Jung Han. Innovations in Food Packaging
Подождите немного. Документ загружается.


Preservative packaging for fresh meats, poultry, and fin fish
213
Fish
Unlike red meats, cooked fish is usually perceived as tender by consumers (Cardello
et al., 1982). Consequently, aging does not enhance the acceptability of fish muscle.
The primary factor determining the texture of fish is the final pH, which affects the
water content of the muscle. Water content increases with increasing pH, and muscle
of unusually high pH can have an undesirable "sloppy" texture (Love, 1988). High
C02 concentration can have the reverse effect, as C02 dissolved in the tissue lowers
pH, thus reducing the water-holding capacity and consequently increasing water
extrusion (Davis, 1998). Dipping fish fillets in a sodium chloride solution can reduce
this effect (Pasteroriza
et al., 1998).
Spoilage of fish due to changes in flavor and odor during storage is almost invari-
ably a consequence of microbial activity. However, oxidative rancidity may contribute
to spoilage, and for this reason high
O2
MAP is not appropriate for use with fatty fish
(Sivertsvik
et
al.,
2002). The early development of rancid odors and flavors in fatty
fish such as salmon and trout is delayed or prevented by packaging in VP or low O2
MAP (Randell
et al., 1999).
Delay
of
microbial spoilage
Red
meats
Muscle tissue provides a rich medium for the support of bacterial growth, and
spoilage bacteria can grow at temperatures below that at which muscle tissue starts to
freeze (Lowry and Gill, 1984). Consequently, meat that is stored at chiller tempera-
tures will inevitably be spoiled by the activities of a spoilage microflora, whether or
not it has been previously rendered unacceptable to consumers by undesirable
changes in appearance, odor, or flavor.
The composition of the bacterial flora at the onset of spoilage is determined by the
qualities of the tissue on which the bacteria are growing, the atmospheric composi-
tion, and the numbers and composition of the microflora at the time of packaging.
A
spoilage flora will generally be dominated by those organisms that can grow most rap-
idly in the environment provided by the tissue and the pack atmosphere (Gill, 1986).
If the initial numbers of bacteria on the meat are small, the growth rate advantage of
the dominant species will be expressed over a relatively large number of generations
before the onset of spoilage. Then, slower-growing organisms will be a trivial fraction
of the flora present at spoilage. However, if the initial number of bacteria is high,
slower-growing organisms may persist as a substantial part of the flora and contribute
to the spoilage process.
The spoilage flora of red meats exposed to air is invariably dominated by species of
Pseudomonas (Gill and Newton, 1977). These organisms are strictly aerobic and pref-
erentially utilize glucose for growth, although many other substances can be utilized
as well. While glucose is available to the pseudomonads, the utilization of other sub-
stances is prevented by catabolite repression (Nychas
et
al., 1988). Red meat muscle
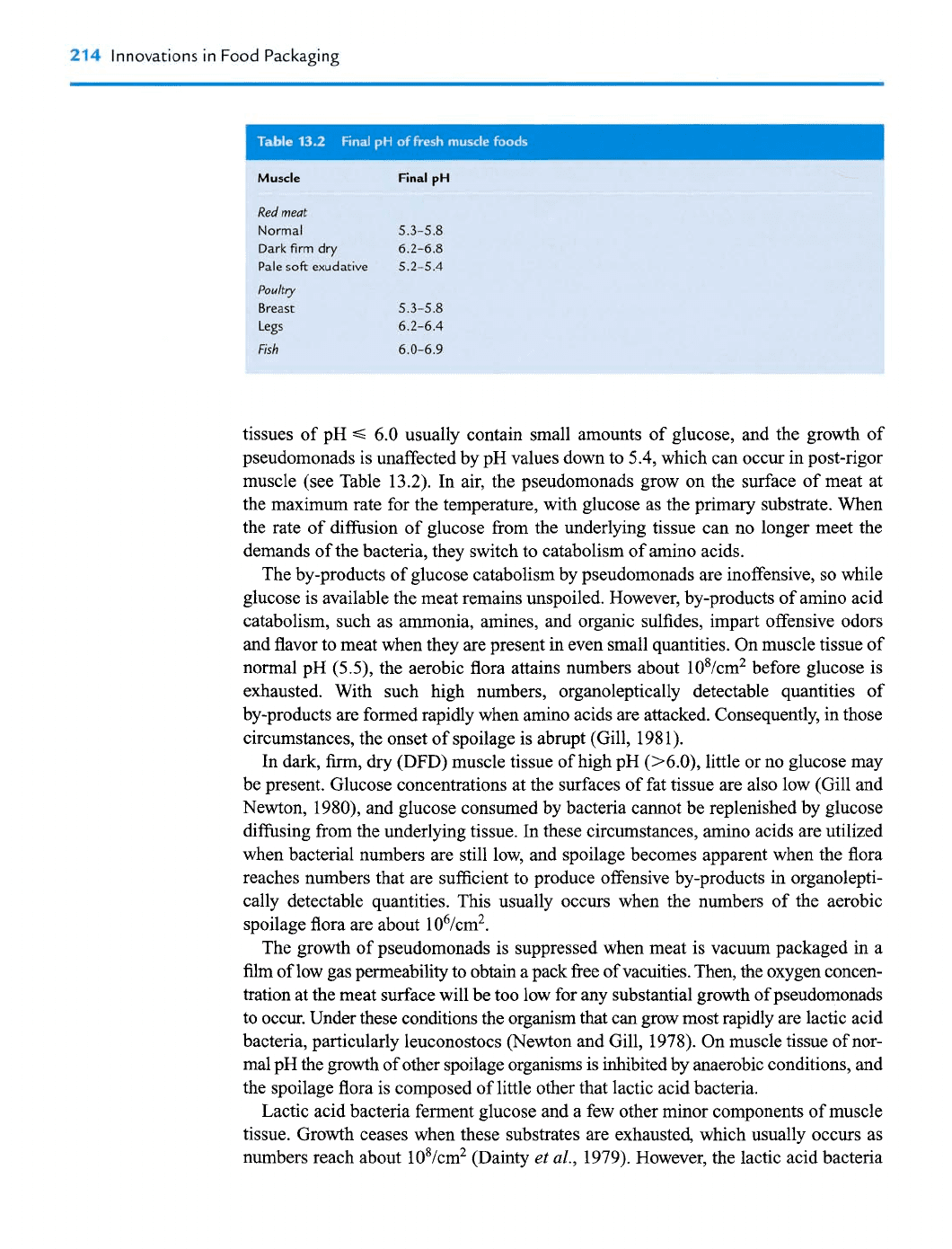
214
Innovations in Food Packaging
Mude
Final
pH
1
Redmeat
Normal
5.3-5.8
Dark firm dry
6.2-6.8
Pale soft exudative
5.2-5.4
Poultry
.
