Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

21.3 Neighboring Systems 1099
CH
3
H
3
C
H
H
OTs
CH
3
H
3
C
H
H
OAc
(AcOH)
CH
3
COOH
PROBLEM 21.16 Work through the reaction of the (R,R) optically active isomer of
3-phenyl-2-butyl tosylate in acetic acid (see below). This time the product is the
optically active acetate shown.
HO
HO
+
+
C
H
C
H
..
FIGURE 21.29 Two examples of carbon–carbon double bonds acting as intramolecular
nucleophiles.
21.3b Carbon–Carbon Double Bonds as Neighboring Groups
Carbon–carbon double bonds are also nucleophiles and can act as neighboring
groups in intramolecular displacement reactions. Among the examples you already
know are the sequence of intramolecular carbocation additions leading to steroids
(p. 560) and intramolecular carbene additions (p. 434). In each of these reactions, a
carbon–carbon double bond acts as a nucleophile toward an empty carbon 2p
orbital as Lewis acid (Fig. 21.29).
Neighboring carbon–carbon π bonds can influence the rate and control the stereo-
chemistry of a reaction just as a heteroatom can. For example, the tosylate of anti-
7-hydroxybicyclo[2.2.1]hept-2-ene (7-hydroxynorbornene)
2
reacts in acetic acid 10
7
2
The bicyclo[2.2.1]heptane cage is commonly known as the norbornyl system.This obscure name comes
from a natural product, borneol, which has the structure shown in Figure 21.30, and comes from the cam-
phor tree, common in Borneo.The prefixes syn and anti locate the direction of the OH or OTs group with
respect to the double bond, and nor means “no methyl groups.”Thus, 7-hydroxybicyclo[2.2.1]hept-2-ene
is 7-hydroxynorborn-2-ene, or, because there is only one possible place for the double bond, 7-hydroxy-
norbornene.
1100 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
Borneol Bornane Norbornane
OH
PROBLEM 21.17 There is only one possible place for the double bond in the bicy-
clo[2.2.1]heptyl ring system. Explain why.
times faster than the corresponding syn compound,and a staggering 10
11
times faster
than the tosylate of the saturated alcohol, 7-hydroxynorbornane. The product is
exclusively the anti acetate (Fig. 21.31).
Rate = 10
11
anti Tosylate
syn Tosylate
Rate = 10
4
H O
C
O
CH
3
+
+
Rate = 1
CH
3
COOH
CH
3
COOH
CH
3
COOH
OTsH
H
TsO
OTsH
WEB 3D
WEB 3D
FIGURE 21.31 The anti isomer of
7-norbornenyl tosylates gives the anti
acetic acid.The anti tosylate reacts
10
7
times faster than the syn isomer
