Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

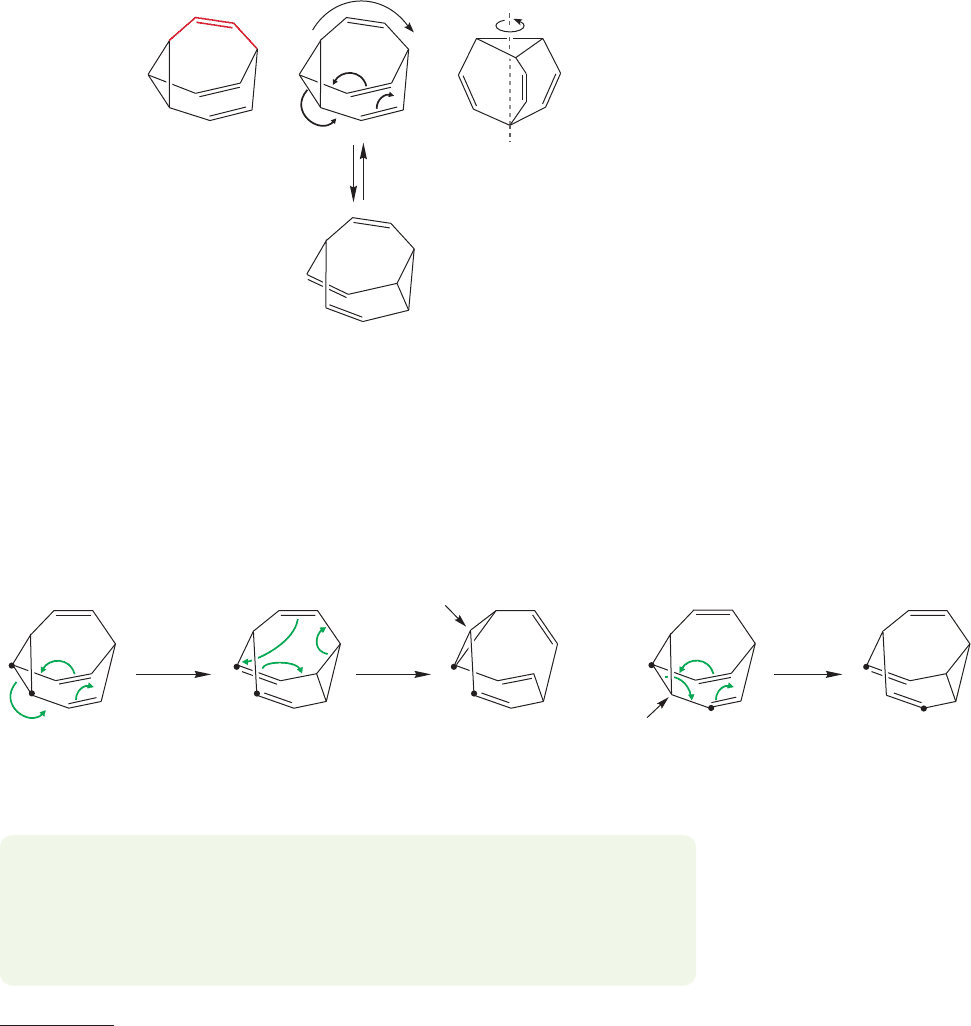
20.7 A Molecule with a Fluxional Structure 1069
But now perhaps you notice the threefold rotational axis in bullvalene. Remove
the color and the three double bonds become indistinguishable. In fact, there is a
Cope rearrangement possible in each of the three faces of the molecule, and every
Cope rearrangement is degenerate; each re-forms the same molecule (Fig. 20.67).
This point is much easier to see if you use a model.
c
3
Cope rearrangement
Turn 90⬚
axis
==
FIGURE 20.67 Bullvalene has a
threefold rotational axis: All three
double bonds are equivalent.The
Cope rearrangement can occur in
each of the three faces of the
molecule and is degenerate in
every case.
That’s quite interesting, but now begin to look at the structural consequences
for bullvalene. As Cope rearrangements occur, the carbons begin to move apart! This
wandering of atoms is shown in Figure 20.68 using dots to track two arbitrary adja-
cent carbons. The figure only begins the process, but it can be demonstrated that
the Cope rearrangement is eventually capable of completely scrambling the 10 CH
units of the molecule.
7
As long as the Cope rearrangement is fast, bullvalene has no
fixed structure; no carbon has permanent nearest neighbors, but is instead bonded
on time average to each of the 9 other methine carbons. It has a fluxional structure.
1
2
2
1
3
4
Cope
in
bottom
face
Cope
in
Redraw-arrow
for location purposes
back
face
Cope
in
bottom
face
=
FIGURE 20.68 A sequence of Cope rearrangements serves to move carbon atoms throughout the molecule.
WORKED PROBLEM 20.20 At somewhat above room temperature the
1
H NMR
spectrum of bullvalene consists of a single broad line at δ 4.2 ppm. Explain.
ANSWER If all the carbon–hydrogen bonds are the same on time average, the
NMR spectrum must consist of a single line. We see the averaged spectrum.
7
In an early paper, Doering noted that a sequence of 47 Cope rearrangements sufficed to demonstrate that
two adjacent carbons could be exchanged without disturbing the rest of the 10-carbon sequence, and that there-
fore all 1,209,600 possible isomers could be reached. No claim was made that this represented the minimum
path. A later computer-aided effort by two Princeton undergraduates (Allan Fisher and Karl Bennett) located
a sequence of 17 Cope rearrangements that could do the same thing.