Breast
5.3-5.8
Legs
6.2-6.4
Fish
6.0-6.9
tissues of pH
S
6.0 usually contain small amounts of glucose, and the growth of
pseudomonads is unaffected by pH values down to 5.4, which can occur in post-rigor
muscle (see Table 13.2). In air, the pseudomonads grow on the surface of meat at
the maximum rate for the temperature, with glucose as the primary substrate. When
the rate of diffusion of glucose from the underlying tissue can no longer meet the
demands of the bacteria, they switch to catabolism of amino acids.
The by-products of glucose catabolism by pseudomonads are inoffensive, so while
glucose is available the meat remains unspoiled. However, by-products of amino acid
catabolism, such as ammonia, amines, and organic sulfides, impart offensive odors
and flavor to meat when they are present in even small quantities. On muscle tissue of
normal pH (5.9, the aerobic flora attains numbers about 10~Icm~ before glucose is
exhausted. With such high numbers, organoleptically detectable quantities of
by-products are formed rapidly when amino acids are attacked. Consequently, in those
circumstances, the onset of spoilage is abrupt (Gill, 1981).
In dark,
firm,
dry
(DFD) muscle tissue of high pH (>6.0), little or no glucose may
be present. Glucose concentrations at the surfaces of fat tissue are also low (Gill and
Newton, 1980), and glucose consumed by bacteria cannot be replenished by glucose
diffusing from the underlying tissue. In these circumstances, amino acids are utilized
when bacterial numbers are still low, and spoilage becomes apparent when the flora
reaches numbers that are sufficient to produce offensive by-products
in
organolepti-
cally detectable quantities. This usually occurs when the numbers of the aerobic
spoilage flora are about 1 06/cm2.
The growth of pseudomonads is suppressed when meat is vacuum packaged in a
film
of low gas permeability to obtain a pack fiee of vacuities.
Then,
the oxygen concen-
tration at the meat surface will be too low for any substantial growth of pseudomonads
to occur. Under these conditions the organism that can
grow
most rapidly are lactic acid
bacteria, particularly leuconostocs (Newton and Gill, 1978). On muscle tissue of nor-
mal pH the growth of other spoilage organisms is inhibited by anaerobic conditions, and
the spoilage flora is composed of little other that lactic acid bacteria.
Lactic acid bacteria ferment glucose and a few other minor components of muscle
tissue. Growth ceases when these substrates are exhausted, which usually occurs as
numbers reach about 10~Icm~ (Dainty
et
al.,
1979). However, the lactic acid bacteria
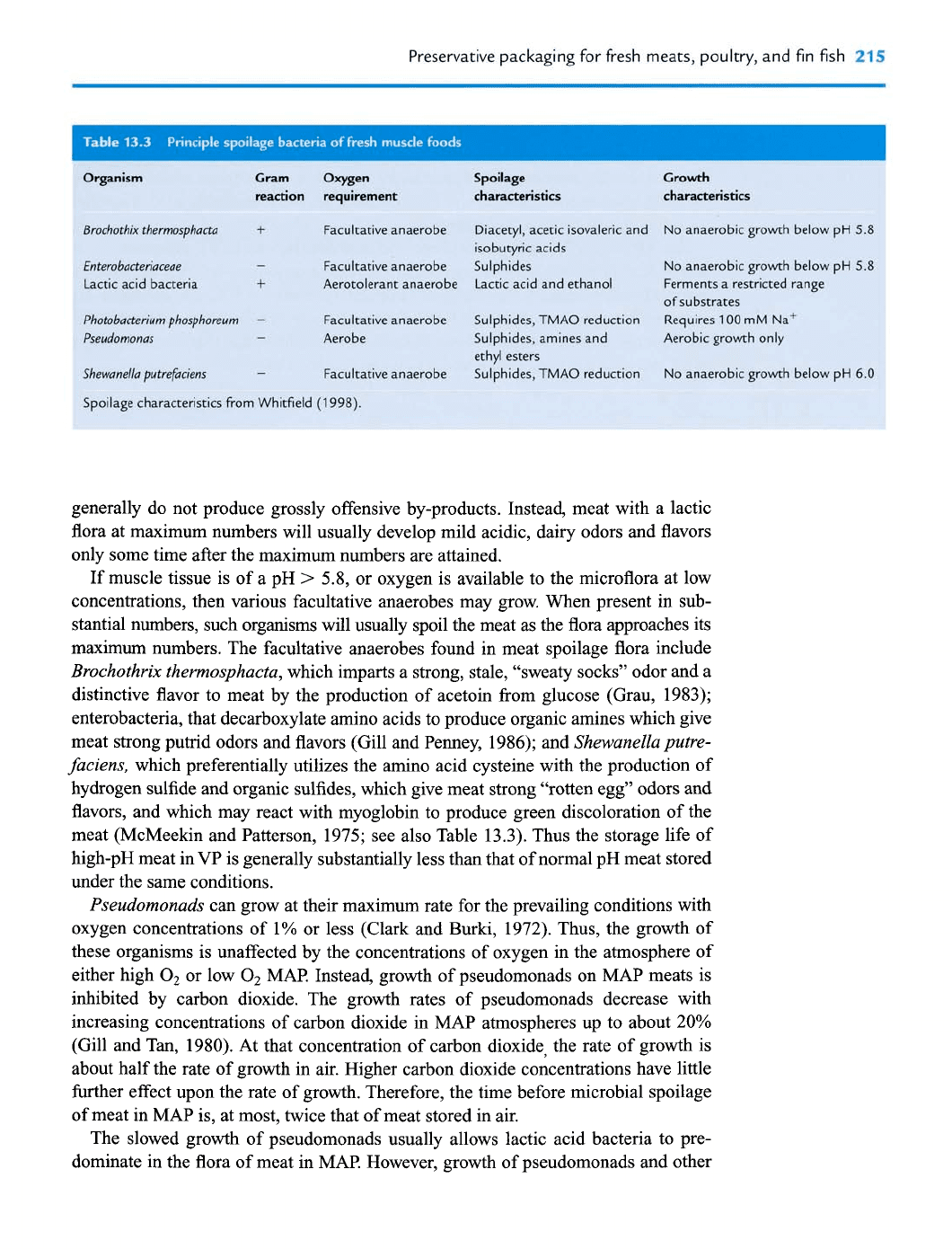
Preservative packaging for fresh meats, poultry,
and
fin fish
215
Organism
Cram
Oxygen Spoilage
Growth
I
reaction requirement characteristics characteristics
Brochothrx thermosphacta
+