and 10
11
times faster than the
saturated compound!
FIGURE 21.30 Norbornane
(bicyclo[2.2.1]heptane) is related
to the natural product borneol,
endo-1,7,7-trimethylbicyclo[2.2.1]
heptan-2-ol.
A consistent mechanistic explanation for the huge difference in reaction rates
derives from the fact that only in the anti compound is the π system of the double
bond in the correct position to assist in ionization of the tosylate. For the double
bond in the syn isomer to assist in ionization would require a frontside S
N
2 displace-
ment, which, as we know, is an unknown process.
The rate enhancement is attributed to anchimeric assistance by the double bond.
Intramolecular displacement of the leaving group is faster than the possible inter-
molecular reactions and is therefore the dominant process. The stereochemistry of
the reaction is explained by noting that addition of acetic acid to the intermediate
ion is also an S
N
2 reaction and must occur from the rear of the departing leaving
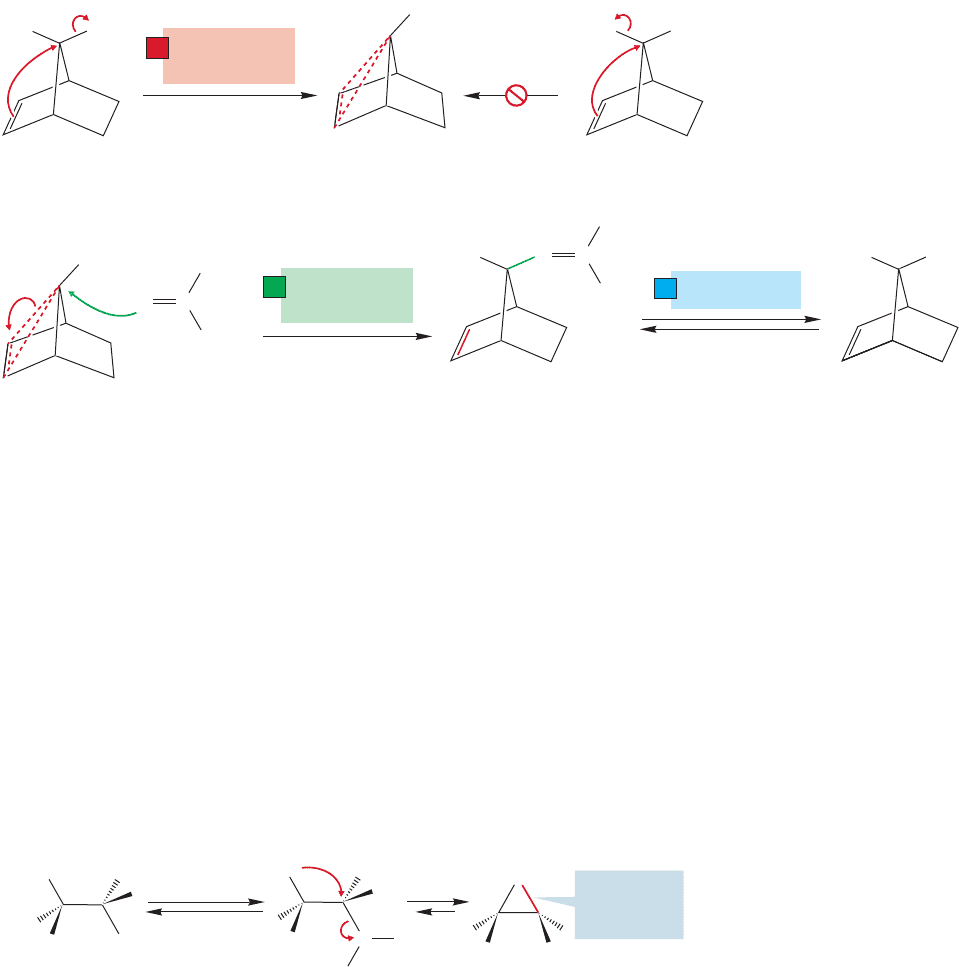
21.3 Neighboring Systems 1101
syn Tosylateanti Tosylate
anti Acetate formed
with retention of
configuration
CH
3
CH
3
COOH
C
OH
..
..
O
..
..
frontside
S
N
2
H
+
OTs
TsO
H
CH
3
C
OH
..
..
+
H H
OAc
Intramolecular
S
N
2
1
Intermolecular
S
N
2
2
Deprotonation
3
O
..
+
H
H
FIGURE 21.32 The carbon–carbon double bond of the anti tosylate, but not of the syn
isomer, can react in an intramolecular S
N
2 reaction to displace the leaving group. Opening
of the cyclic ion must give the anti acetate, the result of net retention.
group. The net result of the two S
N
2 reactions (one intramolecular, one intermol-
ecular) is retention of configuration (Fig. 21.32).
Think a bit now about the nature of the leaving group in the second, intermol-
ecular S
N
2 displacement.Just what is the leaving group, and what do all those dashed
bonds in Figure 21.32 mean? To answer these questions well, we must look closely
at the structure of the postulated intermediate in this reaction. When we formed
three-membered ring intermediates from heteroatoms, there was always a pair of
nonbonding electrons available to do the intramolecular displacement. An example
is the formation of a bromonium ion by displacement of water by neighboring
bromine (Fig. 21.33).
+
HBr
protonation
Br
..
..
S
N
2
Br
..
..
..
OH
..
..
H
H
Br
..
..
..
O
..
+
A normal,
two-electron
bond
..
..
