Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


21.2 Heteroatoms as Neighboring Groups 1089
PROBLEM 21.5 There are many possible labeling experiments that would support (not
prove) the mechanism suggested for the reaction of the chlorosulfide in Figure 21.13.
Design two, one using a methyl group as a label, and the other using an isotopic label.
It is important to be certain you understand the source of the rate increase for the
sulfide compound.Intramolecular reactions often have a large rate advantage over inter-
molecular reactions because there is no need for the nucleophile and substrate to find
each other in solution.Intramolecular assistance in the rate-determining step of a reac-
tion is called anchimeric assistance. Once again, it is important not to be put off by
the elaborate name.The concept is quite simple: Intramolecular nucleophiles are often
more effective displacing agents than intermolecular nucleophiles.What is somewhat
more difficult to anticipate is that an intramolecular displacement by a very weak, but
perfectly situated nucleophile can often compete effectively with intermolecular reactions.
MUSTARD GAS
If we make a seemingly trivial change in our sulfur-
containing molecule, by adding another chlorine, we come to
bis(2-chloroethyl)thioether. This seemingly routine molecule
exposes the dark side of our science. Indeed, a review of the
history of this compound causes one to reflect on the depths
to which human behavior can sink, for this molecule is mus-
tard gas, developed in the early part of the twentieth century
and used in World War I as a replacement for other less effi-
cient toxic agents for which defenses had been worked out.
Mustard gas penetrated the rubber in use in gas masks at the
time and thus was more effective.This mustard is lethal in a
few minutes at 0.02–0.05% concentration. Less severe expo-
sure leads to irreversible cell damage and only delays the
inevitable. Introduced by the Germans at Ypres in 1917, it
was also employed in the China campaign by the Japanese in
World War II. Stories of experiments on prisoners of war are
easy to find, if not easy to read. Nor is there any reason for
national pride in not being the first to use this agent, for we
in America were the developers of napalm, essentially jellied
hydrocarbons, a chemical agent used by U.S. forces in World
War II and the Vietnam War.
We leave it to you to consider whether it is morally
superior to shoot people, or drop an atomic bomb on them,
rather than to dose them with mustard gas or burn them
with napalm. Even more complicated is the question of the
participation by chemists in the development of such agents.
Of course, often it is not easy to see the consequences of
one’s research. But what if one can? Where are the limits?
ClCl
S
Bis(2-chloroethyl)thioether
PROBLEM SOLVING
There are three clues that point to the operation of a neighboring group effect.
The first is an unusual, often “backward” stereochemical result. Generally, this
involves retention of configuration when inversion might have been anticipated.
The second is formation of an unexpected product that appears to be the result
of a rearrangement. The unexpected product arises because an intermediate is
formed that can react further in more than one way. The third clue is an
unexpected increase in the rate of a reaction.The anchimeric assistance provided
by an intramolecular nucleophile often reveals itself in the form of a rate increase.
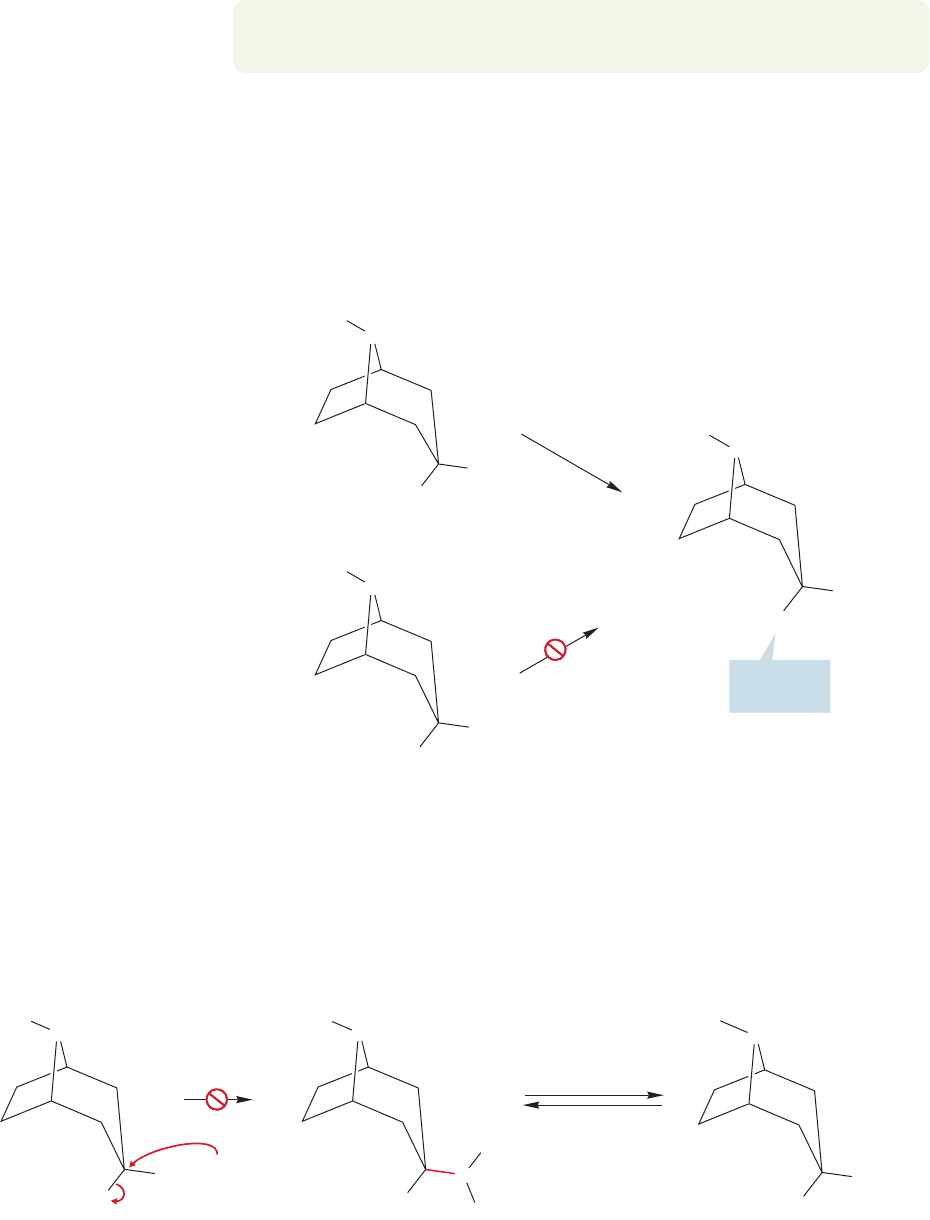
Now let’s look at more heteroatoms with nonbonding electrons that might par-
ticipate in neighboring group reactions. For example, nitrogen bears a lone pair of
electrons, and so can act as an internal nucleophile. Let’s look at the reactions of the
two bicyclic amines in Figure 21.14.Treatment of the endo chloride with ethyl alco-
hol leads to the endo ether, but the exo chloride gives neither the exo nor endo ether
under the reaction conditions. Now we’ll see why.