Facultative anaerobe Diacetyl, acetic isovaleric and No anaerobic growth below pH
5.8
isobutyric acids
Enterobacteriaceae
-
Facultative anaerobe Sulphides
No anaerobic growth below pH
5.8
Lactic acid bacteria
+
Aerotolerant anaerobe Lactic acid and ethanol Ferments a restricted range
of substrates
Photobacterium phosphoreum
-
Facultative anaerobe Sulphides, TMAO reduction
Requires
100
mM Na'
Pseudomonas
-
Aerobe Sulphides, amines and Aerobic growth only
ethyl esters
Shewanella putrefaciens
-
Facultative anaerobe Sulphides, TMAO reduction No anaerobic growth below pH
6.0
Spoilage characteristics from Whitfield
(1
998).
generally do not produce grossly offensive by-products. Instead, meat with a lactic
flora at maximum numbers will usually develop mild acidic, dairy odors and flavors
only some time after the maximum numbers are attained.
If muscle tissue is of a pH
>
5.8, or oxygen is available to the microflora at low
concentrations, then various facultative anaerobes may grow. When present in sub-
stantial numbers, such organisms will usually spoil the meat
as
the flora approaches its
maximum numbers. The facultative anaerobes found in meat spoilage flora include
Bmchothrix thermosphacta, which imparts a strong, stale, "sweaty socks" odor and a
distinctive flavor to meat by the production of acetoin from glucose (Grau, 1983);
enterobacteria, that decarboxylate amino acids to produce organic amines which give
meat strong putrid odors and flavors (Gill and Penney, 1986); and Shewanellaputre-
faciens, which preferentially utilizes the amino acid cysteine with the production of
hydrogen sulfide and organic sulfides, which give meat strong "rotten egg" odors and
flavors, and which may react with myoglobin to produce green discoloration of the
meat (McMeekin and Patterson, 1975; see also Table 13.3). Thus the storage life of
high-pH meat in VP is generally substantially less than that of normal pH meat stored
under the same conditions.
Pseudomonads can grow at their maximum rate for the prevailing conditions with
oxygen concentrations of 1% or less (Clark and
Burki,
1972). Thus, the growth of
these organisms is unaffected by the concentrations of oxygen
in
the atmosphere of
either high
O2
or low
O2
MAP. Instead, growth of pseudomonads on
MAP
meats is
inhibited by carbon dioxide. The growth rates of pseudomonads decrease with
increasing concentrations of carbon dioxide
in
MAP atmospheres up to about 20%
(Gill and Tan, 1980). At that concentration of carbon dioxide, the rate of growth is
about half the rate of growth in air. Higher carbon dioxide concentrations have little
further effect upon the rate of growth. Therefore, the time before microbial spoilage
of meat in
MAP
is, at most, twice that of meat stored in air.
The slowed growth of pseudomonads usually allows lactic acid bacteria to pre-
dominate in the flora of meat in
MAP.