FIGURE 21.33 In a bromonium ion, all bonds are normal two-electron bonds.
Contrast the bromonium ion of Figure 21.33 with the species formed by
intramolecular displacement of tosylate using the π bond. In the bromonium ion,
there are sufficient electrons to form the new σ bond in the three-membered ring.
In the all-carbon species there are not enough electrons. In this cyclic ion, two
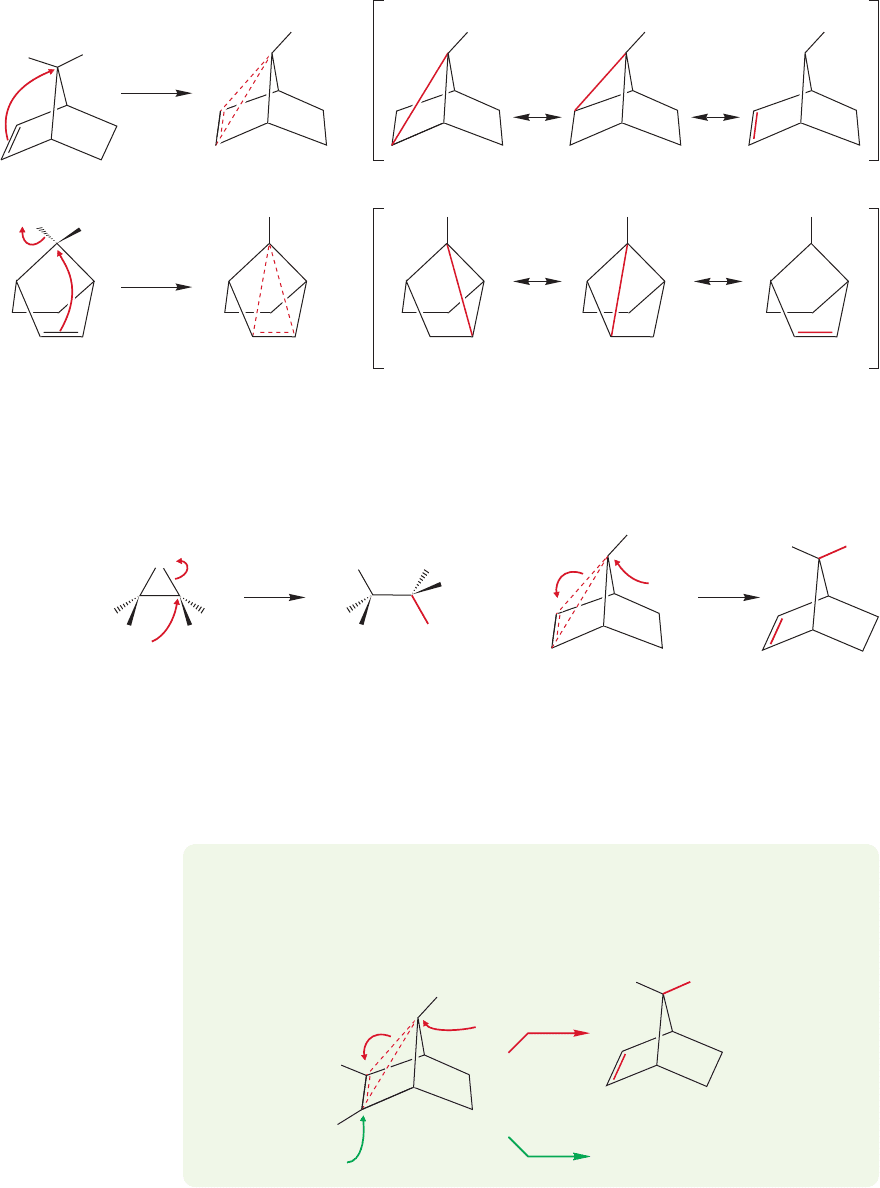
1102 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
=
=
=
H
H
OTs
TsO
+
+
+
+
+
H
+
H
+
H
+
H
H H H
H
FIGURE 21.34 Two views of the
cyclic ion from Figure 21.32 and the
resonance forms contributing to the
ion. Notice the symmetry that is
more clearly shown in the lower view.
+
–
Br
..
..
..
S
N
2
S
N
2
..
Br
..
Nu
–
..
Nu
..
..
..
Nu
H
+
Nu
..
..
..
H
FIGURE 21.35 When a
nucleophile opens a
bromonium ion, a pair of
electrons is displaced.The
same is true for the opening
of the two electron–three
carbon, cyclic ion.
PROBLEM 21.18 If three atoms share the positive charge of the ion in Figure 21.34,
why is it only the 7-position that is attacked by the nucleophile? Explain the lack
of another product. Hint: Look at the structure of the product formed by addi-
tion at the other position in the following reaction:
is a normal, two-electron σ bond that is broken.When the all-carbon three-mem-
bered ring is attacked, it is the two electrons binding the three carbons that act
as the leaving group (Fig. 21.35). The process is still an S
N
2 reaction, however,
and so there is no reason for softening the requirement for backside displacement.
Acceptance of the carbon–carbon double bond as a neighboring group requires you
to expand your notions of bonding,and to accept the partial bonds making up the dashed
three-center, two-electron delocalized system as a valid bonding arrangement.There are
those who resisted this notion mightily, and although they were ultimately shown to be
incorrect, their resistance was anything but foolish. The postulated intermediate in this
reaction is not a normal species and should be scrutinized with the greatest care.
–
..
Nu
–
..
Nu
Why is this “
?”
not formed?
H
Nu
+
H
H
?
H
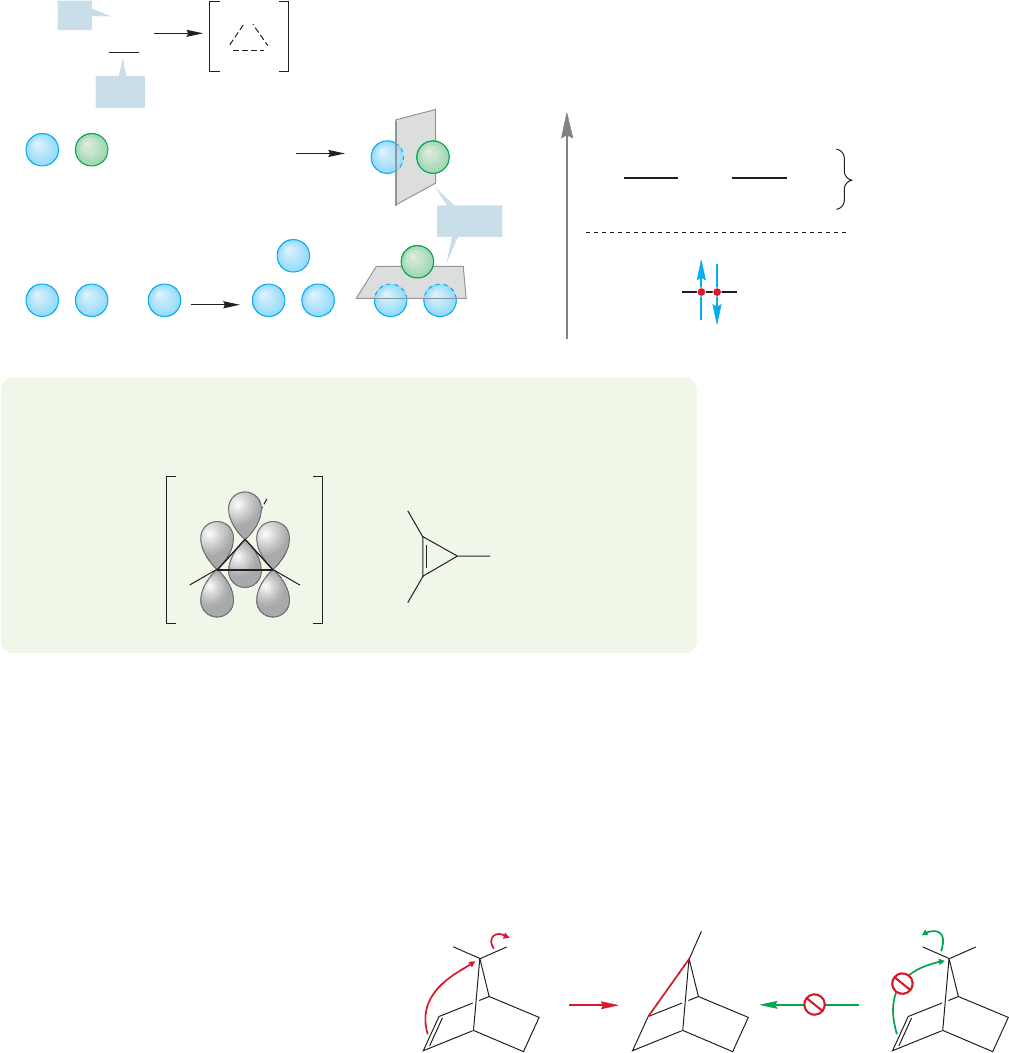
electrons must suffice to bind three atoms (Fig. 21.34).The ion contains an exam-
ple of three-center, two-electron bonding, and the partial bonds are represent-
ed with dashed lines. A resonance formulation shows most clearly how the charge