1090 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
PROBLEM 21.6 Go back and find examples of these three clues in the preceding
reactions.
Still endo—
retention!
N
..
..
..
CH
3
CH
2
OH
H
2
O
50 ⬚C
H
3
C
exo
N
Cl
..
..
..
..
H
3
C
endo
Cl
..
..
..
..
..
..
H
H
..
..
CH
3
CH
2
O
H
3
C
N
H
FIGURE 21.14 Treatment of only one
isomer of this bicyclic amine gives
the related ether. Note the retention
of configuration.
In the reaction of the endo isomer, direct displacement of the chloride by ethyl
alcohol cannot be happening, as this potential reaction leads to a product that
is not observed, the exo ether (Fig. 21.15). The ether is formed, but it is formed
with retention of stereochemistry, not the inversion demanded by the simple
S
N
2 reaction.
N
..
..
HOCH
2
CH
3
S
N
2
N
..
..
OCH
2
CH
3
H
3
C
N
deprotonation
exo
(not the product observed)
endo
H
Cl
..
..
..
..
..
..
H
3
C
H
3
C
H
O
..
+
H
CH
2
CH
3
H
FIGURE 21.15 A simple intermolecular S
N
2 displacement demands inversion of configuration, which does not give the
observed product.
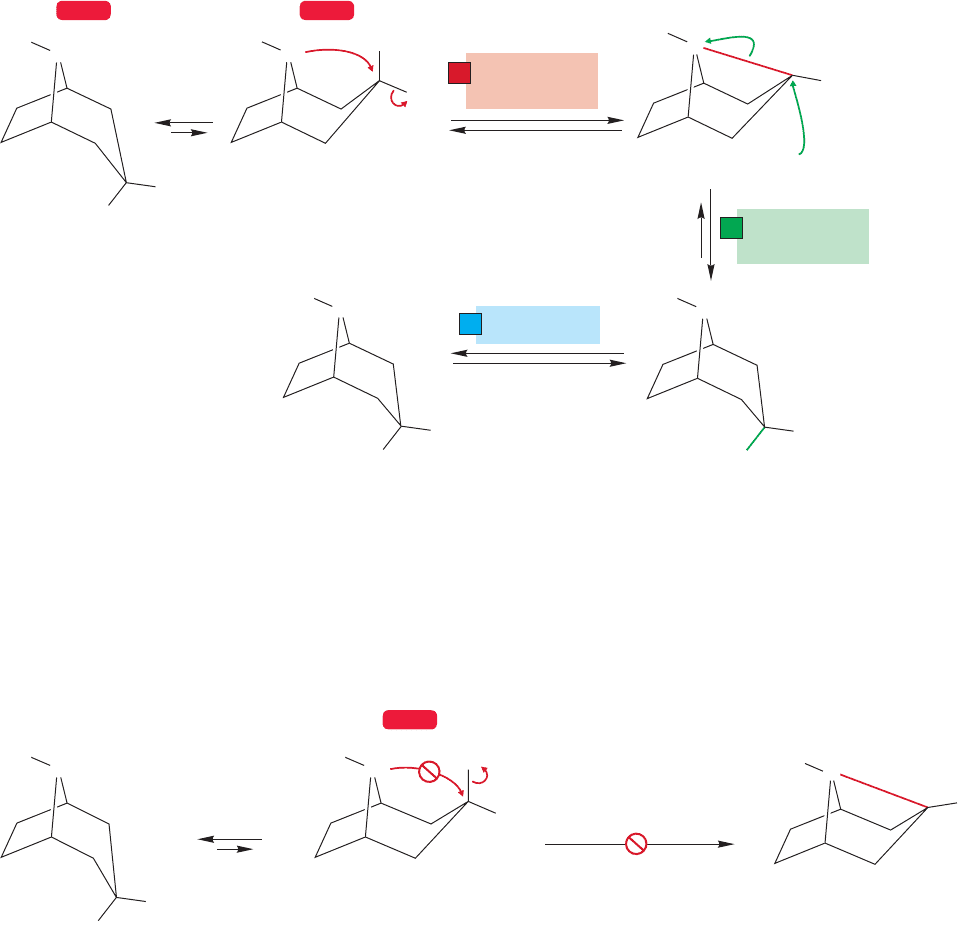
21.2 Heteroatoms as Neighboring Groups 1091
At least one of the clues to the operation of a neighboring group effect is pres-
ent in this reaction.The stereochemistry of the product is the opposite to what one
would expect from a simple displacement (clue 1).
What’s happening? By now you should be alert to the possibility of intramol-
ecular displacement.The nitrogen with its pair of nonbonding electrons is an obvi-
ous possible neighboring group, and it is located in the proper position for a backside
displacement of the chloride (Fig. 21.16). The result is a cyclic ammonium ion, a
ring containing a positively charged nitrogen. If this ring is opened by ethyl alco-
hol, the product ether must be formed with retention of stereochemistry. The
intramolecular S
N
2 displacement by nitrogen is the first inversion reaction, and the
second, intermolecular displacement, the opening of the ammonium ion by ethyl
alcohol, provides the second.Two inversions produce net retention of configuration.
N
N
..
N
..
..
..
CH
3
CH
2
OH
..
H
N
Cl
..
..
..
Cl
..
..
..
N
H
..
..
..
CH
3
CH
2
OH
+
+
H
H
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
CH
3
CH
2
O
..
Intramolecular
S
N
2
1
Intermolecular
S
N
2
2
3
Deprotonation
WEB 3D WEB 3D
FIGURE 21.16 When nitrogen acts as a neighboring group and displaces the leaving group, a cyclic ammonium
ion is formed. The cyclic ion is opened to give the observed product of retention of configuration.