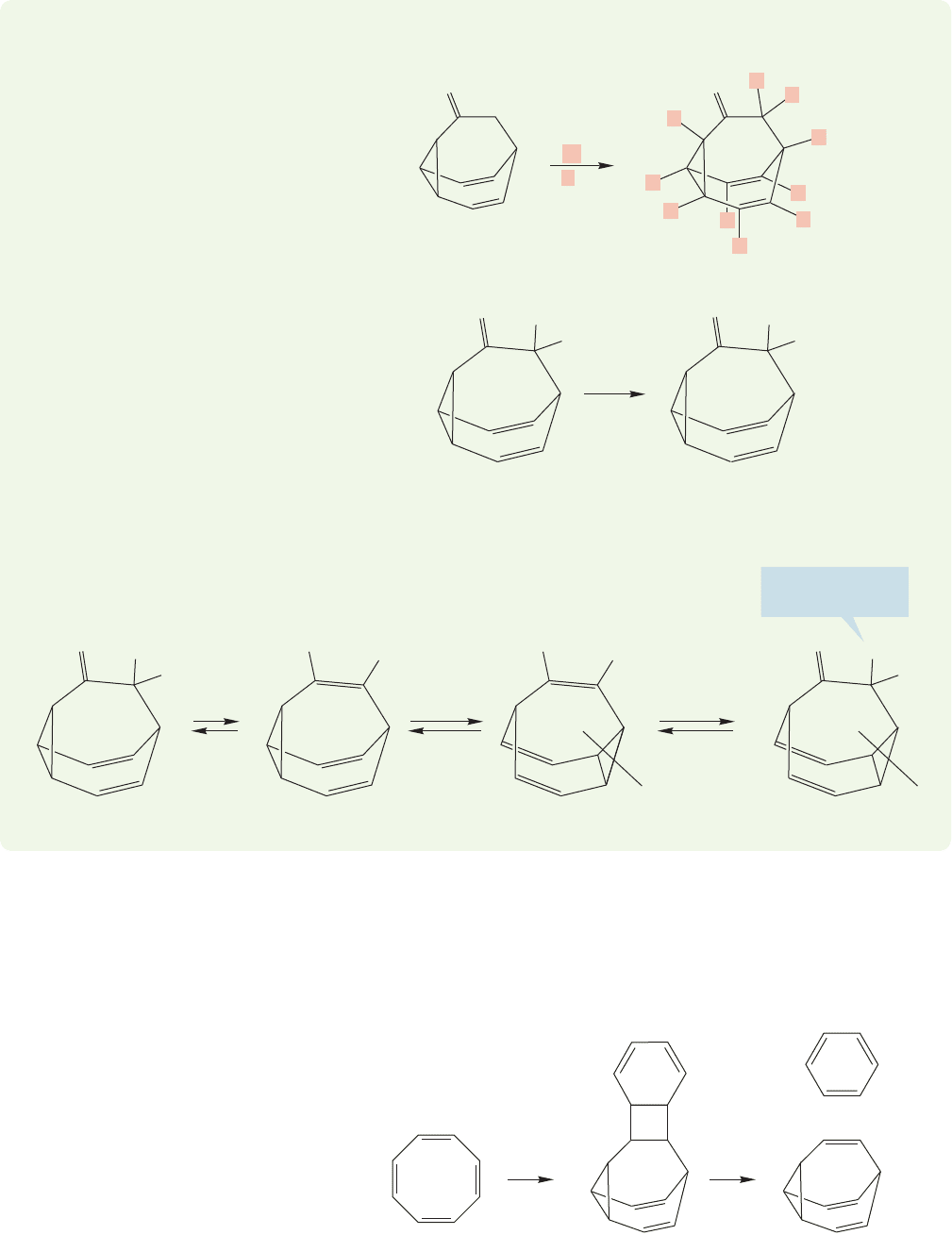
1070 CHAPTER 20 Reactions Controlled by Orbital Symmetry
D
DO
–
O
D
D
D
D
2
O
D
O
D
D
D
D
D
ANSWER Exchange at the α position is routine.
O
H
H
D
D
O
D
2
O
DO
–
WORKED PROBLEM 20.21 Explain why the ketone below exchanges all its hydro-
gens for deuteriums when treated with deuterated base.
However, simple ketones are in equilibrium with their enol forms, and the enol of
this molecule is a substituted bullvalene. The Cope rearrangement makes all the
carbons equivalent, and thus, on time average, all carbon–hydrogens will eventu-
ally come to occupy the exchangeable α position.
Repetition leads to exchange of all the hydrogens for deuterium.
D
D
O
D
DO
H
D
D
O
D
2
OCope
DO
–
H
DO
Now this hydrogen
can exchange
D
Naturally, efforts at synthesis began as soon as the prediction of the properties of
bullvalene appeared in the chemical literature.Long before the efforts at a rational syn-
thesis were sucessful, the molecule was discovered by Gerhard Schröder (b. 1929), a
German chemist then working in Belgium. Schröder was working out the structures
of the several dimers of cyclooctatetraene, and in the course of this effort irradiated
one of the dimers. It conveniently fell apart to benzene and bullvalene (Fig. 20.69).
(C
8
H
8
)(C
16
H
16
)(C
10
H
10
)
(C
6
H
6
)
2
Δ
hν
FIGURE 20.69 Schröder’s accidental
synthesis of bullvalene.
20.8 How to Work Orbital Symmetry Problems 1071
Schröder was alert enough to understand what had happened, and went on to a
highly successful career that included an examination of the properties of bullvalene
and substituted versions of this molecule. Doering’s predictions were completely
confirmed by Schröder’s synthesis. Other neutral, completely fluxional organic mol-
ecules have not appeared, although the phenomenon of fluxionality appears to be
rather more common in organic cations and organometallic compounds.