is shared by the three atoms in the ring. The bromonium ion and the cyclic ion
of Figure 21.34 are very different. When the cyclic bromonium ion is opened, it
21.3 Neighboring Systems 1103
A molecular orbital approach shows how the two electrons can be stabilized
through a symmetrical interaction with an empty carbon 2p orbital. The intellectual
antecedents for this kind of bonding relate to the cyclic H
3
+
molecule in which two
electrons are stabilized in a bonding molecular orbital through a cyclic overlap of hydro-
gen 1s orbitals. We can construct this system by allowing a 1s orbital to interact with
σ and σ* of H
2
as in Figure 21.36 (recall our discussion of the allyl and cyclopropenyl
π systems in Chapter 12).The result is a system of three molecular orbitals: one bond-
ing,two antibonding.Two,but only two, electrons can be accommodated in the bond-
ing orbital. The same orbital pattern will emerge for any triangular array of orbitals.
+
H
HH
H
+
HH
No interaction with 1s
1s
σ, σ*
σ*
σ
σ
+ 1s = 1 σ – 1s = 2
= 3
Nodes
Energy
Nonbonding
Same number
of nodes
1
23
Orbital pattern for a triangular array of H
3
+
+
–
1s
FIGURE 21.36 The construction of
the molecular orbitals of cyclic H
3
+
.
Cyclopropenium ion
+
+
H
H
H
H
=
H
H
PROBLEM 21.19 Work out the π molecular orbitals for the cyclopropenium ion
shown below.
Chemists resisting this notion (the “classicists”) suggested that there was no need for
this new kind of delocalized bonding, and that normal, localized two-electron models
would do as well.Let’s follow their argument.Remember as we go that they must explain
both a rate acceleration for the anti tosylate and the overall retention of stereochemistry.
The two compounds in question here, the syn and anti tosylates, are different, and
there must be some rate difference between them.One can scarcely argue with this point,
and one is reduced to relying on the magnitude and direction of the rate acceleration as
evidence for the delocalized ion. A classicist might argue that in cases such as this, the
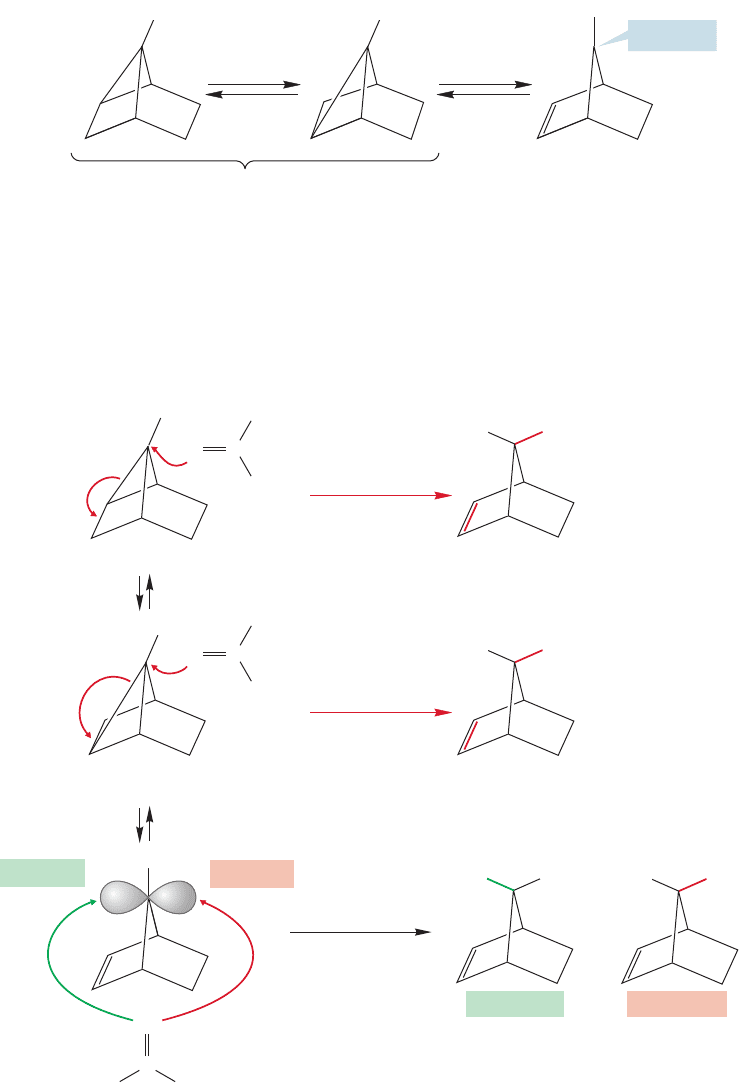
rate increase could easily come from the formation
of a normal, localized or “classical,” carbocationic
intermediate. The anti tosylate has the leaving
group in an excellent orientation to be attacked by
the intramolecular nucleophile, the filled π orbital
of the carbon–carbon double bond (Fig. 21.37).