Why does the exo compound not react in the same way? In this molecule, dis-
placement of the leaving chloride would require a frontside S
N
2 reaction, and this
process is impossible (Fig. 21.17). So, the exo chloride reacts more slowly (and in a
different way) from the endo isomer.
WEB 3D
N
..
N
..
H
3
C
H
3
C
No
frontside
S
N
2!
the cyclic ammonium
ion cannot be formed
this way
H
H
3
C
H
N
+
Cl
..
..
..
Cl
..
..
..
H
FIGURE 21.17 The exo chloride cannot be displaced by nitrogen, as this reaction would require a frontside S
N
2 reaction.
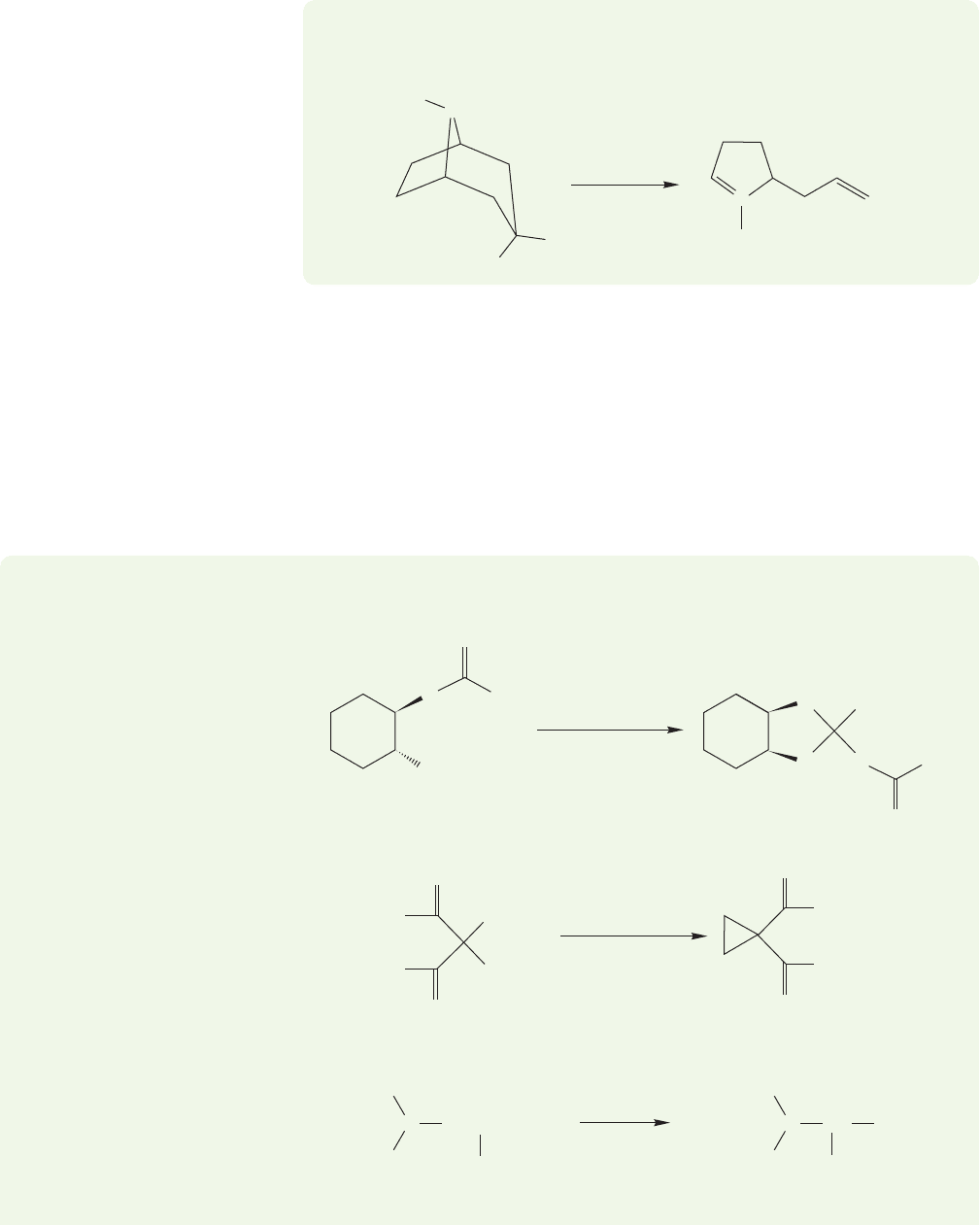
1092 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
PROBLEM 21.7 The exo isomer of Figure 21.14 gives the product shown below.
Suggest a mechanism for this reaction.
N
..
H
3
C
CH
3
CH
2
OH
CH
3
H
2
O
50 ⬚C
N
+
–
Cl
..
..
..
..
H
..
Cl
..
..
It is often difficult to see these reactions in bicyclic systems. The molecules are
not easy to draw, and the extra atoms involved in the cage are distracting. There is
nothing fundamentally difficult about these reactions, however. In fact, one could
argue that once the drawing problems are mastered, the rigidity of the cage is a help.
One need not worry about rotations about single bonds, or about finding the proper
orientation for the reacting molecules.The stereochemical relationships are defined
and preserved by the rigid bicyclic molecules. Here are some problems for practice.
It will help to use models.
WORKED PROBLEM 21.8 Write mechanisms for the following reactions:
CH
3
CH
2
OH
CH
2
CH
2
Br
CH
3
CH
3
COO
–
OTs
+
Na
+
Na
O
*(a)
(b)
(c)
..
..
N
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
CH
3
CH
3
CH
3
CH
2
O
CH
3
CH
2
CH
3
CH
2
CH
2
CHCH
3
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
+
KHO
–
..
..
..
..
OCH
2
CH
3
H
O
..
..
O
..
..
CH
3
CH
2
O
..
..
..
..
OCH
2
CH
3
CH
3
Cl
..
..
..
..
..
..
N
..
..