However, growth of pseudomonads and other

216
Innovations in Food Packaging
strictly aerobic organisms, such as acinetobacteria, is not wholly prevented, and fac-
ultatively anaerobic organisms continue to grow. Therefore, the flora of meat in MAP
usually includes more or less substantial fractions of some of those organisms, with
the meat being spoiled by offensive by-products from pseudomonads, enterobacteria,
andlor B. thermosphacta (Stiles,
199 1).
In CAP with a nitrogen atmosphere, spoilage develops as in VP. However, in an
atmosphere of carbon dioxide, growth of facultative anaerobes on high-pH meat is
inhibited either severely or wholly at temperatures near -lS°C, the optimum for
storage. Under such conditions, a flora of lactic acid bacteria develops irrespective of
the meat pH, and the storage life can be considerably longer than that of similar meat
in VP stored at the same temperature (Gill and Penney,
1988).
Poultry
The pH of poultry breast muscle is usually 5.5, but leg muscle and skin are invariably
of higher pH
-
6.2-6.4
and
7
respectively (McMeekin,
1977).
Vacuum packaging of
whole carcasses is unsatisfactory, because of the vacuity of the body cavity. Vacuum
packaging of bone-in portions is also often unsatisfactory because of bridging of the
packaging film. Moreover, poultry meat is always relatively heavily contaminated
with spoilage bacteria during carcass dressing and breaking processes (Barnes,
1976).
In these circumstances, the flora that develops on the meat in VP, high O2
MAP,
or low
O2 MAP is similar. The flora is rich in lactic acid bacteria, but includes large, some-
times dominant, fractions of enterobacteria which cause putrid spoilage of the prod-
uct after relatively short storage times (Jones et al.,
1982;
Bohnsack et al.,
1988).
However, when poultry is packaged in CAP under COz, growth of the enterobacteria
is inhibited and a flora dominated by lactic acid bacteria develops (Gill et al.,
1990).
Slow growth of the enterobacteria does occur after extended storage, and the meat is
ultimately spoiled by the activities of those organisms. Nonetheless, the storage life of
poultry in CAP under C02 is three to four times that of the same product in
VP.
Fish
The initial microflora of fish is far more variable than that of red meats and poultry.
The flora can differ greatly, in both numbers and composition, depending on the
source of the fish. The microflora on fish from colder sea waters generally num-
bers
<
lo5
cfdcm2, and is composed mostly of gram-negative species that grow most
rapidly in media with sodium chloride at concentrations about
2% (ICMSF,
2000).
Such
organisms include Pseudomonas, Psychrobacter, Vibrio, Shewanella, and Photobac-
terium. Fish from warm sea waters generally carry larger numbers of bacteria, with
gram-positive organisms such as Baccillus, Clostridium, Lactobacillus, Micrococcus
and B. thermosphacta forming large, or predominant, fractions of the flora, along with
gram-negative organisms of the types found on fish from colder waters. Fish from fresh
waters carry similar flora, although Vibrio and Photobacterium are usually absent
while Aeromonas is often present (ICMSF,
2000).

Preservative packaging for fresh meats, poultry, and fin fish
217
The microbial spoilage of fish
in
air is similar to that of red meats, and it is also due
to the activities of gram-negative species, particularly pseudomonads,
S.
putrefaciens,
and Photobacterium phosphoreum. These organisms degrade amino acids to produce
ammonia, amines, organic sulfides, and hydrogen sulfide. However, microbial
spoilage occurs faster
in
fish than in red meat, as the final pH of fish muscle is nor-
mally greater than pH 6 and little glucose is available in fish muscle. This situation
results in fast growth rates for pH-sensitive spoilage organisms and early exhaustion
of available glucose, with initiation of catabolism of amino acids and other nitroge-
nous compounds. Longer shelf lives are associated with fish from tropical waters, in
which the initial flora is dominated by gram-positive organisms, which generally
cause less offensive spoilage.
A major factor contributing to the spoilage of fish is the osmoregulatory molecule
trimethylamine oxide (TMAO) in fish muscle (Whitfield, 1998). Gram-negative bac-
teria, such as
S.
putrefaciens and
P
phosphoreum, use TMAO as an alternative termi-
nal electron acceptor to oxygen during respiration. That reduces the odorless TMAO,
(CH3)3N0, to trimethylamine (TMA), (CH3)3N, which imparts a strong ammonia
odor to the meat. The TMAO reductases of
S.
putrefaciens, and
P
phosphoreum are
constitutively expressed, but expression is repressed in the presence of 02, resulting
in increased TMA production in micro-aerobic and anaerobic conditions (Easter
et
al., 1983). The activity ofTMAO reductase is
pH
sensitive, and at high C02 concen-
trations may be reduced because of acidification of fish muscle by dissolved C02
(Gibson and Davis, 1995).
An
increase in pH, consequent on the production of ammo-
nia from amino acids, also inhibits TMAO reductase and reduces its contribution to
spoilage (Gibson and Davis, 1995).
The elasmobranchs (sharks and rays) use urea rather than TMAO as an osmoregula-
tor, and muscle from these fish may contain up to 2.5% urea. Microbial spoilage then
occurs primarily because of the production of ammonia as a result of bacterial urease
activity (Jemmi et al., 2000).
