Jung Han. Innovations in Food Packaging
Подождите немного. Документ загружается.


Centralized packaging systems for meats
233
iron absorbs any O2 present in the package headspace and is oxidized to the ferric
state; typically, this requires moisture as an activating agent. Ageless FX-100 sachets
have been commercially successful for various food product applications, although
the kinetics of this system at very low O2 concentrations (300-600 ppm) and low tem-
perature (ca.
-
1.5OC) in meats is not fully known (Jeyarnkondan et al., 2000).
Another 02-scavenging system is the Cryovac OS1000, a multi-layer flexible film
with a co-extruded sealant from the Sealed Air Corporation (Saddle Brook, NJ, USA).
A proprietary 02-scavenging polymer is incorporated within the sealant and therefore
is invisible to consumers, unlike the sachets and labels. The polymer is dormant until
it is activated by a
UV
light system (Cryovac 4100
UV
triggering unit, Sealed Air
Corporation) just before packaging. Unlike the Ageless FX-100 sachets, it does not
require moisture as an activating agent; therefore, the product is unaffected. The man-
ufacturing company claims that the system can reduce O2 levels within the modified
atmosphere of the package from 0.5-1% to a few parts per million, in 4 to 10 days
(Jeyamkondan et al., 2000).
Applied research
Scholtz et al. (1992b) compared the performance of a commercial bulk pre-packaging
system, Cryovac
GFII,
manufactured by Darex Africa (Pty) Ltd. (Kemptonpark,
South Africa), to that of a laboratory-based system in terms of quality attributes such
as microbiology, color, odor, and acceptability of PVC-overwrapped pork retail cuts.
A
similar retail shelf life of
3
days was achieved after 0,7 or 14 days bulk storage with
both packaging systems. The color of the samples from both systems was pale to nor-
mal during the trial. After 14 days of storage, samples from both systems were still
acceptable and had a fresh meat odor. These results showed that the commercial
system tested may be applied successfully for 100% C02 bulk packaging of PVC-
overwrapped pork retail cuts.
Gill and Jones (1994) studied the storage life of master-packaged beefsteak under
various atmospheres at
-
1.5OC. Steaks stored under 100% C02 or 100% N2 for less
than 4 days were only slightly desirable because of the formation of metmyoglobin on
the surface due to the presence of small amounts of residual O2 in packages. By
4
days
of storage, metmyoglobin was reduced to deoxymyoglobin by the meat tissue (action
of the enzyme metmyoglobin reductase), and the product acquired the potential to
develop the desirable red color upon final exposure to O2 during retail display. This
phenomenon is known as transient discoloration, and occurs with beef shortly after
packaging in anoxic atmospheres where there are O2 residuals
G
1000ppm. Master
packaging under a C02-O2 (1: 2 V/V) atmosphere gave an initial acceptable appear-
ance, but the color began to deteriorate after 12 days of storage, and it was concluded
that the combination may be suitable for storage of less than 4 days. Master packag-
ing in 100% N2 gave a shelf life of 4 weeks, and 2 days of subsequent retail display,
while master packaging in 100% C02 gave about 7 weeks of shelf life in the master
pack and 2 days of subsequent retail display. This study also emphasized that these
storage lives can only be obtained with products of high initial microbiological quality.

234
Innovations in
Food
Packaging
-
-
-
- -
-
- -
- -
-
Jeremiah and Gibson (1997) investigated the effects of 100% C02 atmospheres
on the flavor and texture profiles of master-packaged pork cuts at
-
1.5OC. Flavor
became inappropriate, unbalanced and unblended after 12 days of storage, and it was
concluded that off-flavor development, most likely caused by lactic acid bacteria, con-
stitutes the limiting factor for shelf-life extension of fresh pork under modified atmos-
phere conditions. As in the previous study, the importance of using cuts with very low
initial bacterial numbers was stressed. In a follow-up study, Nattress
et
al. (1998)
found that off-flavor development coincides with a shift in the dominant microflora
from non-aciduric to aciduric lactic acid bacteria.
Gill and McGinnis (1995) used FreshPax 200R from Multisorb Technologies, Inc.