The syn tosylate does not.
S
N
2
no frontside
S
N
2 possible
+
H OTs TsO H
H
synanti
FIGURE 21.37 A “classical”
displacement reaction by the π bond
of the anti isomer would give a
localized ion that could not be
formed from the syn isomer.
1104 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
As in the symmetrical displacement to form the delocalized ion, the required
attack on the leaving group from the rear could occur only in the anti compound.
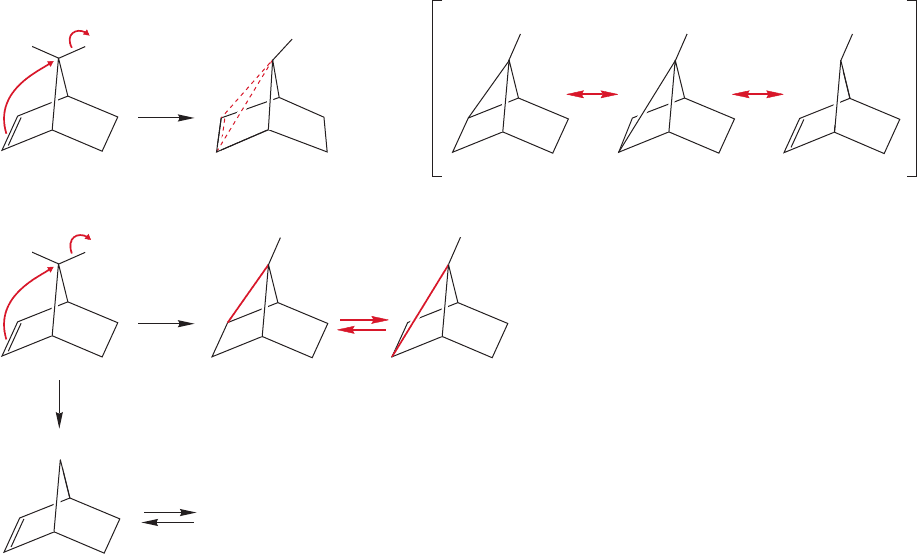
Of course,there are two equivalent intermediates possible (A and B,Fig. 21.38),and
it was suggested that they were in equilibrium. The compound with the charge at
the 7-position (C) might also be a partner in the equilibrium. Be sure you see the
difference between the three equilibrating structures of Figure 21.38 and the three
resonance forms contributing to the single structure of Figure 21.34.
rapid
equilibrium
A racemic pair of ions
+
ABC
+
H
+
7-Position
HH
FIGURE 21.38 The first-formed ion
A equilibrates with B and, perhaps, C.
Addition of acetic acid to ions A and B of Figure 21.38 must give the anti acetate,
because the opening is an S
N
2 reaction.Attack on ion C is more problematic,because
it is difficult to explain the stereospecificity of the reaction. Still, it must be admit-
ted that the syn and anti faces of the ion are different, and in principle, this differ-
ence could lead to a stereospecific reaction (Fig. 21.39).
+
CO
OH
A
B
C
+
..
..
CH
3
1. S
N
2
2. deprotonation
anti Acetate
anti Acetate
syn Acetate
1. S
N
2
2. deprotonation
1. add
anti Face
syn Face
2. deprotonate
CO
OH
..
..
..
..
..
..
..
..
CH
3
If ion C is involved,
why only anti acetate—
why not syn as well?
+
H
C
OH
H
3
C
H
OAc
H
OAc
anti Acetate
H
OAc
AcO
O
..
..
H
H
H
+
FIGURE 21.39 Addition of acetic acid
to the equilibrating ions A and B
must lead to anti acetate. Attack at
the 7-position (ion C) to give only
the anti acetate presents some
difficulties.