Because of the high pH of most fish muscle, growth of the facultatively anaerobic
S.
putrefaciens is not prevented by vacuum packaging, so that organism may dominate
the spoilage process in vacuum-packaged fish (Jorgensen and Huss, 1989). However,
P
phosphoreum, utilizing TMAO to maintain respiratory metabolism, may be largely
responsible for the spoilage of fish such as cod inVP (Dalgaard et al., 1993). The rate
of growth for
P
phosphoreum and
S.
putrefaciens is not greatly reduced by anaerobic
conditions (Jorgensen et al., 1988). Consequently, the extension of shelf life achieved
by vacuum packaging of fish is often small (Davis, 1998).
There has been some investigation of the use of high O2
MAP
with fish, with the aim
of reducing
TMA
production by repressing TMAO reductase expression. Boskou and
Debevere (1997) have demonstrated that TMA production by
S.
putrefaciens growing
on a fish extract can be inhibited by 10% oxygen. Guldager et al. (1998) have also
reported inhibition of TMA formation on refrigerated cod fillets under 40% oxygen.
However, such systems can do little to delay other forms of spoilage caused by grarn-
negative organisms.
Although the growth of
S.
putrefaciens is greatly inhibited by high concentrations of
carbon dioxide, low O2 MAP also may give only a modest extension of fish storage

218
Innovations in Food
Packaging
life (Dalgaard, 1995). This can occur because
P
phosphoreum is relatively insensitive
to carbon dioxide and may spoil fish in MAP after a storage life little longer than that
of the same product stored in air.
There appear to be no reports on the use of CAP with fresh fish, although a large
extension of storage life has been reported for smoked fish packaged in CAP under
carbon dioxide (Penney et al., 1994). Fish packaged in MAP under
100%
carbon
dioxide have been reported to develop spoilage flora rich in lactic acid bacteria,
whereas with lower concentration of the gas flora were composed mainly of gram-
negative species (Lannelongue et al., 1982).
Microbiological safety
Muscle tissue from healthy mammals, poultry, and fish is sterile. Consequently
pathogens, like spoilage flora, are introduced to flesh during processing and handling
of the carcass. Thus, two groups of pathogens may contaminate muscle foods. Those
groups are pathogens present in the environment of the animal and transferred to the
carcass from the skin or the intestinal tract during processing, and human-associated
pathogens transferred during handling.
Concerns about the microbiological safety of raw meats in preservative packaging
are related to the possibility that pathogenic organisms may be less sensitive to pre-
servative atmospheres than are spoilage bacteria. If that should be the case, then
pathogens may reach higher numbers than usual, or initiate toxin production, while
the meat remains unspoiled as assessed by microbiological or sensory criteria
(Hintlian and Hotchkiss, 1986). Also, it has been suggested that a growth-rate advan-
tage conferred on a pathogen by a preservative atmosphere might be enhanced by sup-
pression of the usual competitive inhibition of the pathogen by the developing
microflora (Farber, 199 1).
Mesophilic pathogens can grow on meats in
VP
and
MAP,
but some are inhibited
by high concentrations of C02 near their minimum growth temperatures (Siliker and
Wolfe, 1980; Gill and DeLacy, 199 1). Thus, whether or not their growth is advantaged
relative to that of the spoilage flora, the safety of raw meats in preservative packaging
is dependent on the growth of mesophilic pathogens being certainly prevented by stor-
age of the product at chiller temperatures.
At chiller temperatures, most meat-associated pathogens can not grow, with the