(a)
(b)
O
H
H
O
OH
OH
Δ
Δ
PROBLEM 20.23 Provide mechanisms for the following reactions:
Summary
The Cope rearrangement, a [3,3] sigmatropic shift, is far more than just a chem-
ical curiosity. It is a general reaction of almost all 1,5-hexadienes, and occurs
almost no matter how the skeletal hexadiene form is perturbed and disguised.
All sorts of chemical “spinach” can be added, often making it quite difficult to
find the bare bones of the hexadiene system, but the reaction will persist.
Moreover, as we have just seen, digging deep into the Cope reaction, following
the intellectual implications of substitution, leads to the marvelous molecule
bullvalene, without precedent in organic chemistry.
20.8 How to Work Orbital Symmetry Problems
PROBLEM SOLVING
All people and all problems are, of course, different. Nonetheless, there are some
general principles that apply, and it is worth summarizing ways to approach—
and hopefully solve—orbital symmetry problems, which are separable into three
varieties.
20.8a Electrocyclic Reactions
1. Look for rings opening or closing within a single molecule. Sound like simple advice?
Yes, it is, but in all these problems the major difficulty is one of recognition, and it is
well worth your time to be a little “too straightforward,” and to “think simple.”
PROBLEM 20.22 Why is the second step of Figure 20.69, the photochemical
fragmentation, so easy?
Fluxional molecules are the
result of electrons and atoms
moving much like the blur of a
spinning skater.
1072 CHAPTER 20 Reactions Controlled by Orbital Symmetry
2. Once you are sure that the question involves an electrocyclic reaction,draw an arrow
formalism.
3. Next, work out the molecular orbitals of the open-chain partner.
4. Count the number of electrons involved in the reaction. Be careful to take account
of any charges.
5. Knowing the number of electrons allows you to find the HOMO of the open-chain
partner.
6. Finally, work out the motion,conrotatory or disrotatory, that allows the ring to close
through a bonding—not antibonding—interaction. If there are 4n electrons
involved in the reaction, then the thermal process will be conrotatory and the
photochemical process will be disrotatory. If there are 4n 2 electrons, then the
thermal process will be disrotatory and the photochemical will be conrotatory.
20.8b Cycloaddition Reactions
1. Look for a ring forming from two molecules or breaking open to give two new mol-
ecules. When you find this situation, you know you have a cycloaddition reaction.
2. Draw an arrow formalism.
3. Work out the π molecular orbitals for the two partners.
4. Identify the HOMOs and LUMOs.
5. Examine either possible HOMO–LUMO interaction. It is generally easier to look
at ring closing, rather than ring opening. Your final answer will be good for either
direction (of course!).
6. Count the number of electrons involved in the reaction.
7. If there are 4n electrons involved,then the thermal process will require a rotation in order
to occur and the photochemical process will not. If there are 4n 2 electrons involved,
then the thermal process will not require a rotation and the photochemical will.
20.8c Sigmatropic Shift Reactions These reactions are by far the hardest
for students to “get.”
1. The major difficulty is one of recognition. Is some piece of the molecule flying
around, departing from one place and reattaching somewhere else? If so, that’s
probably a sigmatropic shift reaction. Once you are sure it is a shift, identify—
mark—the shifting atom or group.
2. Where does it depart from? Where does it arrive? Draw an arrow formalism.
3. Now let’s work out a picture of the transition state. Break the bond attaching the
migrating group to the position from which it departs in a homolytic fashion.
Neither ions nor polar transition states are involved.Ask yourself what species is left
behind? What species is migrating?
4. Work out the molecular (or atomic) orbitals for both partners.
5. Remember that the migrating group was attached through a bonding interaction.
That fact allows you to sketch in the symmetry of the HOMO of the portion of the
molecule along which the atom or group is migrating.
6. Can the migrating group reattach reasonably (can it reach?) in a bonding fashion?
If so, the reaction is allowed. If it must reattach through an antibonding interaction,
the reaction is forbidden.
7. Count the number of electrons involved in the reaction.
20.9 Summary 1073
8. If there are 4n electrons involved, then the thermal hydrogen (or other) shift must
be antarafacial in order to occur and the photochemical process will occur in a
suprafacial fashion. If there are 4n 2 electrons, then the thermal process will be
suprafacial and the photochemical process will be antarafacial.
Now practice! Use the schemes above to work the following three problems.
PROBLEM 20.24 Is the following reaction allowed or disallowed? Give a detailed
analysis.
Δ
+
+
PROBLEM 20.25 Is the following thermal reaction allowed or disallowed? Give a
detailed analysis.
..
–
Δ
H
2
CCH
2
..
–
H
2
CCH
2
Δ
H
2
C
H
CH
3
H
3
C
H
3
C
CH
3
H
3
C
PROBLEM 20.26 Is the following thermal reaction allowed suprafacially or
antarafacially? Give a detailed analysis.
20.9 Summary
New Concepts
The central insight of Woodward–Hoffmann theory is that
one must keep track of the phase relationships of orbital
interactions as a concerted reaction proceeds. If bonding
overlap can be maintained throughout, the reaction is allowed;
if not, the reaction is forbidden by orbital symmetry. This
notion leads to a variety of mechanistic explanations for
reactions that can otherwise be baffling. Some of these
pericyclic reactions are discussed in the section on Reactions,
Mechanisms, and Tools.
The notion of a fluxional structure is introduced through
the (CH)
10
molecule bullvalene. In this molecule, a given
carbon atom does not have fixed nearest neighbors, but instead
is bonded, over time, to each of the other nine carbons in the
molecule. In bullvalene, the fluxionality is the result of rapid
degenerate Cope rearrangements.