CH
3
CH
2
CH
3
CH
2
CH CH
2
OH
H
2
O
(continued)
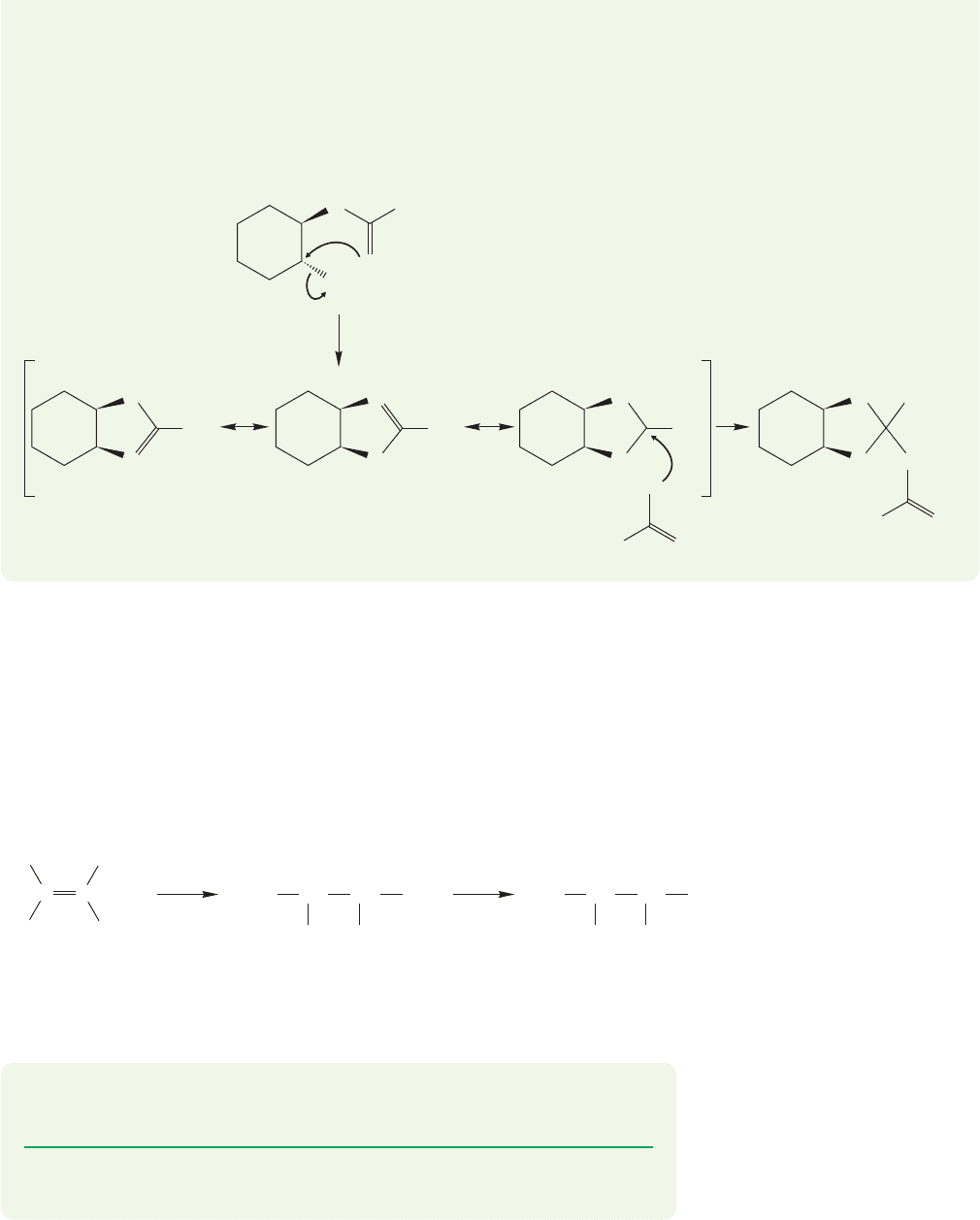
21.2 Heteroatoms as Neighboring Groups 1093
ANSWER (a) The internal nucleophile is the carbonyl oxygen. Notice that the
trans stereochemistry of the starting material allows for a rearside S
N
2 displace-
ment of the good leaving group, tosylate. Displacement yields a cyclic cation that
is nicely stabilized by resonance. Acetate ion adds to the carbon sharing the pos-
itive charge, and gives the product.
O
..
..
..
O
..
..
O
..
..
OTs
S
N
2intramolecular
CH
3
O
..
..
H
3
C
O
..
..
H
3
C
+
O
..
..
..
O
CH
3
+
–
O
..
O
..
..
CH
3
+
O
..
..
O
..
..
CH
3
O
..
..
O
O
..
..
..
..
CH
3
..
..
The halogens are another set of heteroatoms with nonbonding electrons.
Neighboring group reactions of halogens are almost entirely restricted to iodine,
bromine, and chlorine, and very often reveal themselves through the stereochem-
istry of the resulting products. As an example, we will work through the conversion
of cis-2-butene into 2,3-dibromobutane through the synthetic scheme shown in
Figure 21.18.
C
C
H
3
C
H
3
C
H
H
..
..
OH
CH
3
Br
..
..
..
CH
3
CH CH
H
3
C
CH
3
Br
..
..
..
CH CH
Br
..
..
..
..
..
H
2
O
Br
2
HBr
..
..
..
FIGURE 21.18 A possible synthesis of 2,3-dibromobutane.
PROBLEM 21.9 What’s wrong with fluorine? Why are neighboring group effects
involving fluorine very rare?
PROBLEM 21.10 What is a much easier way of making the 2,3-dibromobutane of
Figure 21.18?