(Buffalo,
NY,
USA), an iron-based 02-scavenger in a sachet, to prevent transient dis-
coloration. This scavenging system is composed of activated iron-oxide powder
mixed with acids, salts and humectants to promote oxidation of iron, which removes
residual O2 from the package atmosphere avoiding meat discoloration. These authors
also reported that O2 concentration in the pack atmosphere must be reduced
to <lOppm within 30 minutes at 2OC, or within 2 hours at -1.5OC for acceptable
blooming of ground beef to occur.
Tewari (2000) studied the storage life of master-packaged beef tenderloins
and
pork
loins in combination with other technologies such as 02-scavenging systems, the
CAPTECH process, and a N2-based refrigeration unit able to maintain the tempera-
ture at -1.5
2
0.5OC. Preliminary results showed that Ageless FX-100 sachets
needed to be placed inside the retail packs rather than in the master bag. Eight sachets
were found to be optimal when they were placed below the absorption pads under
each retail cut. Four packaged retail cuts were master-packaged, filled with 4.5 1 of
N2, sealed using a CAPTRON system, and stored at
-
1.5
*
OS°C using a liquid
N2-fueled refrigeration system. The storage life of beef and pork cuts was about
10 weeks, with
3
days of subsequent retail display life.
Conclusions
A variety of centralized packaging systems for retail-ready meat is a commercial real-
ity, and each of them satisfies different requirements. Thus, high O2 MAP is suitable
for local distribution due to the limited shelf-life, whereas master packaging in the
absence of 02, combined with appropriate temperature control, good manufacturing
practices, and suitable packaging materials can achieve 610 weeks of storage life,
besides being the most economical system due to the grouping of individual packs
(Jeyarnkondan
et
al., 2000).
The case-ready program has been a reactionary process built on market demand, satis-
fymg cost-cutting requirements at specific segments of the supply and distribution chan-
nels. Communications between packer and retail buyer, and between retail buyer and store
meat manager, are key to the success of the system, because they allow prediction of sales
on a store-by-store basis and provide better pricing strategies and promotional activities
(Pizzico, 2002). In the USA, a January 2001 survey by the Cryovac Division of Sealed
Air

Centralized packaging systems
for
meats
235
Corporation showed that of the 127 000 grocery stores in the country, about 25 000 car-
ried case-ready poultry, 6000 carried case-ready pork, 10 000 carried case-ready ground
beef, and 1000 carried case-ready total muscle cuts. The report also found that stores
making the move to case-ready products have experienced, on average, a 3.8% growth
in
sales. Based on these numbers, it is clear that this sector of the industry has
nowhere to go but up, pushed both by consumers and improved productivity
(Quigley, 2002).
References
Anonymous (1989). Fresh meat package combines. VSP and MAP. Food Eng. 61,72-75.
Ailey, C. G., Jayas, D. S., Holley, R.
A.,
Jeremiah,
L.
E. and Gill, C. 0. (1997).
Design, fabrication, and testing of a returnable, insulated, nitrogen refrigerated ship-
ping container for distribution of fresh red meat under controlled C02 atmosphere.
Food Res. Intl30,743-753.
Brody, A. L. (1996). Integrating aseptic and modified atmosphere packaging to fulfill a
vision of tomorrow. Food Technol. 50(4), 56-66.
Cole, A. B. Jr (1986). Retail packaging systems for fresh red meat cuts. Reciprocal Meat
Conference. Proc.
Am.
Meat Sci. Assoc. 39, 106-1 11.
Gill, C.
0.
and Harrison, J. C.
L.
(1989). The storage life of chilled pork packaged under
carbon dioxide. Meat Sci. 26,3 13-324.
Gill, C. 0. and Jones, T. (1994). The display life of retail-packaged beef steaks after their
storage in master packs under various atmospheres. Meat Sci. 38,385-396.
Gill, C. 0. and McGinnis, J. C. (1995). The use of oxygen scavengers to prevent transient
discolouration of ground beef packaged under controlled oxygen-depleted atmos-
pheres. Meat Sci. 41, 19-27.
Gill, C. 0. and Molin, G. (1991). Modified atmospheres and vacuum packaging. In: Food
Preservatives (N.J. Russell and G.W. Gould, eds), pp. 172-199. Blackie
&
Sons Ltd.,
London,
UK.
Habok, M.
N.
N.
(1999). Modification and testing of a nitrogen refrigerated, controlled
atmosphere container for distribution of fresh red meats. MSc thesis, Department of
Biosystems Engineering, University of Manitoba, Winnipeg, Canada.