21.3 Neighboring Systems 1105
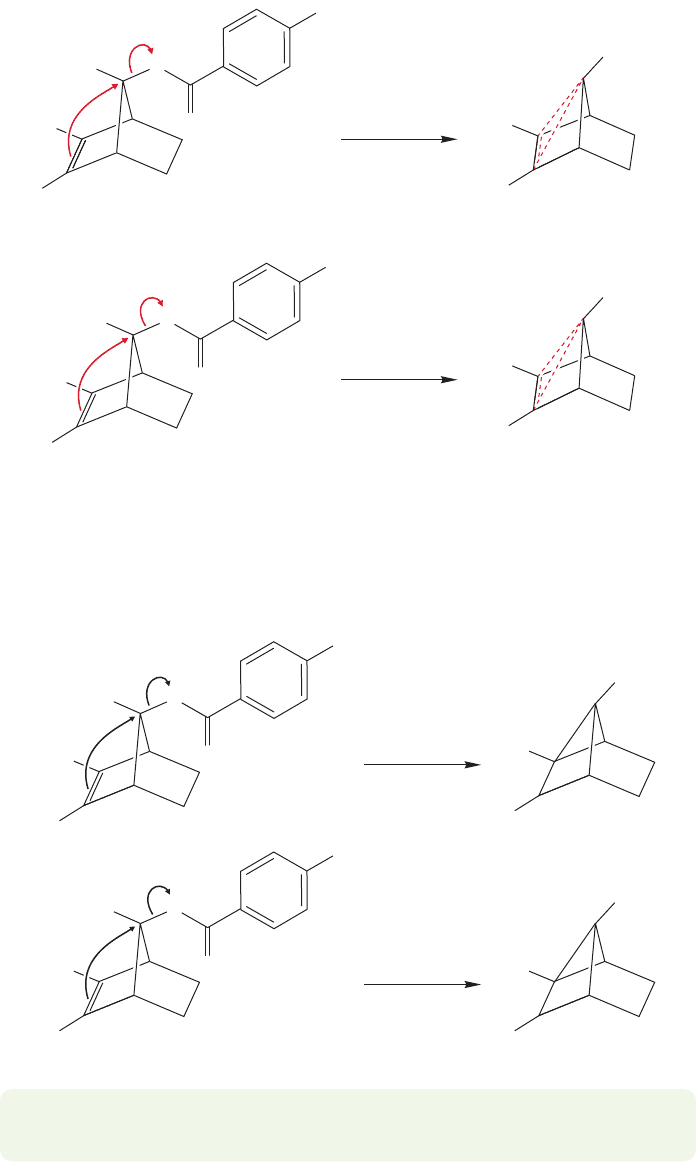
Stop right now and compare the two mechanistic hypotheses (Fig. 21.40). In one,
there is a symmetrical displacement of the leaving group to give a resonance-stabilized
delocalized intermediate. In the other, either the leaving group is displaced to give a
carbocationic intermediate containing a three-membered ring (A and B) in equilibrium
with at least one other ion, C, or a simple S
N
1 ionization to C takes place.
Symmetrical, delocalized
intermediate
+
+
+
+
H OTs
S
N
2
H OTs
S
N
2=
Unsymmetrical, normal localized
pair of equilibrating carbocations
A and B
A and B
C
S
N
1
H
H
H
+
+
+
H H H
FIGURE 21.40 A comparison of the symmetrical, delocalized intermediate with the localized ions A, B, and C.
The whole difference between the two mechanisms is the formulation of the
structure of the intermediate! The structures in the two versions of the mechanism
for this reaction are only very subtly different. In one, the constituent structures are
contributing resonance forms to a single intermediate delocalized species; in the
other they are equilibrating molecules, each with its own separate existence.
Are we now down to questions of chemical trivia? Are we counting angels on
pin heads? Many thought so, but we maintain that they were quite misguided in
their criticisms of those engaged in the argument. All questions of mechanism come
down to the existence and structures of intermediates, and to the number of barri-
ers separating energy minima.
This argument has been recast many times in related reactions. Not all questions
have been resolved, but those that have been have generally involved two kinds of
evidence. Sometimes a direct observation of the ion in question was made, usually
under superacid conditions (p. 679) at low temperature.In other reactions,an exper-
iment like the following one provides evidence for symmetrical participation to pro-
duce a delocalized intermediate.
The reactions in acetic acid of the monomethyl and dimethyl derivatives of
the p-nitrobenzoate of anti-bicyclo[2.2.1]hepten-7-ol have been examined. The
1106 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
rate = 1.0
rate = 13.3
A more stable bridged ion—
one resonance form is a
tertiary carbocation
H
H
H
O
NO
2
H
H
NO
2
H
3
C
..
..
O
..
..
O
..
..
O
..
..
CH
3
COOH
CH
3
COOH
H
3
C
+
H
H
H
+
H
H
FIGURE 21.41 Formation of a
symmetrical, delocalized ion from the
methyl-substituted molecule would
be faster than ion formation from
the parent.
CH
3
COOH
CH
3
COOH
rate = 1.0
rate = 13.3
+
H
H
H
H
H
O
NO
2
+
H
H
H
NO
2
Secondary carbocation
Tertiary carbocation
H
3
CH
3
C
..
..
O
..