Many stereochemical labeling experiments are described in
this chapter. A careful labeling experiment is able to probe
deeply into the details of a reaction mechanism. Examples
include W. R Roth’s determination of the suprafacial nature of
the [1,5] shift of hydrogen and Doering and Roth’s experiments
on the structure of the transition state for the [3,3] sigmatropic
shift known as the Cope rearrangement.
Key Terms
antarafacial motion (p. 1055)
bullvalene (p. 1068)
conrotation (p. 1037)
Cope rearrangement (p. 1059)
cycloaddition reaction (p. 1043)
degenerate reaction (p. 1031)
disrotation (p. 1037)
electrocyclic reaction (p. 1036)
fluxional structure (p. 1063)
pericyclic reaction (p. 1031)
sigmatropic shift (p. 1050)
suprafacial motion (p. 1055)
Woodward–Hoffmann theory (p. 1031)

1074 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Reactions, Mechanisms, and Tools
20.10 Additional Problems
Certain polyenes and cyclic compounds can be interconverted
through a pericyclic process known as an electrocyclic reaction.
Examples include the 1,3-butadiene–cyclobutene and 1,3-
cyclohexadiene–1,3,5-hexatriene interconversions (Figs. 20.5
and 20.16).
Orbital symmetry considerations dictate that in 4n-electron
reactions the thermal process must use a conrotatory motion,
whereas the photochemical reaction must be disrotatory. Just
the opposite rules apply for reactions involving 4n 2 elec-
trons. The key to analyzing electrocyclic reactions is to look at
the way the p orbitals at the end of the open-chain π system
must move in order to generate a bonding interaction in the
developing σ bond.
Cycloaddition reactions are closely related to electrocyclic
reactions.The phases of the lobes in the HOMOs and LUMOs
must match so that bonding interactions are preserved in the
transition state for the reaction. Sometimes a straightforward
head-to-head motion is possible in which the two π systems
approach each other in parallel planes to produce two bonding
interactions. Such reactions are typically easy and are quite
common. An example is the Diels–Alder reaction (Fig. 20.21).
In other reactions, the simple approach of HOMO and
LUMO involves an antibonding overlap. In such reactions, a
rotation is required in order to bring the lobes of the same sign
together (Fig. 20.28). Although in principle this rotation main-
tains bonding interactions, in practice it requires substantial
amounts of energy, and such reactions are rare.
Sigmatropic shifts involve the migration of hydrogen or