Jeremiah, L. E. and Gibson, L. L. (1997). The influence of controlled atmosphere storage
on the flavor and texture profiles on display-ready pork cuts. Food Res. Intl 30,
117-129.
Jeyamkondan, W. (1999). Design and evaluation of a portable, nitrogen-refrigerated, jack-
eted container for storage and distribution of chilled meat. MSc thesis, Department
of Biosystems Engineering, University of Manitoba, Winnipeg, Canada.
Jeyamkondan, W., Jayas, D. S. and Holley,
R.
A. (2000). Review of centralized packaging
systems for distribution of retail-ready meat.
J
Food Prot. 63,796-804.
Labuza, T.
P.
(1996). An introduction to active packaging for foods. Food Technol. 59(4),
68-7 1.
Labuza, T.
P.
and Breene, W. M. (1989). Applications of "active packaging" for improve-
ment of shelf-life and nutritional quality of fresh and extended shelf-life foods.
J
Food Proc. Pres. 13, 1-69.

236
Innovations
in
Food Packaging
-
-
-
-
Nattress, F. M., Worobo, R. J., Greer,
G.
C. and Jeremiah, L. E. (1998). Effect of lactic acid
bacteria on beef flavour.
Proc. 44th Intl. Congress Meat Sci. Technol.
44,321-327.
Penny,
N.
and Bell, R. G. (1993). Effect of residual oxygen on the colour, odour and taste
of carbon-dioxide packaged beef, lamb and pork during short term storage at chill
temperatures.
Meat Sci.
33,245-252.
Pizzico, B. (2002). The key to case ready.
Meat
&
Poultry
48(2), 38-41.
Quigley, L. (2002). Canadian style case ready.
Meat
&
Poultry
48(2), 30-36.
Scholtz, E. M., Jordaan, E., Kriiger, J. and Nortjt, R.T. (1992a). The influence of different
centralized pre-packaging systems on the shelf-life of fresh pork.
Meat Sci.
32,
11-29.
Scholtz, E. M., Kriiger,
J.,
NortjC,
G.
and NaudC, R. (1992b). Centralized bulk pre-packaging
of pork retail cuts.
Intl.
Z
Food Sci. Technol.
27,391-398.
Seideman, S. C., Cross,
H.
R., Smith,
G.
C. and Durland,
P.
R. (1984). Factors associated
with fresh meat color: a review.
1
Food Qual.
6,211-237.
Shay, B. J. and Egan,
A. F. (1987). The packaging of chilled red meats.
Food Technol. Aust.
39,283-285.
Tewari, G. (2000). Centralized packaging of retail meat cuts. PhD thesis, Department of
Biosystems Engineering, University of Manitoba, Winnipeg, Canada.
Tewari, G., Jayas, D. S. and Holley, R.
A.
(1999). Centralized packaging of meat cuts: a
review.
1
Food Prot.
62,4 1 8-425.
Varnam,
A.
H.
and Sutherland, J.
P.
(1995).
Meat and Meat Products: Technology,
Chemistry and Microbiology,
2nd edn. Chapman
&
Hall, London, UK.
Zhao,
Y.,
Wells, J.
H.
and McMillen, K. W. (1994). Applications of dynamic modified
atmosphere packaging systems for fresh red meats: a review.
1
Muscle Foods
5,
299-328.
Edible
films
and
Jung H. Hun and Aristippos Cennadios
Introduction
.................................
.....
239
Historical and current uses of edible films and coatings
.......................... 241
Filmcornposition
........................................................ 242
Functionsandadvantages
.................................................
245
Scientific parameters
...................................................
253
Practical parameters for commercialization
....................................
255
Conclusions
............................................................ 258
References
..............................................................
259
Introduction
Films are generally defined as stand-alone thin layers of materials. They usually consist
of polymers able to provide mechanical strength to the stand-alone
thin
structure. Sheets
are thick films. Therefore, there is not an obvious difference in material composition
between films and sheets other than their thickness. Films can form pouches, wraps,
capsules, bags, or casings through further fabrication processes. Coatings are a partic-
ular form of films directly applied to the surface of materials. Removal of coating layers
may be possible; however, coatings are typically not intended for disposal separately
from the coated materials. Therefore, coatings are regarded
as
a part of the
final
product.