exception of the cold tolerant Aeromonas hydrophila, Listeria monocytogenes and
Yersinia entemcolitica (Table 13.4). However, their growth on muscle tissue with a pH
of under 6 is inhibited or prevented by storage under anaerobic conditions at chiller
temperatures (Palumbo, 1988; Grau andvanderlinde, 1990). The growth of cold-tolerant
pathogens is also inhibited by high concentrations of C02 (Garcia de Fernando
et al., 1995). In CAP under COz, growth of these pathogens does not occur on high-
pH meat stored at 5°C or lower (Gill and Reichel, 1989). Suppression of competitive
inhibition is not a factor of importance on rich substrates, such as meat, as interactions
between bacteria do not affect rates of growth until the flora approaches maximum
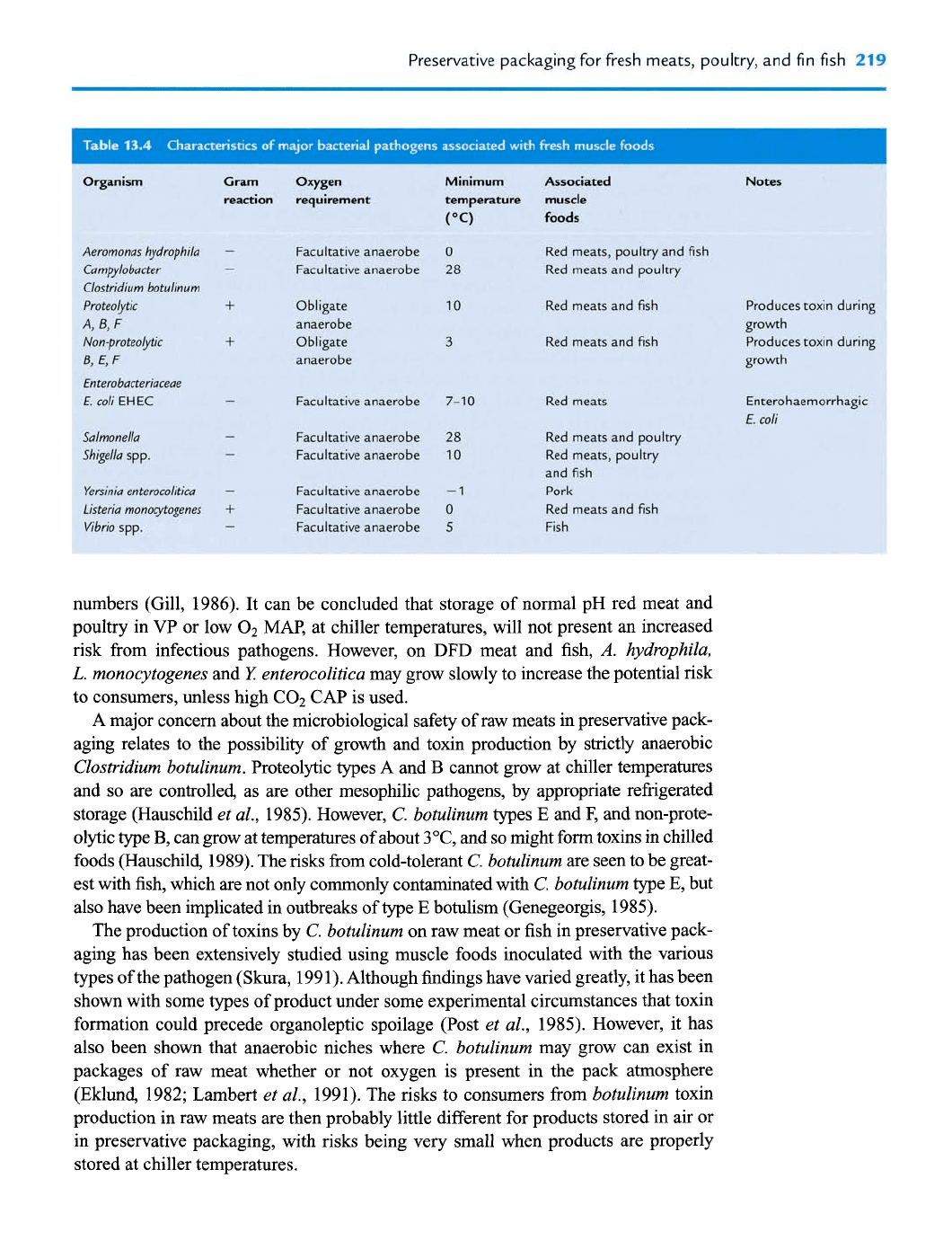
Preservative packaging for fresh meats, poultry, and fin fish
219
:
'Organism
fleromonas hydrophila
Campylobacter
?~~lostridium botulinum
I
Proteolytic
B~
*,Nan-proteohic
i:s,
E,
F
g~nterobacteriaceae
1
E.
coli
EH
EC
.I;
:
Salmonella
?-,
.,Shigella
spp.
;I
i;Yersinia enterocolitica
I.Listeria monoqvtogenes
..
;v
Vibrio
spp.
Gram
reaction
Oxygen Minimum
requirement temperature
("(3
Facultative anaerobe
0
Facultative anaerobe 28
Obligate
10
anaerobe
Obligate
3
anaerobe
Facultative anaerobe
7-1
0
Facultative anaerobe 28
Facultative anaerobe
10
Facultative anaerobe
-
1
Facultative anaerobe
0
Facultative anaerobe
5
Associated
muscle
foods
Red meats, poultry and fish
Red meats and poultry
Notes
Red meats and fish Produces toxin during
growth
Red meats and fish Produces toxin during
growth
Red meats Enterohaemorrhagic
E.
coli
Red meats and poultry
Red meats, poultry
and fish
Pork
Red meats and fish
Fish
numbers (Gill,
1986).
It can be concluded that storage of normal pH red meat and
poultry in
VP
or low O2 MAP, at chiller temperatures, will not present an increased
risk from infectious pathogens. However, on
DFD
meat and fish,
A.
hydrophila,
L.
monocytogenes
and
I:
enterocolitica
may grow slowly to increase the potential risk
to consumers, unless high C02 CAP is used.
A major concern about the microbiological safety of raw meats in preservative pack-
aging relates to the possibility of growth and toxin production by strictly anaerobic
Clostridium botulinum.
Proteolytic types A and
B
cannot grow at chiller temperatures
and so are controlled,
as
are other mesophilic pathogens, by appropriate refrigerated
storage (Hauschild
et al.,
1985).
However,
C. botulinum
types
E
and
F,
and non-prote-
olyt~c type
B,
can grow at temperatures of about 3"C, and so might form toxins in chilled
foods (Hauschild,
1989).
The risks from cold-tolerant
C. botulinum
are seen to be great-
est with fish, which are not only commonly contaminated with
C. botulinum
type E, but
also have been implicated in outbreaks of type E botulism (Genegeorgis,
1985).
The production of toxins by
C. botulinum
on raw meat or fish in preservative pack-
aging has been extensively studied using muscle foods inoculated with the various
types of the pathogen (Skura,
1991).
Although findings have varied greatly, it has been
shown with some types of product under some experimental circumstances that toxin
formation could precede organoleptic spoilage (Post
et al.,
1985).
However, it has
also been shown that anaerobic niches where
C. botulinum
may grow can exist in
packages of raw meat whether or not oxygen is present in the pack atmosphere
(Eklund,
1982;
Larnbert
et al.,
1991).
The risks to consumers from
botulinum
toxin
production in raw meats are then probably little different for products stored in air or
in preservative packaging, with risks being very small when products are properly
stored at chiller temperatures.