another atom along a π system. Allowed migrations can be
either suprafacial (leave and reattach from the same side of the
π system) or antarafacial (leave from one side and reattach from
the other) depending on the number of electrons involved in
the migration. The important point is that in an allowed shift,
bonding overlap must be maintained at all times both to the
lobe from which the migrating group departs and to that to
which it reattaches. Steric considerations can also be important,
especially for hydrogen, which must migrate using a small 1s
orbital (Figs. 20.43 and 20.44).
Syntheses
The photochemical 2 2 dimerization of alkenes.
Common Errors
+
hν
For many students the big problem in this material is determin-
ing the kind of reaction involved. The way to start a problem in
this area is to spend some time and thought identifying what
kind of reaction is taking place. Cycloadditions are generally
easy to find, although even here confusion exists between these
reactions and electrocyclic processes. Sigmatropic shifts can be
even harder to uncover. Often it is not easy to identify exactly
what has happened in a sigmatropic reaction. When one atom
or a group of atoms has translocated from one part of the
molecule to another, there is often a rather substantial
structural change. The product sometimes doesn’t look much
like the starting material. The temptation is to use other
reagents to make the change, but in a sigmatropic shift every-
thing is “in house.”
PROBLEM 20.27 Analyze the photochemical and thermal
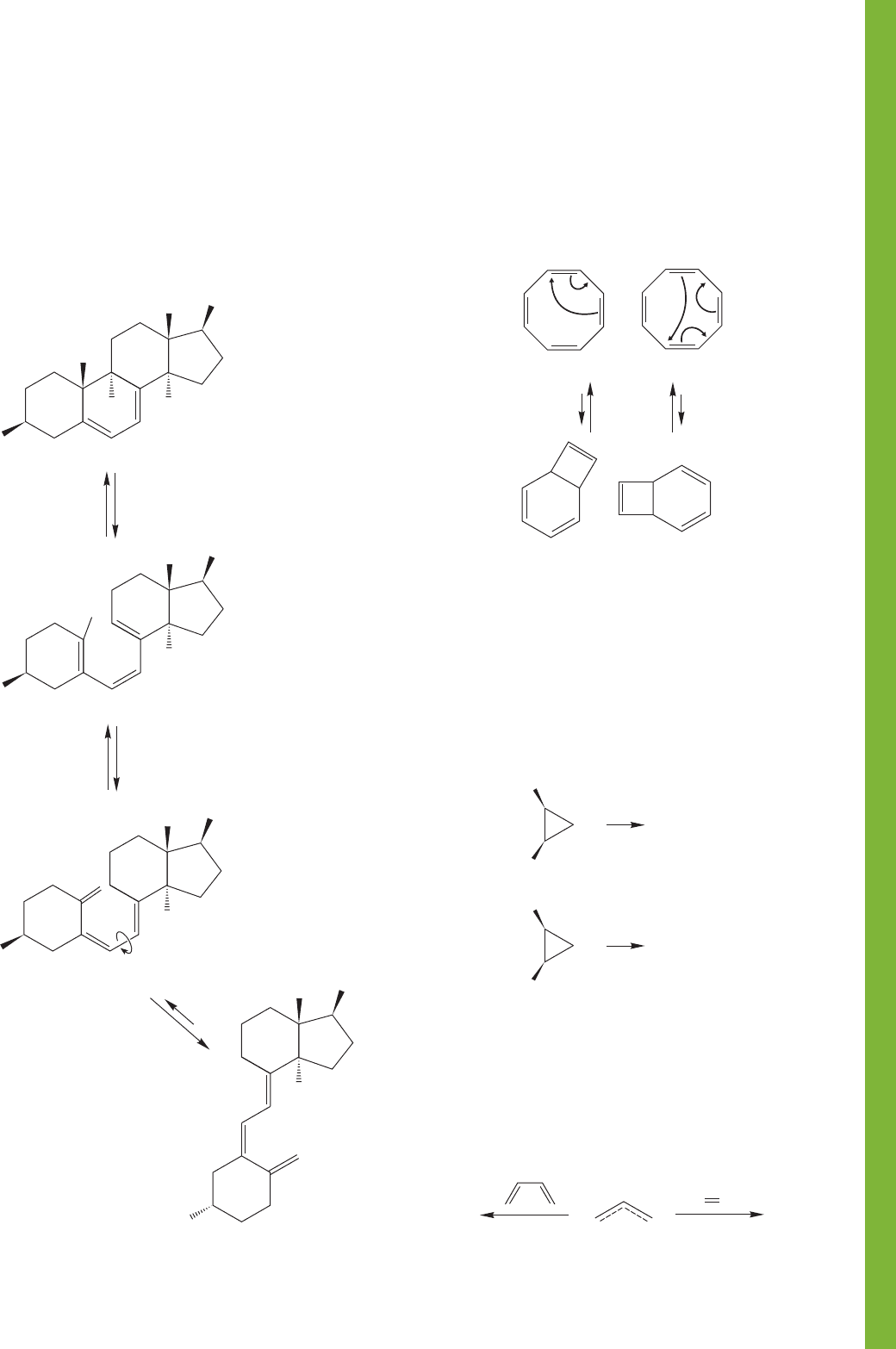
[1,7] shifts of hydrogen in 1,3,5-heptatriene. Be sure to look at
all possible stereoisomers of the starting material. What kind of
[1,7] shift will be allowed thermally? What kind photochemi-
cally? How will the starting stereochemistry of the triene affect
matters? Top views of the π molecular orbitals of heptatrienyl
are given.
Energy
CH CH
+
+
+
+
+
+
+
–
–
–
0
+
+
+
+
+
–
–
–
+
+
–
0
+
0
–
0
+
+
–
–
+
–
–
+
–
+
–
0
+
–
+
+
–
+
–
+
–
+
Top views of the π
molecular orbitals
of heptatrienyl
(+ means blue,
– means green)
1,3,5-Heptatriene
H
2
C CH CH
3
CH CH
20.10 Additional Problems 1075
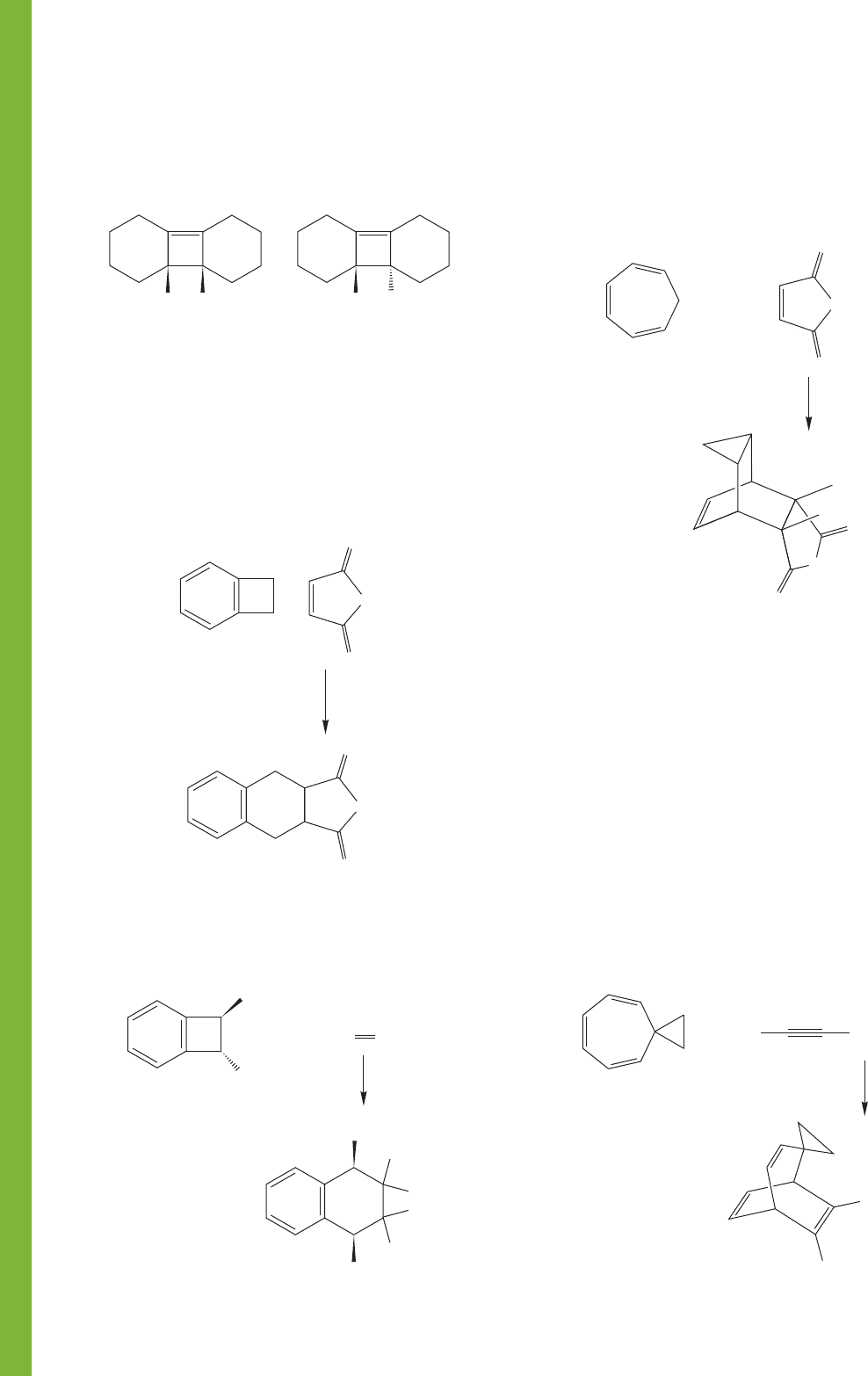
PROBLEM 20.29
The thermally induced interconversion of
cyclooctatetraene (1) and bicyclo[4.2.0]octa-2,4,7-triene (2) can
be described with two different sets of arrows. Although these
two arrow formalisms may seem to give equivalent results, one
of them is, in fact, not a reasonable depiction of the reaction.