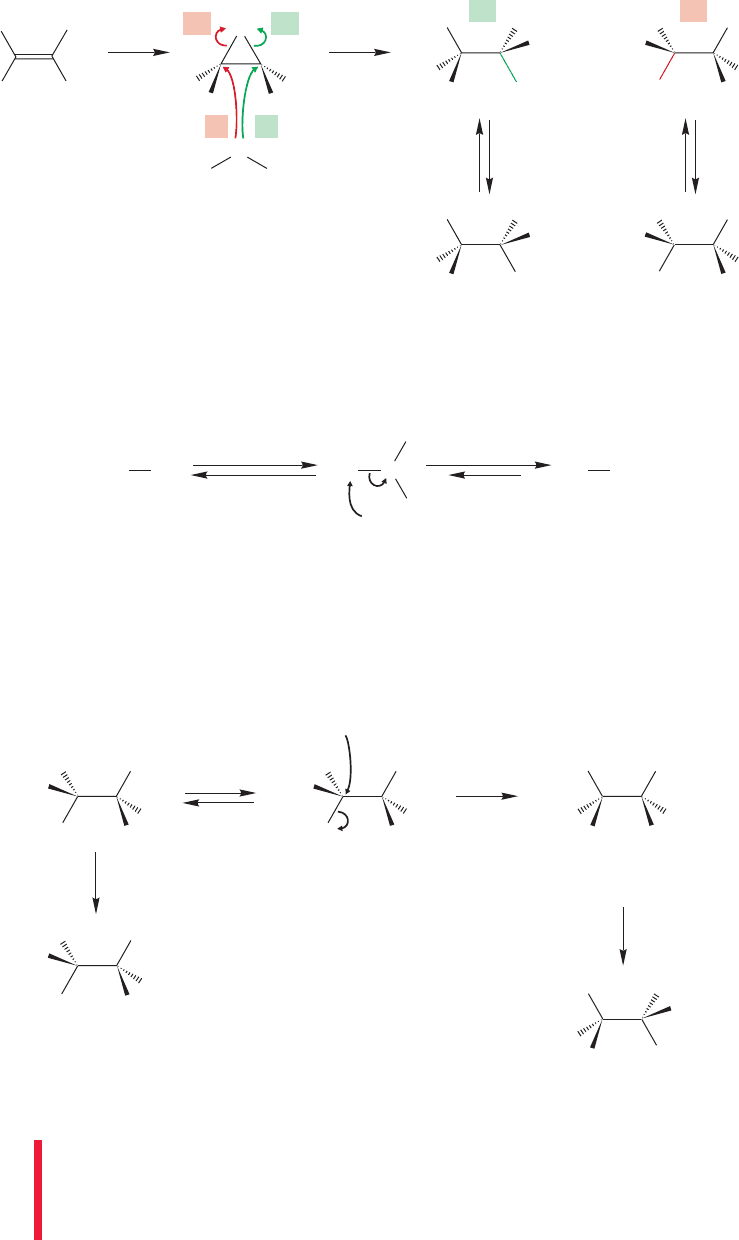
Treatment of a primary or secondary alcohol with hydrogen bromide gives the
corresponding bromide through the mechanism shown in Figure 21.20.
1094 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
O
..
..
+
+
+
CH
3
CH
3
deprotonations
An enantiomeric pair of bromohydrins
H
3
C
H
3
C
H
H
H
(a)
(a)
(a)
(b)
(b)
(b)
H
HH
CH
3
CH
3
Br
2
H
3
C
H
3
C
H
H
H
H
Br
..
..
..
..
H
2
O
Br
..
..
..
Br
..
..
..
..
OH
2
H
2
O
CH
3
CH
3
..
..
H
3
C
H
3
C
H
H
H
H
Br
..
..
..
Br
..
..
..
..
..
OH
HO
+
+
..
FIGURE 21.19 Formation of a
bromonium ion, followed by opening
by water, gives a pair of enantiomeric
bromohydrins.
H
H
..
R
Br
ROH O
R
HBr S
N
2
protonation
displacement
..
..
H
2
O
..
..
+
Br
..
..
..
+
–
..
..
..
..
FIGURE 21.20 The mechanism for
formation of an alkyl bromide from
a primary or secondary alcohol and
hydrogen bromide.
The S
N
2 reaction of Figure 21.20 would give meso-2,3-dibromobutane from the
bromohydrins of Figure 21.19, but it is the racemic mixture,not the meso compound
that is produced (Fig.21.21). Be absolutely certain you see this difference. If you are
lost, reread Chapter 4, which works through the differences between meso com-
pounds and racemic mixtures.
..
..
CH
3
HBr
HBr
Eclipsed—
energy maximum
Staggered—
energy minimum
meso-2,3-Dibromobutane
not formed
racemic 2,3-Dibromobutane
This is the product that
is actually formed; note
retention of stereochemistry!
H
3
C
H
H
Br
CH
3
..
H
3
C
H
2
O
H
3
C
S
N
2
H
H
Br
..
..
..
H
2
O
CH
3
..
..
H
3
C
H
H
Br
..
..
..
HO
CH
3
H
3
C
H
H
Br
..
..
..
Br
..
..
..
Br
..
..
..
+
Br
..
..
..
..
–
+
CH
3
H
H
Br
..
..
..
Br
..
..
..
..
..
..
FIGURE 21.21 Reaction of
the bromohydrin of Figure
21.19 by S
N
2 displacement
should give meso-2,3-
dibromobutane.This
compound is not the actual
product.
In the bromination of cis-2-butene, the initial intermediate is the cyclic bromo-
nium ion (p. 415). The ring is opened by water to give the racemic pair of bromo-
hydrin enantiomers shown in Figure 21.19.
Two conventions are being used here. First, the figures often carry through with
only one enantiomer of a pair. Be sure to do Problem 21.11. Second, for clarity,
eclipsed forms are often drawn. Be sure you remember that these are not really
energy minima, but energy maxima.