The use of edible films and coatings as carriers of active substances has been sug-
gested as a promising application of active food packaging (Cuq
et
al., 1995; Han,
2000,2001). In fact, the use of edible filmsand coatings for food packaging without
active substances is arguably also an application of active food packaging, since the
edibility and biodegradability of the films are additional functions not offered by con-
ventional packaging materials (Cuq
et
al., 1995; Han, 2002).
Edible films and coatings
Edible films and coatings are produced from edible biopolymers and food-grade addi-
tives. Film-forming biopolymers can be proteins, polysaccharides (carbohydrates and
Innovations in Food Packaging
ISBN:
0-12-3
1
1632-5
Copyright
0
2005 Elsevier Ltd
All
rights of reproduction in
any
form reserved

--
,nnovations in Food Packaging
Figure
15.1
Edible film made from carboxymethyl cellulose.
gums) or lipids (Gennadios
et
al., 1997; see Figure 15.1). Plasticizers and other additives
are combined with the film-forming biopolymers to modify the physical properties or
functionality of films.
The film-forming mechanisms of biopolyrners include intermolecular forces such
as covalent bonds (e.g., disulfide bonds and cross-linking) andlor electrostatic,
hydrophobic or ionic interactions. Fabrication procedures initiate these film-forming
mechanisms. For the resulting films or coatings to be edible, the film-forming mech-
anism involved in fabrication should be an appropriate food process, namely pH mod-
ification, salt addition, heating, enzymatic modification, drying, use of food-grade
solvents, and addition of other food-grade chemicals. The control of fabrication process
conditions is very important because changes in treatment conditions can alter kinet-
ics and reaction mechanisms (Guilbert
et
al., 1996, 1997).
Edible films and coatings enhance the quality of food products, protecting them from
physical, chemical, and biological deterioration (Kester and Fennema, 1986). The appli-
cation of edible films and coatings can readily improve the physical strength of food
products, reduce particle clustering, and improve visual and tactile features on prod-
uct surfaces (Cuq
et
al., 1995; Cisneros-Zevallos
et
al., 1997). It can also protect food
products from moisture migration, microbial growth on the surface, light-induced
chemical changes, oxidation of nutrients etc. (Kester and Fennema, 1986). Most com-
monly, edible films and coatings function as barriers against oils, gases or vapors, and
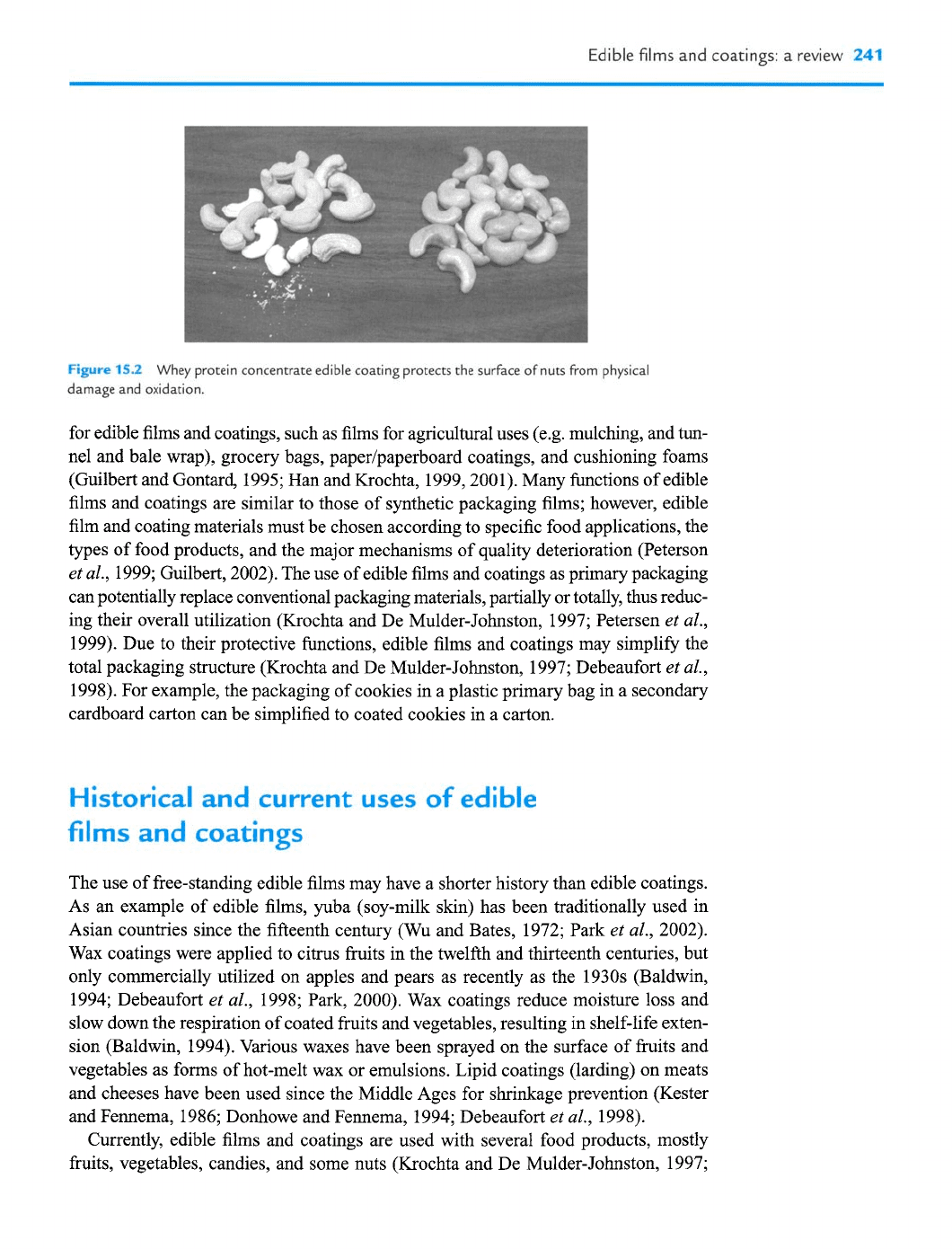
as carriers of active substances, such as antioxidants, antimicrobials, colors, and flavors
(Kester and Fennema, 1986; Gennadios and Weller, 1990; Guilbert and Gontard, 1995;