Explain.
PROBLEM 20.28 Vitamin D
3
(3) is produced in the skin as a
result of UV irradiation. It was once believed that 7-dehydro-
cholesterol (1) was converted directly into 3 upon photolysis. It
is now recognized that there is an intermediate, previtamin D
3
(2), involved in the reaction. This metabolic process formally
incorporates two pericyclic reactions, 12and 23.
Identify and analyze the two reactions.
UU
HH
HO
CH
3
CH
3
C
8
H
17
H
HO
H
3
C
CH
3
C
8
H
17
H
HO
CH
3
C
8
H
17
H
1
2
s-cis-3
s-trans-3
rotation
HO
CH
3
C
8
H
17
1
(a)
(b)
1
2
=
PROBLEM 20.30 Analyze the thermal ring opening to allyl
ions of the two cyclopropyl ions shown below. What stereo-
chemistry do you expect in the products? Will the reactions
involve conrotation or disrotation? Explain. Do not be put off
by the charges! They only serve to allow you to count electrons
properly.
(a)
An allyl cation
+
Δ
(b)
An allyl anion
–
Δ
H
3
C
H
3
C
H
3
C
H
3
C
..
PROBLEM 20.31 Orbital symmetry permits one of the two fol-
lowing photochemical cycloaddition reactions to take place in a
concerted fashion. What would be the products, which reaction
is the concerted one, and why?
Allyl cation
+
hν hν
CH
2
H
2
C
??
1076 CHAPTER 20 Reactions Controlled by Orbital Symmetry
HH H
H
PROBLEM 20.32 The following two cyclobutenes can be opened
to butadienes thermally. However, they react at very different
rates. Which is the fast one, which is the slow one, and why?
PROBLEM 20.33 These same two cyclobutenes (Problem
20.32) react photochemically to give two monocyclic (one ring)
compounds isomeric with starting material. Predict the prod-
ucts, and explain the stereospecificity of the reaction.
PROBLEM 20.34 Benzocyclobutene (1) reacts with maleic
anhydride when heated at 200 °C to give the single product
shown. Write a mechanism for this reaction.
+
200 ⬚C
O
O
1
O
O
O
O
PROBLEM 20.35 Given your answer to Problem 20.34, explain
the stereochemistry observed in the following reaction:
+
Δ
Ph
Ph
Ph
Ph
C(CN)
2
(NC)
2
C
CN
CN
CN
CN
PROBLEM 20.36 Write a mechanism for the following reac-
tion. Note that no similar reaction appears for cis-1,3,5-hexa-
triene. It may be useful to work backward from the product.
What molecules must lead to it?
+
Δ
O
O
O
O
O
O
H
H
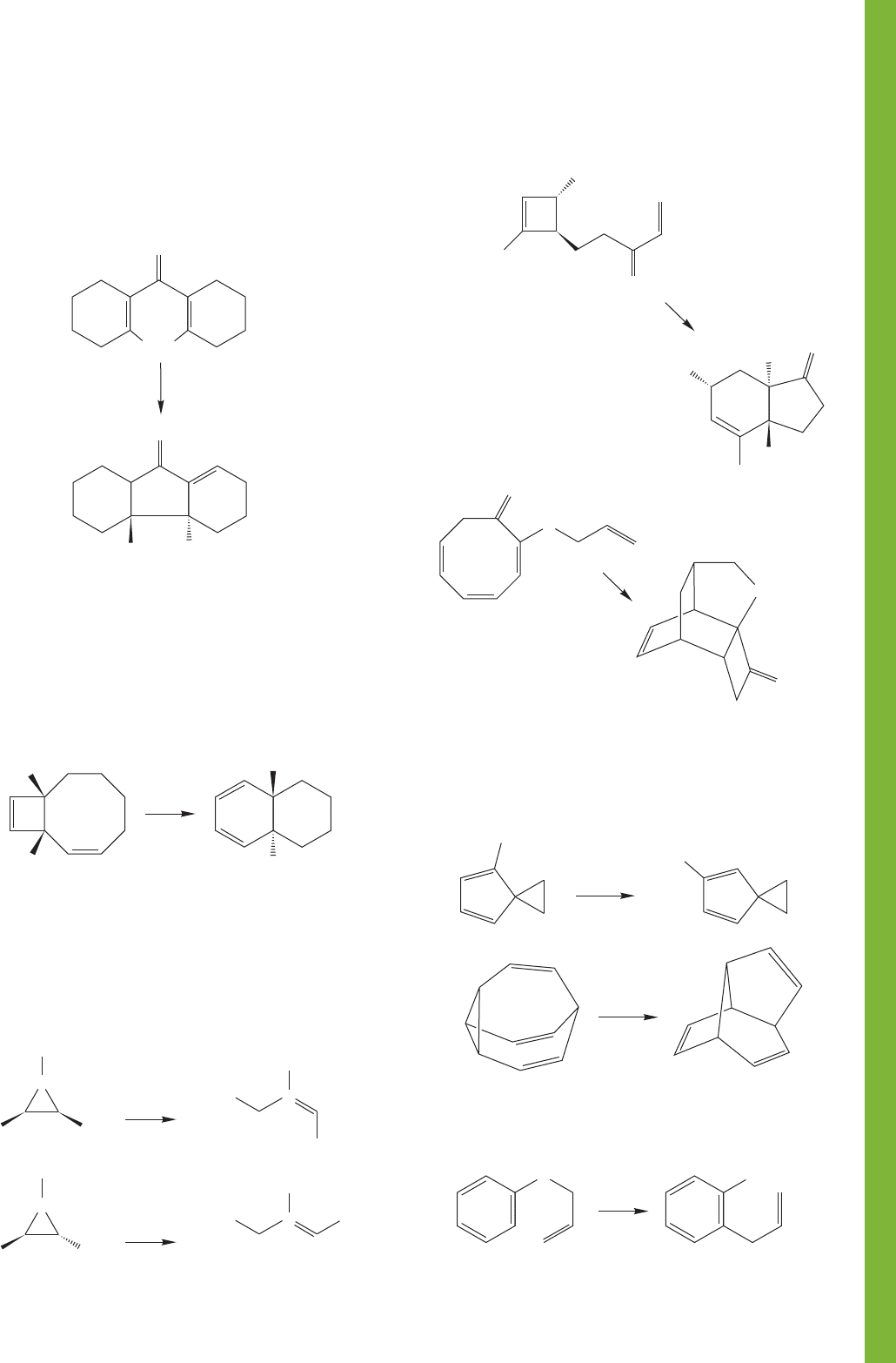
PROBLEM 20.37 Now, here’s an interesting variation on the
reaction of Problem 20.36. Diels–Alder additions typically