CONVENTION ALERT
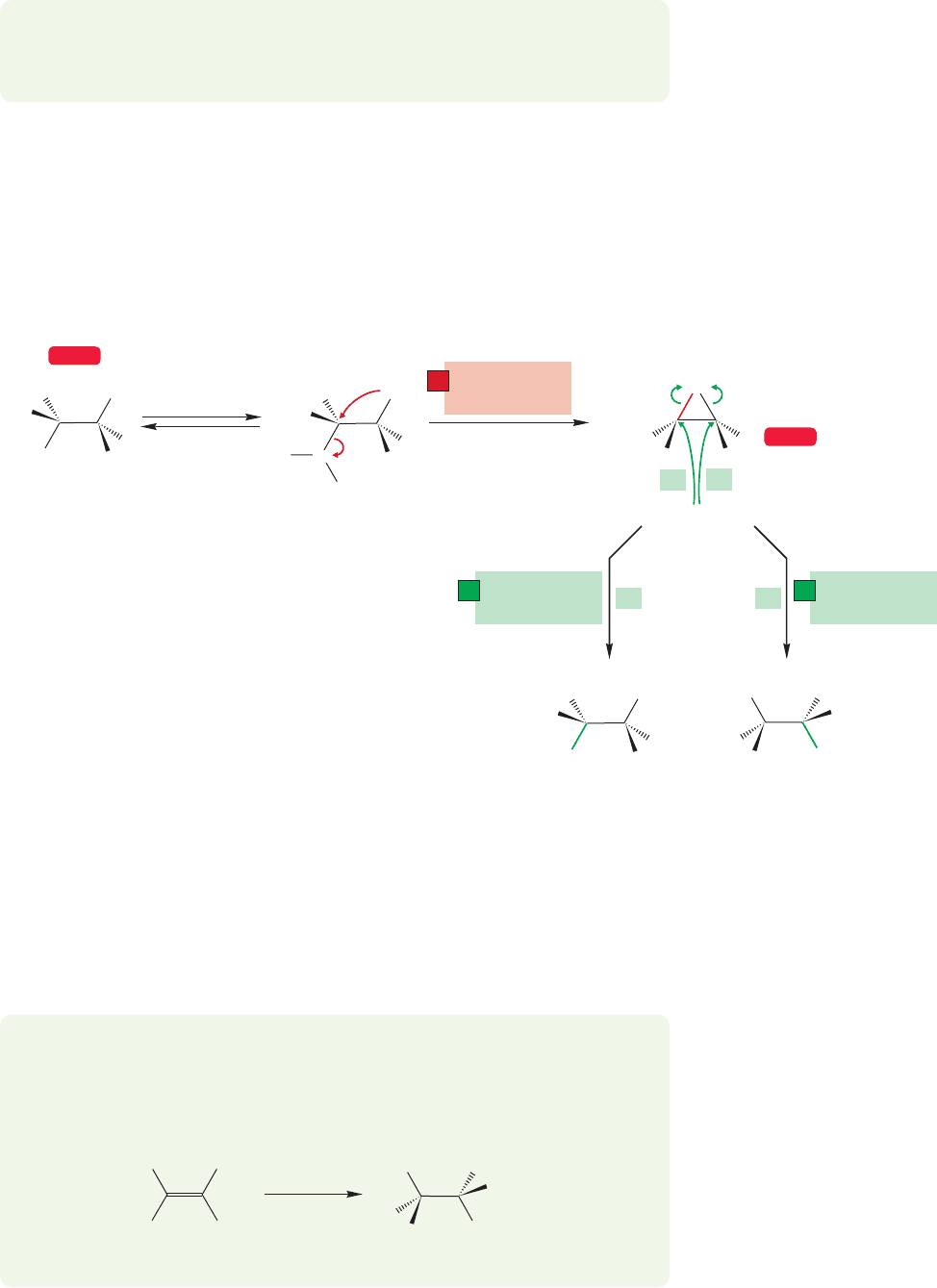
21.2 Heteroatoms as Neighboring Groups 1095
PROBLEM 21.11 Figure 21.21 works the conventional reaction mechanism through
with one of the enantiomers of Figure 21.19. You work it through with the other
enantiomer.
The formation of the “wrong”stereoisomer is the clue to the operation of a neigh-
boring group effect in the reaction of Figure 21.21.The product is formed not with
the inversion of configuration required by the mechanism, but with retention (clue 1).
After the hydroxyl group is protonated, it is displaced not by an external bromide
ion, but by the bromine atom lurking in the same molecule (Fig. 21.22). It is most
important at this point to keep the stereochemical relationships clear. Displacement
must be from the rear, as this is an intramolecular S
N
2 reaction.
+
CH
3
H
3
C
H
(a)
(a)
(b)
(b)
H
H
H
CH
3
protonation
HBr
H
3
C
H
H
An enantiomeric pair of dibromides
An achiral bromonium ion
Br
..
..
..
CH
3
..
..
H
3
C
H
H
Br
..
..
..
..
HO
CH
3
H
3
C
H
H
Br
..
..
..
CH
3
H
3
C
H
H
Br
..
..
..
Br
..
..
..
Br
..
..
..
O
Br
..
..
..
..
–
Br
..
..
..
..
–
Br
..
..
..
..
–
1
Intramolecular
S
N
2
Intermolecular
S
N
2
2
Intermolecular
S
N
2
2
+
Br
..
..
WEB 3D
WEB 3D
FIGURE 21.22 When the bromine acts as a
neighboring group and displaces water, a
bromonium ion is formed. Intermolecular opening
of this cyclic ion by bromide gives the observed
product, a pair of enantiomeric dibromides.
Notice that we have formed the same bromonium ion as in Figure 21.19, though
by a different route.Like all bromonium ions,this one is subject to opening by nucleo-
philic attack. In this case, bromide ion can open the cyclic ion at two points (paths a
and b) leading to the pair of enantiomers shown in Figure 21.22.The product shows
no optical activity not because it is a meso compound,but because it is a racemic mix-
ture of enantiomers.
PROBLEM 21.12 Work through the stereochemical relationships in the following
reactions of trans-2-butene in great detail. Follow the reactions of all pairs of
enantiomers. Contrast them with what you would expect from a reaction not
involving a neighboring group effect.
meso-2,3-Dibromobutane
CH
3
H
3
C
H
H
Br
Br
..
..
..
1. Br
2
/H
2
O
2. HBr
..
..
..
H
HCH
3
H
3
C

1096 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
WORKED PROBLEM 21.13 There is a fundamental difference between the forma-
tion of a cyclic ion using a pair of nonbonding electrons and forming the cyclic
ion using a pair of σ electrons. Carefully draw out the cyclic species for both cases
and explain the difference. Hint: Count electrons.
ANSWER Problem 21.12 contains many examples of formation of a three-
membered ring using a pair of nonbonding electrons.The result is a normal com-
pound in which all bonds are two-electron bonds.
+
H
H
H
3
C
CH
3
CH
3
Br
..
..
..
..
OH
2
H
H
H
3
C
=
Br
..
..
CH
3
H
H
H
3
C
Br
..
..
++
.
.
+
H
H
H
3
C
CH
3
CH
3
A
H
H
H
3
C
=
A
+
CH
3
H
H
H
3
C
A
+
CH
3
H
H
H
3
C
A
+
.
.
.
.
.
.
OH
2
..
When a ring is formed through participation by a σ bond, everything changes. It
isn’t possible to make what we could call a normal three-membered ring because
there are not enough electrons. The two electrons originally in the σ bond must
serve to bind three nuclei.
21.3 Neighboring Systems
21.3a Aromatic Neighboring Groups We have progressed from neighbor-
ing group reactions involving very strong nucleophiles such as alkoxides and sulfur,
to those of less powerful displacing agents such as the halogens. Now we examine
even weaker nucleophiles, the π systems of aromatic molecules and alkenes. We will
finally move on to very weak nucleophiles indeed, and see σ bonds acting as
intramolecular displacing agents.