Krochta and De Mulder-Johnston, 1997; Miller
et
al., 1998; see Figure 15.2). These
protective functions are aimed to enhance the quality of food products, resulting in
shelf-life extension and safety improvement (Gennadios and Weller, 1990).
Biodegradable
films
and coatings
Inherently, edible
film
and coating materials are biodegradable (Krochta, 2002).
In
fact,
biodegradability is one of the greatest benefits of edible films and coatings, along with
edibility (Debeaufort
et
al., 1998). There are several potential non-food applications
Edible
films and coatings: a
review
241
Fipm
15.2
Whey protein concentrate edible coating protects the surface of nuts from physical
damage and oxidation.
for edible films and coatings, such as films for agricultural uses (e.g. mulching, and
tun-
nel and bale wrap), grocery bags, paperlpaperboard coatings, and cushioning foams
(Guilbert and Gontard, 1995; Han and Krochta, 1999,2001). Many functions of edible
films and coatings are similar to those of synthetic packaging films; however, edible
film and coating materials must be chosen according to specific food applications, the
types of food products, and the major mechanisms of quality deterioration (Peterson
et al., 1999; Guilbert, 2002). The use of edible films and coatings as primary packaging
can potentially replace conventional packaging materials, partially or totally, thus reduc-
ing their overall utilization (Krochta and De Mulder-Johnston, 1997; Petersen et al.,
1999). Due to their protective functions, edible films and coatings may simplify the
total packaging structure (Krochta and De Mulder-Johnston, 1997; Debeaufort et al.,
1998). For example, the packaging of cookies in a plastic primary bag in a secondary
cardboard carton can be simplified to coated cookies in a carton.
Historical and current uses of edible
films and coatings
The use of free-standing edible films may have a shorter history than edible coatings.
As an example of edible films, yuba (soy-milk skin) has been traditionally used in
Asian countries since the fifteenth century (Wu and Bates, 1972; Park et al., 2002).
Wax coatings were applied to citrus fruits in the twelfth and thirteenth centuries, but
only commercially utilized on apples and pears as recently as the 1930s (Baldwin,
1994; Debeaufort et al., 1998; Park, 2000). Wax coatings reduce moisture loss and
slow down the respiration of coated fruits and vegetables, resulting in shelf-life exten-
sion (Baldwin, 1994). Various waxes have been sprayed on the surface of fruits and
vegetables as forms of hot-melt wax or emulsions. Lipid coatings (larding) on meats
and cheeses have been used since the Middle Ages for shrinkage prevention wester
and Fennema, 1986; Donhowe and Fennema, 1994; Debeaufort et al., 1998).