take place as shown in Problem 20.36, with the ring-closed
partner (A in the answer to Problem 20.36) actually doing
the addition reaction. However, the molecule shown below is
an exception, as the Diels–Alder reaction takes place in the
normal way to give the product shown. Draw an arrow
formalism for the reaction and then explain why 1 reacts
differently from other cycloheptatrienes.
+
Δ
CN
CN
CN
1
NC
20.10 Additional Problems 1077
PROBLEM 20.38
Write an arrow formalism mechanism for
the following thermal reaction and explain the specific trans
stereochemistry. Careful here—note the acid catalyst for this
reaction.
HH
H
H
O
O
H
3
PO
4
PROBLEM 20.39 Write an arrow formalism mechanism
for the following reaction and explain the observed trans
stereochemistry:
Δ
H
H
H
H
PROBLEM 20.40 Explain the following differences in product
stereochemistry:
Δ
Ph
Ph
N
CH
3
H
3
C
..
..
N
H
3
C
CH
3
–
+
Δ
Ph
Ph
N
CH
3
H
3
C
..
..
N
H
3
C
–
+
CH
3
PROBLEM 20.41 Provide mechanisms for the following trans-
formations:
Δ
Δ
O
H
H
O
O
O
(b)
(a)
COOCH
2
CH
3
CH
3
CH
2
OOC
CH
3
CH
2
OOC
CH
3
CH
2
OOC
O
O
PROBLEM 20.42 Provide mechanisms for the following trans-
formations. Part (a) is harder than it looks (Hint: There are two
intermediates in which the three-membered ring is gone) and
(b) is easier than it looks (Hint: What simple photochemical
sigmatropic shift do you know?).
Δ
hν
(b)
(a)
CH
3
H
3
C
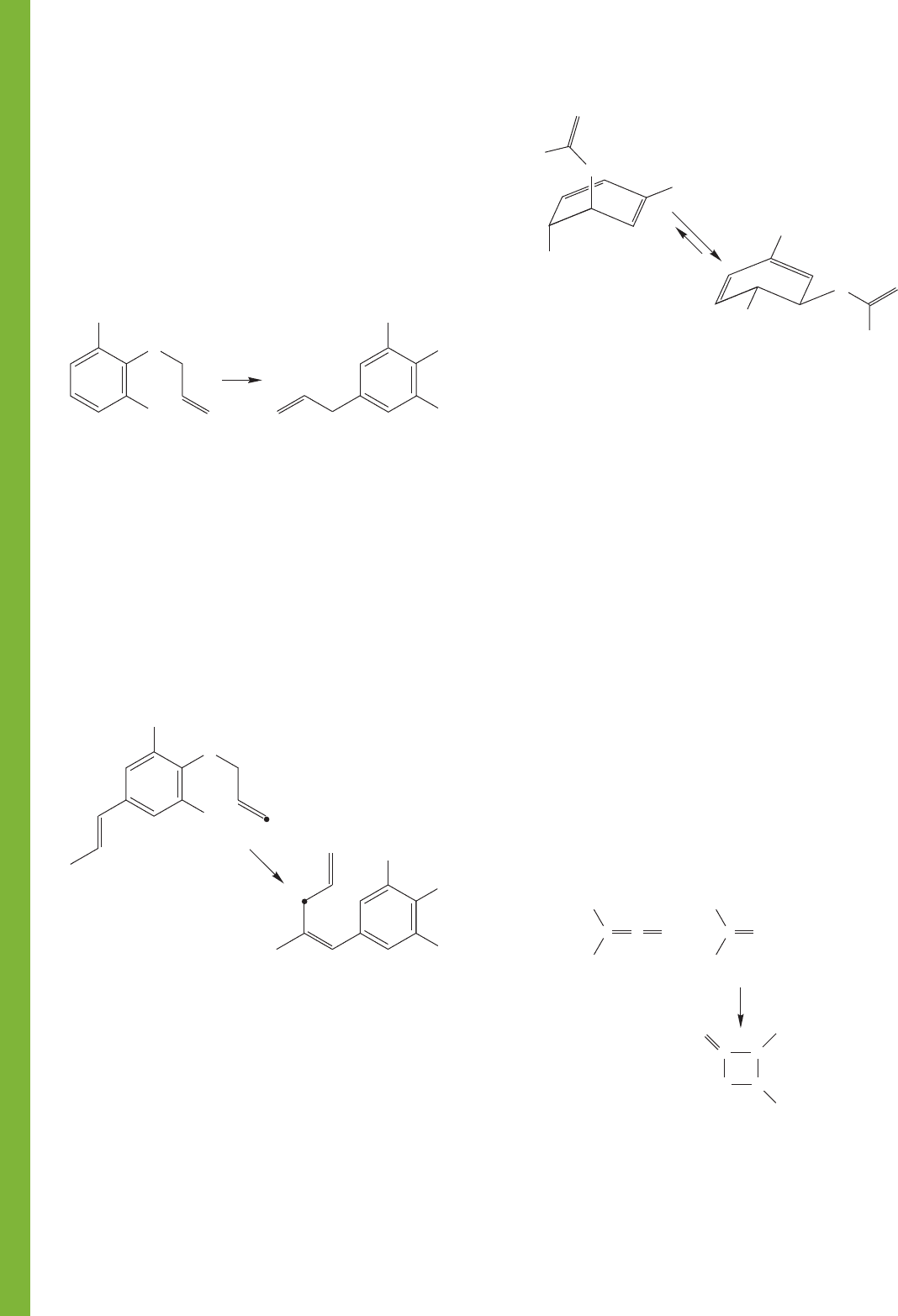
PROBLEM 20.43 In Problem 20.23 (b), we saw an example
of a [3,3] sigmatropic shift known as the Claisen rearrange-
ment. This reaction involves the thermally induced intramol-
ecular rearrangement of an allyl phenyl ether to an o-allylphenol.
Δ
OH
O
(continued)
1078 CHAPTER 20 Reactions Controlled by Orbital Symmetry
Δ
OH
O
CH
3
CH
3
CH
3
CH
3
Δ
OH
O
CH
3
CH
3
CH
3
CH
3
(a) It might be argued that this rearrangement is not a
concerted process, but instead involves a pair of radical
intermediates. Write such a mechanism and design a
labeling experiment that would test this hypothesis.
(b) If both the ortho positions of the allyl phenyl ether are
blocked with methyl groups, the product of this rearrange-
ment is the p-allylphenol.This version of the Claisen
reaction is often called the para-Claisen rearrangement.
Explain why the rearrangement takes a different course in