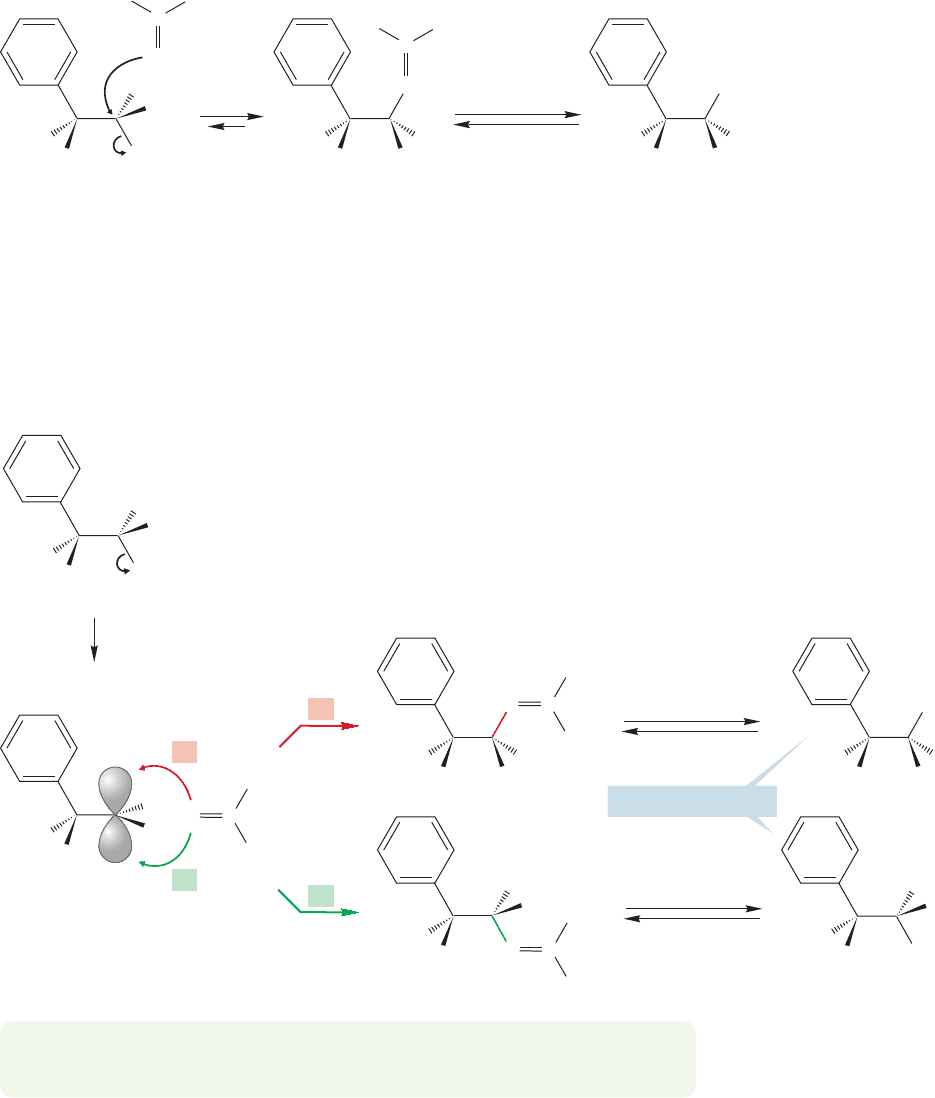
The π systems of aromatic rings can act as neighboring groups.The reactions of
the 3-phenyl-2-butyl tosylates are good examples. D. J. Cram [1919–2001, award-
ed the Nobel Prize in Chemistry in 1987 for work on crown ethers (p. 254)] and
his co-workers showed that in acetic acid, the optically active 3-phenyl-2-butyl tosy-
late shown in Figure 21.23 gives a racemic mixture of the corresponding 3-phenyl-
2-butyl acetates.
But this result is not what we expect from a simple analysis of the possible dis-
placement reactions, either S
N
2 or S
N
1 substitution. The S
N
2 reaction must give
CH
3
Racemic mixture of acetatesOptically active
H
3
C
H
H
CH
3
H
3
C
H
H
+
(AcOH)
CH
3
COOH
OAc
CH
3
H
3
C
H
H
OTs
AcO
FIGURE 21.23 This optically
active isomer of 3-phenyl-2-
butyl tosylate gives a racemic
mixture of the corresponding
acetates.
Neighboring pi: Not to be confused
with neighborly pies!
21.3 Neighboring Systems 1097
inversion in the substitution step, and would form the optically active acetate shown
in Figure 21.24.
Optically active tosylate
CH
3
H
3
C
H
H
OTs
–
OTs
C
H
3
C
O
..
..
OH
..
..
S
N
2
H
3
C
CH
3
H
H
C
H
3
C
O
..
OH
..
..
+
H
3
C
CH
3
H
H
OAc
deprotonate
Optically active acetate
(not formed)
FIGURE 21.24 Simple S
N
2 displacement by acetic acid fails to predict the correct product.
Note the eclipsed forms drawn in this figure! They are there for clarity only and will be
converted into the more stable staggered forms.
Optically active
Still optically active
Both optically active
+
C
OH
O
..
..
..
..
CH
3
H
3
C
H
H
OAc
CH
3
CH
3
CH
3
C
OH
CH
3
H
3
C
H
H
H
3
C
H
OTs
H
S
N
1
H
3
C
CH
3
H
H
O
+
..
C
OH
CH
3
H
3
C
CH
3
H
H
O
H
3
C
CH
3
H
H
OAc
deprotonation
deprotonation
(a)
(b)
(a)
(b)
+
..
..
..
..
..
FIGURE 21.25 An S
N
1 ionization
also fails to form the product with
the correct stereochemistry. The S
N
1
reaction must give a mixture of two
optically active acetates.
Ionization in an S
N
1 process gives an intermediate open carbocation that can be
attacked at either face by acetic acid to give the two different optically active prod-
ucts of Figure 21.25.
PROBLEM 21.14 What kind of stereoisomers are the two products at the right of
Figure 21.25?
Because neither the S
N
1 nor the S
N
2 reaction produces the observed product,
some other mechanism must be operating in this reaction. One of the clues for a
neighboring group participation is present—an unexpected stereochemical result,
clue 1—so let’s look around for possible internal nucleophiles.