..
O
..
..
O
..
..
H
H
FIGURE 21.42 There would also be
a rate increase in the formation of
the unsymmetrical, localized ion
postulated by the classicists.
relative to that of the parent, which can produce only a less stable secondary carbo-
cation (Fig. 21.42).The observed rate increase of 13.3 for the monomethyl analogue
is accommodated by both mechanisms.
monomethyl compound reacts faster than the parent (Fig. 21.41), as predicted by either
mechanism. A delocalized ion would be stabilized because one of its contributing
resonance forms is tertiary.Another way of putting this is to note that the methyl group
makes the double bond a stronger nucleophile. In the localized mechanism, a terti-
ary carbocation is formed and this would surely accelerate the rate of the reaction
PROBLEM 21.20 Draw resonance forms for the monomethyl intermediate of
Figure 21.41.
21.3 Neighboring Systems 1107
+
H
H
H
H
NO
2
H
O
..
..
O
..
..
+
H
H
3
C
H
H
NO
2
H
3
C
O
..
..
O
..
..
+
H
3
C
H
3
C
H
3
C
H
NO
2
O
..
..
O
..
..
H
3
C
Relative rate
= 13.3
Relative rate = 1.0
Predicted rate (13.3)
2
= 177
Both methyl groups stabilize
the cation at the same time
CH
3
COOH
CH
3
COOH
CH
3
COOH
H
H
H
FIGURE 21.43 Reaction of the
dimethyl compound to give a
delocalized ion should be still faster
than the reaction of the monomethyl
compound.The two methyl groups
simultaneously stabilize the cation.
+
..
..
Predicted rate = 2 13.3 = 26.6
H
H
+
H
O
O
..
..
NO
2
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
COOH
FIGURE 21.44 In the localized, unbridged ions, the two methyl groups can never stabilize the ion at the same time.
Now consider the dimethyl compound. If a delocalized intermediate is formed
through a symmetrical displacement, both methyl groups exert their influence at the
same time.If one methyl group induces a rate effect of 13.3,two should give an effect
of 13.3 × 13.3
= 177 (Fig. 21.43).
On the other hand, if it is the formation of a localized tertiary ion that is impor-
tant, the second methyl increases the possibilities by a factor of 2, but the two methyl
groups never exert their influence at the same time. The rate change should approxi-
mate a factor of 2 (Fig. 21.44).
1108 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
The fundamental difference between a mechanism involving a symmetrical delo-