Currently, edible films and coatings are used with several food products, mostly
fruits, vegetables, candies, and some nuts (Krochta and De Mulder-Johnston, 1997;

242
Innovations in Food Packaging
Petersen
et
al.,
1999). Collagen films are used for sausage casings, and some hydroxy-
methyl cellulose films as soluble pouches for dried food ingredients. In general, there
are more applications of coatings
than
of films. Shellac and wax coatings on fruits and
vegetables, zein coatings on candies, and sugar coatings on nuts are the most common
commercial practices of edible coatings (Krochta and De Mulder-Johnston, 1997).
The pharmaceutical industry uses sugar coatings on drug pills and gelatin films for
soft capsules (Gennadios, 2002; Krochta, 2002). The use of cellulose ethers (such as
carboxymethyl cellulose, hydroxypropyl cellulose, and methylcellulose) as ingredients
in coatings for fruits, vegetables, meats, fish, nuts, confectionery, bakery, grains, and
other agricultural products is increasing (Nussinovitch, 2003).
Film
composition
Film-forming materials
The main film-forming materials are biopolymers, such as proteins, polysaccharides,
lipids, and resins. They can be used alone or in combinations. The physical and chem-
ical characteristics of the biopolymers greatly influence the properties of resulting
films and coatings (Sothornvit and Krochta, 2000). Film-forming materials can be
hydrophilic or hydrophobic. However, in order to maintain edibility, solvents used are
restricted to water and ethanol (Peyron, 1991).
Proteins are commonly used film-forming materials. They are macromolecules with
specific amino acid sequences and molecular structures. The secondary, tertiary, and
quaternary structures of proteins can be easily modified by heat denaturation, pressure,
irradiation, mechanical treatment, acids, alkalis, metal ions, salts, chemical hydrolysis,
enzymatic treatment, and chemical cross-linking. The most distinctive characteristics of
proteins compared to other film-forming materials are conformational denaturation,
electrostatic charges, and amphiphilic nature. Many factors can affect the conformation
of proteins, such as charge density and hydrophilic-hydrophobic balance. These factors
can ultimately control the physical and mechanical properties of prepared films and
coatings. Protein film-forming materials are derived
from
many different animal and
plant sources, such as animal tissues, milks, eggs, grains, and oilseeds (Krochta, 2002).
Polysaccharide film-forming materials include starch, non-starch carbohydrates,
gums, and fibers. The sequence of polysaccharides is simple compared to proteins,
which have 20 common amino acids. However, the conformation of polysaccharide
structures is more complicated and unpredictable, resulting
in
much larger molecular
weights than proteins. Most carbohydrates are neutral, while some gums are mostly
negatively charged. Although this electrostatic neutrality of carbohydrates may not
affect significantly the properties of formed films and coatings, the occurrence of rel-
atively large numbers of hydroxyl groups or other hydrophilic moieties in the struc-
ture indicate that hydrogen bonds may play significant roles in film formation and
characteristics. Some negatively charged gums, such as alginate, pectin, and carboxy-
methyl cellulose, show significantly different rheological properties in acidic than in
neutral or alkaline conditions.
