refractory periodonti-
tis patients with evidence of ongoing disease despite
optimal mechanical therapy.
It is biologically sound and good medical practice
to base systemic antimicrobial therapy on appropriate
microbiologic data. In addition, antibiotics should not
be administered systemically before completion of
thorough mechanical debridement (patients with
acute signs of disease such as periodontal abscesses,
or acute necrotizing gingivitis, with fever and malaise,
may be the exception). Therefore, in most cases, the
initial mechanical therapy should be carried out and
evaluated before microbiological testing. The original
treatment plan, may be modified six to twelve weeks
after initial therapy, taking into account how the peri
-
odontal tissues reacted to the non-specific reduction
of the bacterial mass by root instrumentation and oral
hygiene. Microbial samples from the deepest pocket
in each quadrant can give a good picture of the pres-
ence and relative importance of putative pathogens in
the oral flora (Mombelli et al. 1991b, 1994). Microbial
testing should be comprehensive and sensitive
enough to determine the presence and relative pro-
portion of the most important periodontal organisms.
Since the antimicrobial profiles of most putative peri-
odontal pathogens are quite predictable, susceptibil-
ity testing is not routinely performed. One should
keep in mind, however, that some important microor
-
ganisms may demonstrate resistance to tetracyclines,
(3-lactam drugs or metronidazole. It is recommended
to start drug administration immediately following a
mechanical re-instrumentation. In practical terms this
often means that the patient commences antibiotic
therapy in the evening after the last surgical proce-
dure. Even if no further mechanical therapy is indi-
cated from a clinical point of view, the pockets should
be re-instrumented to reduce the subgingival bacterial
deposits as much as possible and to disrupt the sub-
gingival biofilm.
After resolution of the periodontal infection, the
patient should be placed on an individually tailored
maintenance care program. Optimal plaque control by
the patient is of paramount importance for a favorable
clinical and microbiologic response to systemic an-
timicrobial therapy (Kornman et al. 1994).
Local antimicrobial therapy in clinical trials
A variety of methods to deliver antimicrobial agents
into periodontal pockets have been devised and sub-
jected to numerous kinds of experiments. The phar-
macokinetic shortcomings of rinsing, irrigating and
similar forms of drug placement, and the lack of sig-
nificant clinical effects have already been discussed.
This section will deal with clinically tested drug de-
livery systems that fulfill at least the basic pharmacok
inetic requirements of sustained drug release. Much
of what has been stated about difficulties in the inter
-
pretation of studies dealing with the systemic use of
antibiotics applies to the studies conducted with local
delivery devices. Again, comparison of various forms
of therapy is complicated because studies vary with
regard to sample size, selection of subjects, range of
parameters, controls, duration of the study, and the
inclusion of only one form of local drug delivery. Most
of the evidence for a therapeutic effect of local delivery
devices comes from trials involving patients with pre-
viously untreated adult periodontitis. Some protocols
compare local drug delivery to a negative control,
such as the application of only the carrier without the
drug. These studies may be able to show a net effect
of the drug, but they are not able to demonstrate a
benefit over the most obvious alternative – scaling and
504
• CHAPTER 23
root planing — and the question remains as to how
much value the procedure has
in addition
to mechani-
cal treatment. If a study is unable to demonstrate a
significant difference between local drug delivery and
scaling and root planing, this is not automatically a
proof of equivalence of the two treatments. Equiva-
lence testing requires statistical testing of the power of
the data, taking into account the size of the study
sample. Only very few studies have addressed the use
of local drug delivery in recurrent or persistent peri-
odontal lesions — the potentially most valuable area
for their application. The following paragraphs dis-
cuss the predominant products available today for
local antimicrobial therapy. Further developments
currently under
way
will undoubtedly lead to the
commercial availability of additional products in the
future.
Minocycline ointment and microspheres
A 2%
minocycline
ointment (Dentomycin; Cyanamid,
Lederle Division, Wayne, NJ, US) has been distributed
commercially in some countries for several years. The
efficacy of this product has been assessed in a series of
clinical trials mainly conducted in adult patients in
Japan. In a randomized, double-blind study of 103
adults with moderate to severe periodontitis, the
safety and efficacy of subgingivally applied 2% mino-
cycline ointment was tested at four Belgian universi-
ties (van Steenberghe et al. 1993). All patients were
treated by conventional scaling and root planing at
baseline. In addition, the patients received either the
test or a control ointment in four consecutive sessions
at an interval of two weeks (baseline and 2, 4, 6).