calized ion and the traditional mechanism, which postulates a localized, less sym-
metrical ion, is that in the delocalized case both methyl groups exert their stabilizing
influence at the same time.In the pair of equilibrating open, localized ions they can-
not both act simultaneously.
The observed rate for the dimethyl compound is 148, much closer to that
expected for the delocalized intermediate than for the localized ion.
Summary
We have progressed from a discussion of obvious neighboring groups,heteroatoms
with nonbonding electrons, to less obvious internal nucleophiles, the filled π
orbitals of double bonds. In every case, a pair of internal electrons does an initial
intramolecular S
N
2 displacement, which is followed by a second, external (inter-
molecular) S
N
2 reaction. The result is overall retention of configuration.
21.4 Single Bonds as Neighboring Groups
Surely,one of the weakest nucleophiles of all would be the electrons occupying the low-
energy bonding σ orbitals. Yet there is persuasive evidence that even these tightly held
electrons can assist in the ionization of a nearby leaving group. For example, when the
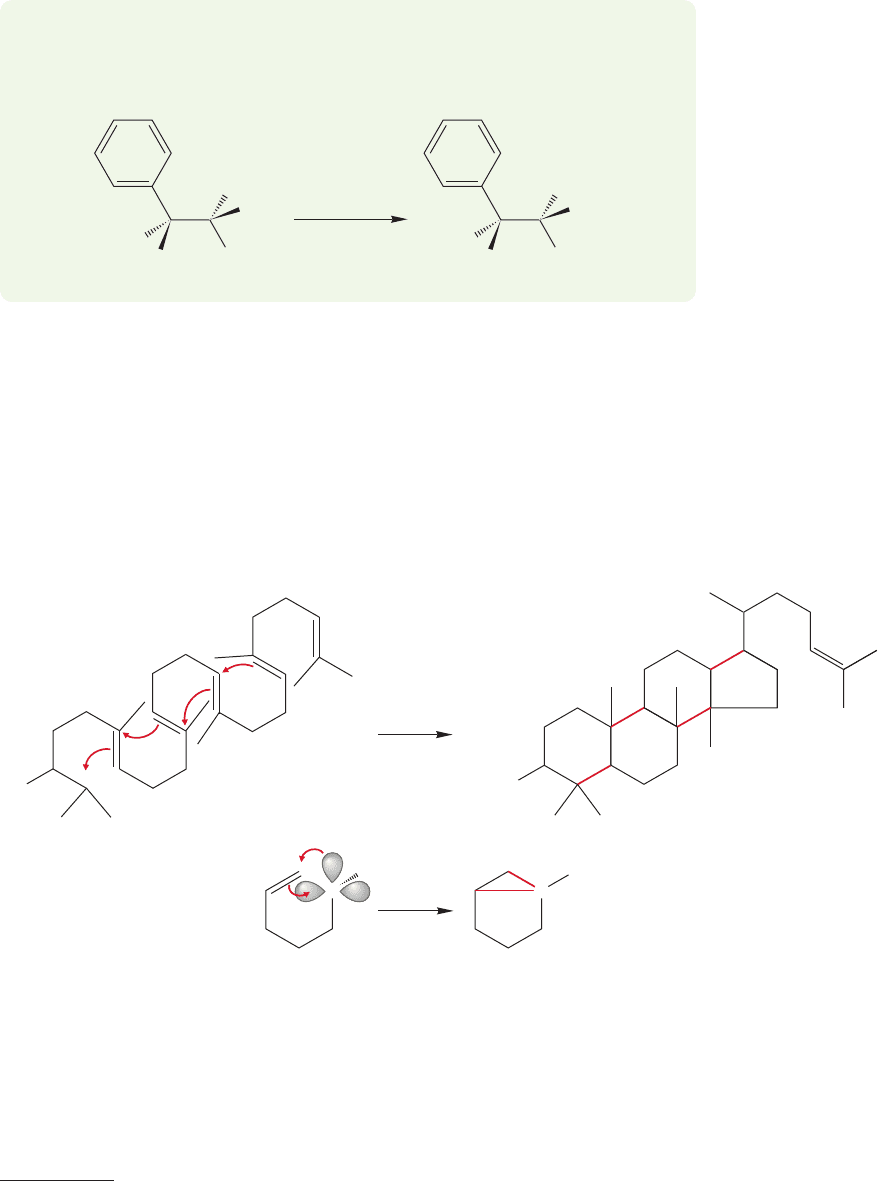
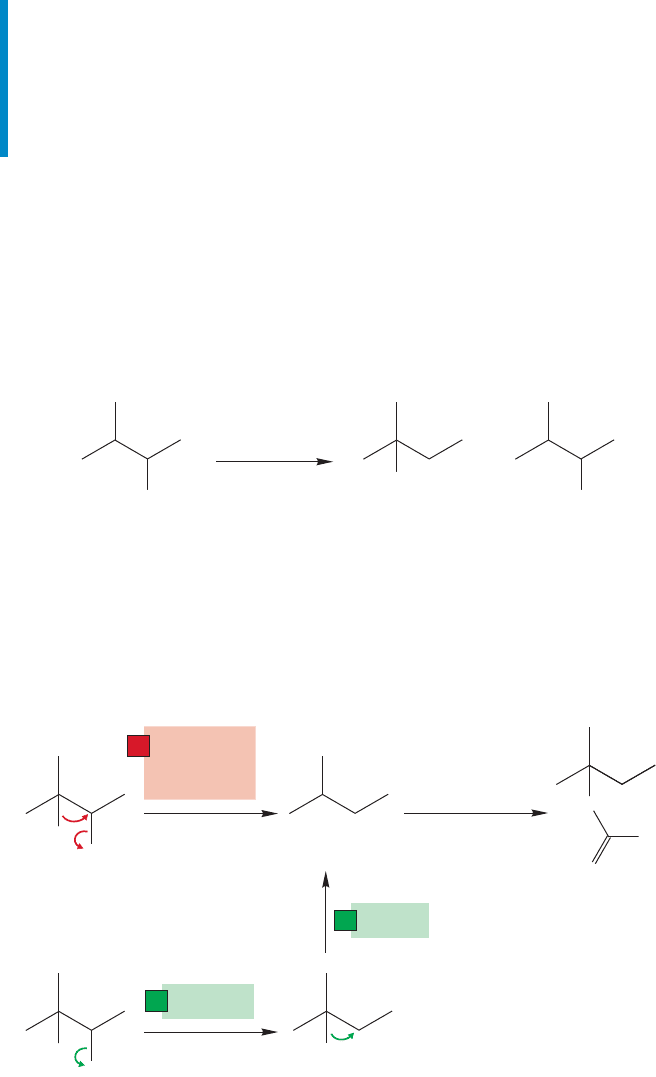
tosylate of 3-methyl-2-butanol is treated with acetic acid the result is formation of the
rearranged tertiary acetate, not the “expected” secondary acetate (Fig. 21.45).
(97%) (3%)
OTs OAc
OAc
(AcOH)
+
CH
3
COOH
25 ⬚C
FIGURE 21.45 The reaction of the
tosylate of 3-methyl-2-butanol in
acetic acid involves a rearrangement.
This result is easily rationalized through a hydride shift to form the relatively
stable tertiary carbocation.The interesting mechanistic question is whether the sec-
ondary carbocation is an intermediate, or whether the hydride moves as the leaving
group departs (Fig. 21.46).Thus, the timing of the steps becomes an issue. Is formation
of the tertiary carbocation a two- or one-step process? This example recapitulates
OTs
H
1. CH
3
COOH
2. deprotonation
–
OTs
+
+
OTs
H
Secondary
carbocation
Tertiary
carbocation
H
..
..
O
O
CH
3
..
..
..
–
..
–
1
1
H migration
as
–
OTs
leaves
Ionization
2
H shift
FIGURE 21.46 Two possible
mechanisms for the acetolysis of
3-methyl-2-butyl tosylate.The green
route is a two-step process. The red
path is a one-step process.