Summary
Few foods are as rapidly perishable as fresh muscle foods. Although freezing allows
long term preservation of meat and fish, frozen products do not command the pre-
mium price and consumer acceptance of fresh products. Preservative packaging under
atmospheres other than air can dramatically increase the shelf life of muscle foods
while preserving sensory characteristics, allowing producers to access markets that
are unreachable without these technologies.
The success of preservative packaging for controlling the spoilage of fresh muscle
foods depends largely on the three factors of pack atmosphere, temperature of storage,
and product pH. The maximum achievable shelf life with any packaging system requires
chiller temperatures approaching
-
1.5"C. The reliability of any packaging system for
delaying spoilage is only as good as the temperature control in the distribution chain for
the product. Temperature is also the critical factor in assuring product safety. The poten-
tial threat posed by
C.
botulinum
can be eliminated by ensuring the product temperature
remains below 3°C. Similarly, though cold-tolerant pathogens can grow slowly at tem-
peratures below 4"C, their growth is inhibited by chiller temperatures and
high
C02.
The preservation of red meats and poultry of normal pH
(<6)
in preservative pack-
aging is accomplished by establishing conditions in which the spoilage flora will be
composed primarily of lactic acid bacteria, as opposed to gram-negative bacteria with
higher spoilage potentials. This is achieved by the creation of an anaerobic environment
andlor slowing the growth of gram-negative organisms with C02. Unfortunately, con-
sistent establishment of a spoilage flora dominated by lactic acid bacteria requires a
pH of below
6.
Consequently, the shelf life for meat of high pH and fish is generally
much less than that of normal pH meat, although substantial extensions in shelf life
compared to air storage can still be achieved.
Of the three gases commonly used in MAP systems, only C02 plays a role in con-
trolling microbial spoilage. Oxygen at high concentrations preserves the color of
myoglobin, but when it is present in atmospheres the growth of gram-negative organisms
of high spoilage potential will occur. High oxygen concentrations can also be used
with fish, to inhibit TMAO reduction, but the gains in storage life are limited since
other spoilage activities of bacteria are not similarly inhibited. Nitrogen serves solely
to prevent pack collapse. CAP systems containing carbon dioxide from which all oxy-
gen has been removed consistently achieve the longest shelf lives. For chilled meats
in preservative packaging, the only limitation on the use of CAP is myoglobin color
changes. These may be prevented by the rigorous exclusion of oxygen or the use of
low concentrations of carbon monoxide. Concerns about the safe use of carbon
monoxide with red meat and fish can apparently be overcome. Should regulations
regarding the use of carbon monoxide change, the need for high O2 MAP could dis-
appear and systems of that type could become redundant.
References
Allen, C.
E.
and Foegeding,
E.
A.
(1981).
Some
lipid characteristics
and
interactions in
muscle foods.
Food
Technol.
35(5),
253-257.

Preservative packaging for fresh meats, poultry, and fin fish
221
Barnes,
E.
M. (1976). Microbiological problems of poultry at refrigerator temperatures
-
a review.
J Sci. FoodAgric.
27,777-782.
Bohnsack, U., Knippel, G. and Hopke, H.-U. (1988). The influence of a C02 atmosphere
on the shelf-life of fie h poultry.
Fleischwirtsch. Intl
68,
1553-1 557.
S
Boskou, G. and Debevere,
J.
(1997). Reduction of trimethylamine oxide by
Shewanella
spp. under modified atmospheres in vitro.
Food Microbiol.
14,543-553.
Cardello, A.
V, Sawyer,
E
M., Maller, 0. and Digrnan,
L.
(1982). Sensory evaluation of the
texture and appearance of 17 species of North Atlantic fish.
J Food Sci.
47,18 18-1 823.
Chen, H. M., Meyers, S.
P.,
Hardy,
R.
W. and Biede, S. L. (1984). Color stability of astax-
anthin pigmented rainbow trout under various packaging conditions.
J Food Sci.
49,
1337-1340.
Clark, D. S. and Bruki, T. (1972). Oxygen requirements of strains of
Pseudomonads
and
Achromobacter. Can. J Microbiol.
18,321-326.
Cornforth, D. (1994). Color-its basis and importance. In:
Quality Attributes and their
Measurement in Meat, Poultry and Fish Products
(A. M. Pearson and T. R. Dutson,
eds), pp. 34-78. Blackie Academic, London, UK.
Dainty, R. M., Shaw, B. G., Harding, C.
0.
and Michanie, S. (1979). The spoilage of vac-
uum packaged beef by cold tolerant bacteria. In:
Cold Tolerant Microbes in Spoilage
and the Environment
(A. D. Russell and R. Fuller, eds), pp. 83-100. Academic Press,
London, UK.
Dalgaard,
P.
(1995). Modelling of microbial activity and prediction of shelf life for packed
fresh fish.
IntlJ Food Microbiol.
26,305-3 17.
Dalgaard,
P., Gram,
L.
and Huss, H. H. (1993). Spoilage and shelf-life of cod fillets packed
in vacuum or modified atmospheres.
Intl J Food Microbiol.
19,283-294.
Davis,
H.
K.
(1998). Fish and shelfish. In:
Principles andApplications ofModijiedAtmos-
phere Packaging of Foods,
2nd edn. (B.
A.
Blakistine, ed.), pp. 194-239. Blackie
Academic, London, UK.