this case. In addition, would the label you suggested in (a)
be useful in this case for distinguishing concerted from non-
concerted (radical) mechanisms?
PROBLEM 20.44 Propose an arrow formalism mechanism for
the following reaction. Be sure that your mechanism accommo-
dates the specific labeling results shown by the dot (
•
14
C).
Also, be sure that any sigmatropic shifts you propose do not
involve impossibly long distances.
O
COO
OOC
–
–
OH
1
2
OOC
–
–
O
OH
COO
Perhaps the role of the enzyme is to bind the predominant
pseudodiequatorial conformer 2. The enzyme–substrate com-
plex then undergoes a rate-determining conformational change
to convert the substrate into the pseudodiaxial form 1, which
rapidly undergoes rearrangement.
(a) Explain why 1 can rearrange to prephenate but 2 cannot.
(b) Suggest a reason why conformer 2 is preferred over con-
former 1. Hint: Focus on the OH. What can it do in 2 that
it cannot do in 1?
(c) Draw an appropriate Energy versus Reaction progress dia-
gram that illustrates the situation outlined above, that is, a
reaction in which the rate-determining step is the conver-
sion of 2 into 1. What would the Energy versus Reaction
progress diagram look like if the rate-determining step were
the conversion of 1 into prephenate?
PROBLEM 20.46 In 1907, Staudinger reported the reaction of
diphenylketene (1) and benzylideneaniline (2) to give β-lactam
3. Although this reaction is formally a 2 2 cycloaddition
reaction, there is ample experimental evidence to suggest that
the reaction is not concerted, but is instead a two-step process
involving a dipolar intermediate.
PROBLEM 20.45 We saw on page 1063 that chorismate
undergoes a [3,3] sigmatropic shift (Claisen rearrangement) to
prephenate catalyzed by the enzyme chorismate mutase. This
enzyme-catalyzed reaction occurs more than 1 million times
faster than the corresponding nonenzymatic reaction. How does
the enzyme effect rate enhancement? Both enzymatic and
nonenzymatic reactions proceed through a chairlike transition
state. However, the predominant conformer of chorismate in
solution, as determined by
1
H NMR spectroscopy, is the pseu-
dodiequatorial conformer 2, which cannot undergo the reaction.
+
Ph
Ph
C
Ph
2
CCH
N
O
CCO
Ph
Ph
Ph
H
C NPh
20 ⬚C
12
3
(a) Propose an arrow formalism mechanism for this cycloaddi-
tion reaction. Don’t forget to include the dipolar intermediate.