Assessment of clinical response was made at weeks 4
and 12. A significantly greater reduction of probing
depth was observed in the test group. An additional
multicenter study evaluated the long-term safety and
efficacy of subgingivally administered minocycline
ointment when used intermittently as an adjunct to
subgingival debridement in chronic periodontitis.
The
repeated subgingival administration of minocycline
ointment yielded an adjunctive improvement
after
subgingival instrumentation in both clinical and
microbiologic variables over a 15-month period (van
Steenberghe et al. 1999). One study assessed the effect
of a weekly repeated local application of minocycline
ointment for 8 weeks after placement of expanded
polytetrafluoroethylene membranes to guide regen-
eration of periodontal tissue. Although bacterial colo-
nization of treated sites could not be prevented, the
mean clinical attachment gain of the test group was
significantly greater than that of the control group
(
Yoshinari et al. 2001).
The subgingival delivery of minocycline in differ
ent
forms, for example in bioabsorbable 10% minocy-
cline-loaded microcapsules, has also been investi-
gated. Proof of principle studies involving relatively
small numbers of patients with chronic periodontitis
indicated that such local subgingival delivery systems
may reduce bleeding on probing better than scaling
and root planing alone and may induce a microbial
response more favorable for periodontal health than
scaling and root planing (Yeom et al. 1997). The effi-
cacy and safety of locally administered microencapsu
-
lated minocycline was assessed in a multicenter trial
including 748 patients with moderate to advanced
periodontitis. Minocycline microspheres plus scaling
and root planing provided substantially more probing
depth reduction than either scaling and root planing
alone or SRP plus vehicle. The difference reached
statistical significance after the first month and was
maintained throughout the 9 months of the trial (Wil
-
liams et al. 2001).
Doxycycline hyclate
in a
biodegradable polymer
A two syringe mixing system for the controlled release
of doxycycline
(Atridox; Block Drug, Jersey City, NJ,
US) has been evaluated in a number of investigations,
and has become available commercially in several
countries. Syringe A contains the delivery vehicle,
which is a bioabsorbable, flowable polymeric formu-
lation composed of poly(DL-lactide) dissolved in N-
methyl-2-pyrrolidone, and syringe B contains 50 mg
of doxycycline hyclate. The constituted product con-
tains 10% w/w doxycycline hyclate. The clinical effi-
cacy and safety of Atridox was compared to placebo
control, oral hygiene, and scaling and root planing in
two multicenter studies. Each study entered 411 pa-
tients who demonstrated moderate to severe adult
periodontitis. Comparisons showed the treatment to
be statistically superior to placebo control and oral
hygiene and equally effective as scaling and root plan
-
ing in reducing the clinical signs of adult periodontitis
over a 9-month period (Garrett et al. 1999). Clinical
changes resulting from local delivery of doxycycline
hyclate or traditional scaling and root planing were
evaluated in a group of patients undergoing suppor-
tive periodontal therapy. Attachment level gains and
probing depth reductions were similar at 9 months
after therapy (Garrett et al. 2000).
The effect of Atridox, applied after no more than 45
minutes of debridement without analgesia in subjects
with moderately advanced chronic periodontitis, was
compared to 4 hours of thorough deep scaling and
root planing in a study involving 105 patients at three
centers. Interestingly clinical parameters indicated a
better result for the pharmaco-mechanical treatment
approach after 3 months, although considerably less
time had been invested than for conventional me-
chanical therapy (Wennstrom et al. 2001).
Metronidazole gel
Dialysis tubing, acrylic strips, and poly-OH-butyric
acid strips have been tested as solid devices for deliv
-
ery of
metronidazole.
The most extensively used device
for metronidazole application is a gel consisting of a
semi-solid suspension of 25% metronidazole benzoate
in a mixture of glyceryl mono-oleate and sesame oil
(
Elyzol Dental Gel; Dumex, Copenhagen, Denmark).
Applied with a syringe inserted into the pocket, the