Dhananjaya, S. and Stroud, G.D. (1994). Chemical and sensory changes in haddock and
herring stored under modified atmospheres.
Intl
J
Food Sci. Technol.
29,575-583.
Doherty, A. M. and Allen,
P.
(1998). The effect of oxygen scavengers on the color stabil-
ity and shelf-life of C02 master packaged pork.
J Muscle Foods
9,35 1-363.
Dransfield, E. (1994). Tenderness of meat, poultry, and fish.
In:
Quality Attributes and
their Measurement in Meat, Poultry and Fish Products
(A. M. Pearson and
T.
R.
Dutson, eds), pp. 287-3 15. Blackie Academic, London, UK.
Easter, M. C., Gibson, D. M and Ward, F. B. (1983). The induction and location of
trimethylmine-N-oxide reductase in
Alteromonas
sp. NCIMB 400.
J Gen.
Microbiol.
129,3689-3696.
Echevarne,
C.,
Renerre, M. and Labas, R. (1990). Metrnyoglobin reductive activity in
bovine muscle.
Meat Sci.
27,16 1-1 72.
Eklund, M. W. (1982). Effect of C02 atmospheres and vacuum packaging on
Clostridium
botulinum
and spoilage organisms of fisheries products.
In:
Proceedings of the 1st
National Conference on Seafood Packaging and Shipping
(R.
E. Martin, ed.),
pp. 298-33 1. National Fisheries Inst, Washington, DC.
Farber,
J.
M. (1991). Microbiological aspects of modified-atmosphere packaging technol-
ogy
-
a review.
J Food Prot.
54,58-70.

:L
Innovations in Food Packaging
Faustman, C. and Cassens,
R.
G. (1990). The biochemical basis for discoloration in fresh
meat
-
a review.
J
Muscle Foods
1,2 17-243.
Garcia de Fernando, G. D., Mano,
S.
B., Lopez,
D.
and Ordbiiez, J. A. (1995). Effect of
modified atmospheres on the growth of pathogenic psychrotrophic microorganisms
on proteinaceous foods.
Microbiologia
11,7-22.
Genegeorgis, C. (1985). Microbiological safety of the use of modified atmospheres to
extend the storage life of fresh meat and fish.
Intl
J
Food Microbiol.
1,237-25 1.
Gibson, D.
M.
and Davis, H. K. (1995). Fish and shellfish products in sous vide and mod-
ified atmosphere packs. In:
Principles of ModiJied Atmosphere and Sous Vide
Product Packaging
(J. M. Farber and K. L. Dodds, eds), pp. 153-174. Technomic
Publishing, Lancaster, PA.
Gill, C. 0. (1981). Meat spoilage and evaluation of the potential storage life of fresh meat.
J
Food Prot.
46,444-452.
Gill, C.
0.
(1986). The control of microbial spoilage in fresh meats. In:
Advances in Meat
Research,
Vol. 2 (A. M. Pearson and T.
R.
Dutson, eds), pp. 49-88. AVI Publishing,
Westport, CT.
Gill,
C.
0. (1988). The solubility of carbon dioxide in meat.
Meat Sci.
22,65-71.
Gill, C.
0.
(1989). Packaging meat for prolonged chilled storage: the Captech Process.
BZ Food
J.
91,ll-15.
Gill,
C.
0. (1990). Meat and modified atmosphere packaging. In:
The Encyclopedia of
Food Science and Technology
(Y.
H. Hui, ed.), pp. 1678-1 683. Wiley, New York,
NY.
Gill, C. 0. and DeLacy, K. M. (1991). Growth of
Escherichia coli
and
Salmonella
typhimurium
on high-pH beef packed under vacuum or carbon dioxide.
Intl
.l
Food
Microbiol.
13,21-30.
Gill, C. 0. and Jones, T. (1994a). The display life of retail-packaged beef steaks after their
storage in master packs under various atmospheres.
Meat Sci.
38,385-396.
Gill, C. 0. and Jones, T. (1994b). The display life of retail-packs of ground beef after their
storage in master packs under various atmospheres.
Meat Sci.
37,281-295.
Gill, C.
0.
and Jones,
T.
(1996). The display life of retail-packaged pork chops after their
storage in master packs under atmospheres of N2, C02 or O2
+
C02.
Meat Sci.
42,
203-213.
Gill, C.
0.
and McGinnis, J. C. (1995a). The effects of residual oxygen concentration and
temperature on the degradation of the color of beef packaged under oxygen-depleted
atmospheres.
Meat Sci.
39,387-394.
Gill, C. 0. and McGinnis, J. C. (1995b). The use of oxygen scavengers to prevent the
tran-
sient discoloration of ground beef packaged under controlled, oxygen-depleted
atmospheres.
Meat Sci.
41, 19-27.
Gill, C. 0. and Newton, K. G. (1977). The development of spoilage flora on meat stored at
chill temperatures.
J
Appl. Bacteriol.
43, 189-195.
Gill,
C.
0. and Newton, K.G. (1980). Development of bacterial spoilage at adipose tissue
surfaces of fresh meat.
Appl. Environ. Microbiol.
39, 1076-1077.
Gill,
C.
0. and Penney, N. (1986). Packaging conditions for extended storage of chilled,
dark,
firm,
dry beef.
Meat Sci.
18,41-53.
Gill, C. 0. and Penney, N. (1988). The effect of the initial gas volume to meat weight ratio
on the storage life of chilled beef packaged under carbon dioxide.
Meat Sci.
22,5343.