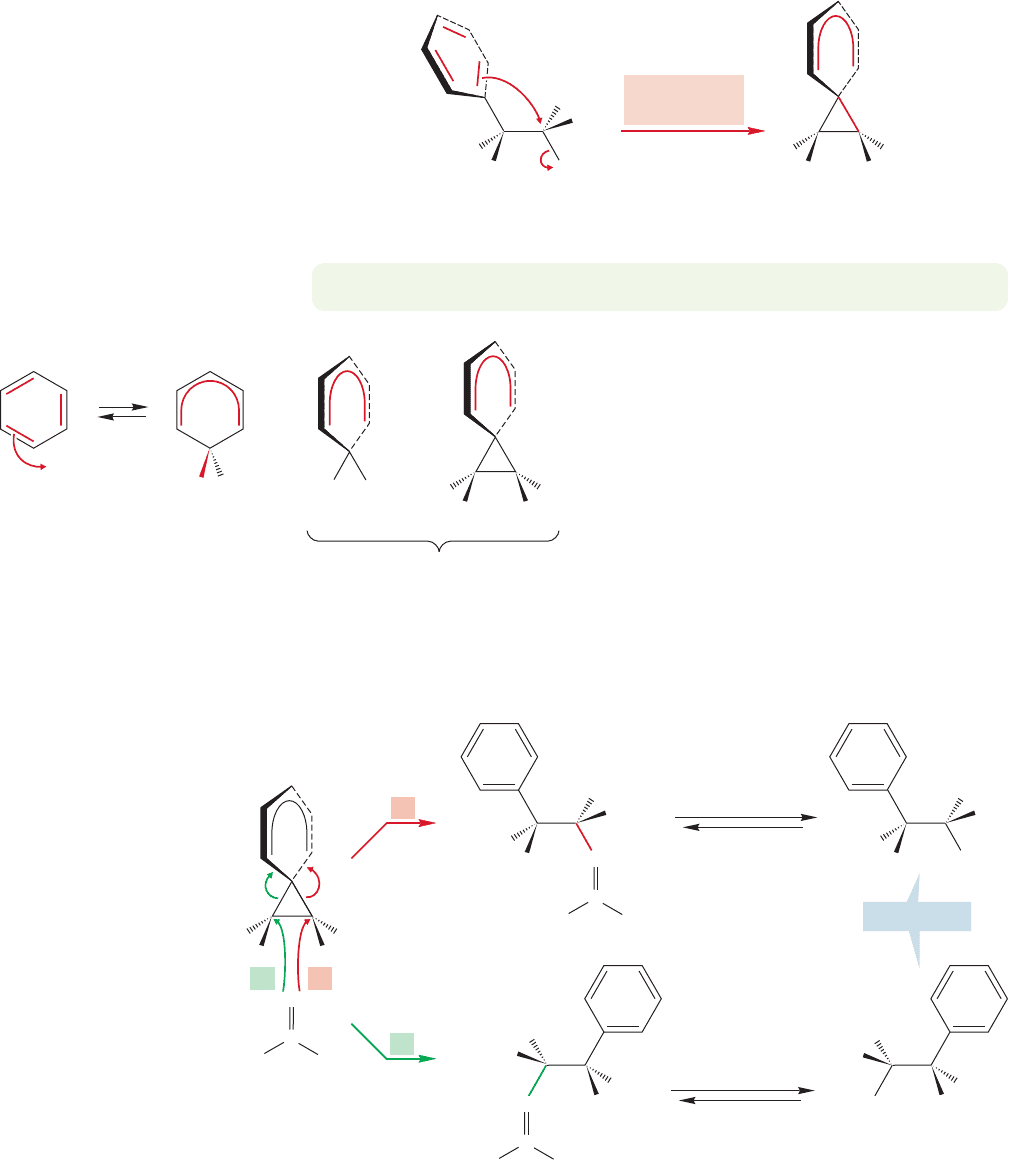
1098 CHAPTER 21 Intramolecular Reactions and Neighboring Group Participation
FIGURE 21.26 If the benzene ring
acts as a neighboring group, a cyclic
phenonium ion intermediate results.
Notice that the intermediate has a
plane of symmetry and is therefore
achiral.
FIGURE 21.27 The phenonium ion
is similar to the intermediate ion
produced in electrophilic aromatic
substitution.
PROBLEM 21.15 Draw resonance forms for the phenonium ion of Figure 21.26.
Note carefully the geometry of the phenoni-
um ion. The two rings are perpendicular, not
coplanar, and the ion is achiral, a meso compound.
All chance of optical activity is lost at this point.
Note also the close correspondence between this
intermediate and the benzenonium ion formed
in an electrophilic aromatic substitution reaction
(Fig. 21.27).
There is nothing particularly odd about this
spirocyclic species. If it is opened in a second,
intermolecular S
N
2 reaction, there are two possi-
ble sites for attack (Fig. 21.28). The two products are mirror images, and the
racemic mixture of enantiomers is exactly what is found experimentally.
Aromatic rings act as nucleophiles toward all kinds of electrophiles in the aromatic
substitution reaction (Chapter 13), so perhaps we can use the π system of the phenyl
ring in 3-phenyl-2-butyl tosylate as a nucleophile. If the π system displaces the tosylate,
it must do so from the rear.The result is the phenonium ion shown in Figure 21.26.
H
3
C
HH
CH
3
intramolecular
S
N
2
A phenonium ion—achiral!
CH
3
H
3
C
H
H
OTs
+
–
OTs
CH
3
H
3
C
H
H
(a)
(a)
(b)
(b)
H
3
C
deprotonation
Enantiomers
CH
3
H
3
C
H
H
CH
3
H
3
C
H
H
C
OH
..
..
O
..
..
H
3
C
C
OH
..
..
O
..
H
3
C
C
OH
..
..
O
..
CH
3
H
3
C
H
H
CH
3
H
3
C
H
H
+
deprotonation
OAc
..
..
AcO
..
..
+
+
FIGURE 21.28 Opening of the phenonium ion in
Figure 21.26 must occur in two equivalent ways (a and
b) to give a pair of enantiomers—a racemic mixture.
H
CH
3
H
3
C
HH
These two species are
very similar
Aromatic substitution
E
+
=
~
~
+
+
+
E
E H