Edible
films
and coatings: a review
243
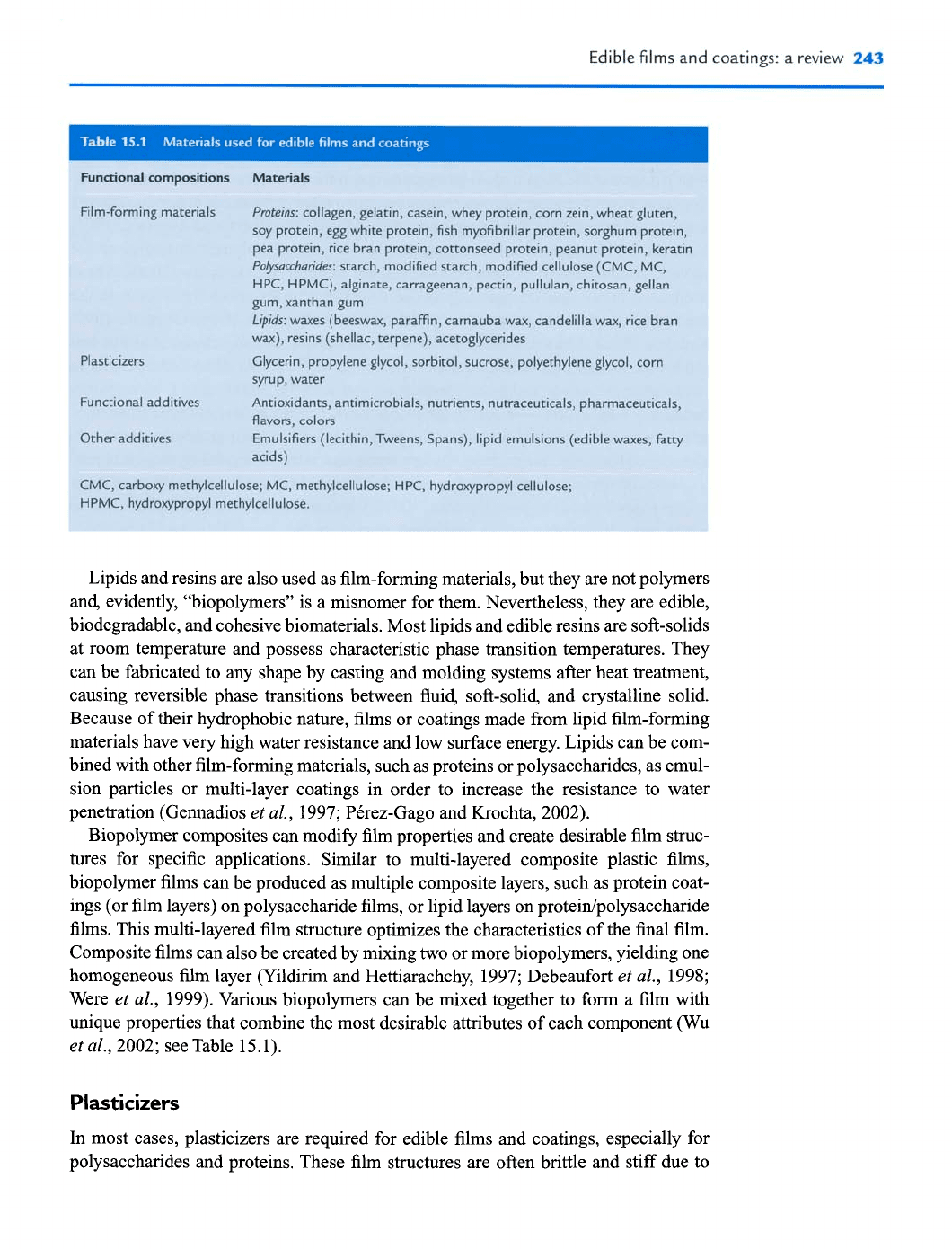
Fumtbnal
compositions Matuials
7lm-forming materials
Proteins:
collagen, gelatin, casein, whey protein, corn zein, wheat gluten,
soy protein, egg white protein, fish myofibrillar protein, sorghum protein,
pea protein, rice bran proteip, cottonseed protein, peanut protein, kerati~
Po5wccharides:
starch,.modified starch, modified cellulose (CMC, MC,
HPC, HPMC), alginate, carrageenan, pectin, pullulan, chitosan, gellan
gum, xanthan gum
tipids:
waxes (beeswax, paraffin, carnauba wax, candelilla
wax,
rice bran
wax),
resins (shellac, terpene), acetoglycerides
Plasticizers
Glycerin, propylene glycol, sorbitol, sucrose, polyethylene glycol, corn
syrup, water
'unctional additives
Antioxidants, antimicrobials, nutrients, nutraceuticals, pharmaceuticals,
flavors, colon
3ther additives
Emulsifiers (lecithin, Tweens, Spans), lipid emulsions (edible waxes,
fatty
acids)
ZMC,
carboxy methylcellulose; MC, methylcellulose; HPC, hydroxypropyl cellulose;
iPMC, hydroxypropyl methylcellulose.
Lipids and resins are also used as film-forming materials, but they are not polymers
and, evidently, "biopolymers" is a misnomer for them. Nevertheless, they are edible,
biodegradable, and cohesive biomaterials. Most lipids and edible resins are soft-solids
at room temperature and possess characteristic phase transition temperatures. They
can be fabricated to any shape by casting and molding systems after heat treatment,
causing reversible phase transitions between fluid, soft-solid, and crystalline solid.
Because of their hydrophobic nature, films or coatings made from lipid film-forming
materials have very high water resistance and low surface energy. Lipids can be com-
bined with other film-forming materials, such as proteins or polysaccharides, as emul-
sion particles or multi-layer coatings in order to increase the resistance to water
penetration (Gennadios
et
al., 1997; Pkrez-Gago and Krochta, 2002).
Biopolymer composites can modify film properties and create desirable film struc-
tures for specific applications. Similar to multi-layered composite plastic films,
biopolymer films can be produced as multiple composite layers, such as protein coat-
ings (or film layers) on polysaccharide films, or lipid layers on protein/polysaccharide
films. This multi-layered film structure optimizes the characteristics of the final film.
Composite films can also be created by mixing two or more biopolymers, yielding one
homogeneous film layer (Yildirim and Hettiarachchy, 1997; Debeaufort
et
al., 1998;
Were
et
al.,
1999). Various biopolymers can be mixed together to form a film with
unique properties that combine the most desirable attributes of each component (Wu
et
al., 2002; see Table 15.1).
Plasticizers
In
most cases, plasticizers are required for edible films and coatings, especially for
polysaccharides and proteins. These film structures are often brittle and stiff due to

extensive interactions between polymer molecules (Krochta, 2002). Plasticizers are
low molecular weight agents incorporated into the polymeric film-forming materials,
which decrease the glass transition temperature of the polymers. They are able to posi-
tion themselves between polymer molecules and to interfere with the polymer-polymer
interaction to increase flexibility and processability (Guilbert and Gontard, 1995;
Krochta, 2002). Plasticizers increase the free volume of polymer structures or the
molecular mobility of polymer molecules (Sothornvit and Krochta, 2000). These
properties imply that the plasticizers decrease the ratio of crystalline region to the
amorphous region and lower the glass transition temperature (Guilbert
et
al.,
1997;
Krochta, 2002). The addition of plasticizers affects not only the elastic modulus and
other mechanical properties, but also the resistance of edible films and coatings to
permeation of vapors and gases (Sothornvit and Krochta, 2000, 2001). Most plasti-
cizers are very hydrophilic and hygroscopic so that they can attract water molecules
and form a large hydrodynamic plasticizer-water complex. For protein and polysac-
charide edible films, plasticizers disrupt inter- and intra-molecular hydrogen bonds,
increase the distance between polymer molecules, and reduce the proportion of crys-
talline to amorphous region (Krochta, 2002). Water molecules in the films function as
plasticizers. Water is actually a very good plasticizer, but it can easily be lost by dehy-
dration at a low relative humidity (Guilbert and Gontard, 1995). Therefore, the addi-
tion of hydrophilic chemical plasticizers to films can reduce water loss through
dehydration, increase the amount of bound water, and maintain a high water activity.
There are two main types of plasticizers (Sothernvit and Krochta, 2000,2001):
1.
Agents capable of forming many hydrogen bonds, thus interacting with polymers
by interrupting polymer-polymer bonding and maintaining the farther distance
between polymer chains
2. Agents capable of interacting with large amounts of water to retain more water mole-
cules, thus resulting in higher moisture content and larger hydrodynamic radius.
However, owing to the hydrophilic nature of water, biopolymers, and plasticizers, and
due to the abundantly existing hydrogen bonds in their structures, it is very difficult to
separate these two mechanisms. Sothornvit and Krochta (2001) suggested that several
factors of plasticizers affect plasticizing efficiency, including sizelshape of plasticizer
molecules, number of oxygen atoms and their spatial distance within the structure of the
plasticizers, and water-binding capacity. Besides the effect of hydrogen bonding, repul-
sive forces between molecules of the same charge or between polarlnon-polar polymers
(e.g. acetylated starch) can increase the distance between polymers, thus achieving the
function of plasticization in the case of charged polymeric film structures. Therefore,
compared to neutral polymer films (e.g. starch films), the flexibility of charged polymer
films (e.g. soy protein, carboxymethyl cellulose or alginate films) may be affected
more significantly by altering pH and salt addition at the same water activity level.
Additives
Edible films and coatings can carry various active agents, such as emulsifiers, antioxi-
dants, antimicrobials, nutraceuticals, flavors, and colorants, thus enhancing food quality
