Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.

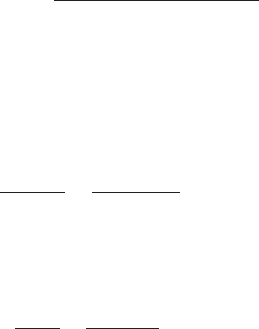
Public Water Supply 375
8.1 Jar test
For the jar test, chemical (coagulant) is added to raw water sample for
mixing in the laboratory to simulate treatment-plant mixing conditions.
Jar tests may provide overall process effectiveness, particularly to
mixing intensity and duration as it affects floc size and density. It can
also be used for evaluating chemical feed sequence, feed intervals, and
chemical dilution ratios. A basic description of the jar test procedure and
calculations is presented elsewhere (APHA 1995).
It is common to use six 2 L Gator jars with various dosages of chemi-
cal (alum, lime, etc); and one jar as the control without coagulant.
Appropriate coagulant dosages are added to the 2 L samples before the
rapid mixing at 100 revolution per minute (rpm) for 2 min. Then the sam-
ples and the control were flocculated at 20 rpm for 20 or more minutes
and allow to settle. Water temperature, floc size, settling characteristics
(velocity, etc.), color of supernatant, pH, etc. should be recorded.
The following examples illustrate calculations involved in the jar
tests; prepare stock solution, jar test solution mixture.
Example 1: Given that liquid alum is used as a coagulant. Specific gravity
of alum is 1.33. One gallon of alum weighs 11.09 pounds (5.03 kg) and contains
5.34 pounds (5.42 kg) of dry alum. Determine: (a) the alum concentration,
(b) mL of liquid alum required to prepare a 100 mL solution of 20,000 mg/L
alum concentration, (c) the dosage concentration of 1 mL of stock solution in
a 2000 mL Gator jar sample.
solution:
Step 1. Determine alum concentration in mg/mL
Step 2. Prepare 100 mL stock solution having a 20,000 mg/L alum
concentration
Let x ⫽ mg of alum required to prepare 100 mL stock solution
Step 3. Calculate mL (y) of liquid alum to give 2000 mg
y 5 3.125 mL
y mL
1 mL
5
2000 mg
640 mg
x 5 2000 mg
x
100 mL
5
20,000 mg
1000 mL
5 640 mg/mL
Alum smg/Ld 5
s5.34 lbd s453,600 mg/lbd
s1 galds3785 mL/gald
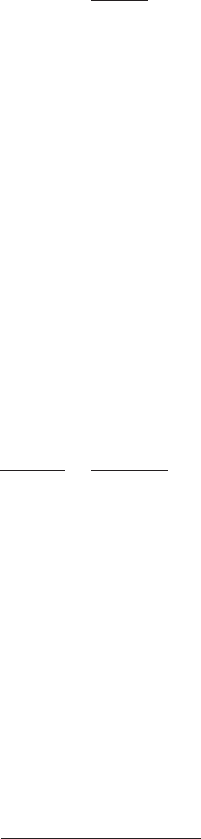
Note: Or add 6.25 mL of liquid alum into the 200 mL stock solution, since
3.125 mL is difficult to accurately measure.
Step 4. Find 1 mL of alum concentration (z) in 2000 mL sample (jar)
Note: Actual final volume is 2001 mL; using 2000 mL is still reasonable.
Example 2: Assuming that 1 mL of potassium permanganate stock solu-
tion provides l mg/L dosage in a 2 L jar, what is the weight of potassium
permanganate required to prepare 100 mL of stock solution? Assume
100% purity.
solution:
Step 1. Find concentration (x mg/L) of stock solution needed
Step 2. Calculate weight ( y mg) to prepare 100 mL (as 2000 mg/L)
Add 200 mg of potassium permanganate into 100 mL of deionized water to
give 100 mL stock solution of 2000 mg/L concentration.
Example 3: Liquid polymer is used in a 2 L Gator jar test. It weighs
8.35 lb/gal. One mL of polymer stock solution provides 1 mg/L dosage.
Compute how many mL of polymer are required to prepare 100 mL of stock
solution.
solution:
Step 1. Calculate (x) mg of polymer in l mL
5 1000 mg/mL
x 5
s8.35 lbds453,600 mg/lbd
s1 galds3785 mL/gald
y 5 200 mg
y mg
100 mL
5
2000 mg
1000 mL
x 5 2000 mg/L
sx mg/Ld s1 mLd 5 s1 mg/Ld sas 2000 mLd
5 10 mg/L
z 5
20,000
2000
smg/Ld
sz mg/Ld s2000 mLd 5 s20,000 mg/Lds1 mLd
376 Chapter 5

Step 2. As in Example 2, find the weight (y mg) to prepare 100 mL of 2000
mg/L stock solution
Step 3. Compute volume (z mL) of liquid polymer to provide 200 mg
Note: Use 0.20 mL of polymer in 100 mL of distilled water. This stock solu-
tion has 2000 mg/L polymer concentration. One mL stock solution added to
a 2 L jar gives 1 mg/L dosage.
8.2 Mixing
Mixing is an important operation for the coagulation process. In prac-
tice, rapid mixing provides complete and uniform dispersion of a chem-
ical added to the water. Then follows a slow mixing for flocculation
(particle aggregation). The time required for rapid mixing is usually 10
to 20 s. However, recent studies indicate the optimum time of rapid
mixing is a few minutes.
Types of mixing include propeller, turbine, paddle, pneumatic, and
hydraulic mixers. For a water treatment plant, mixing is used for coag-
ulation and flocculation, and chlorine disinfection. Mixing is also used
for biological treatment processes for wastewater. Rapid mixing for
coagulant in raw water and activated-sludge process in wastewater
treatment are complete mixing. Flocculation basins after rapid mixing
are designed based on an ideal plug flow using first-order kinetics. It is
very difficult to achieve an ideal plug flow. In practice, baffles are
installed to reduce short-circuiting. The time of contact or detention
time in the basin can be determined by:
For complete mixing
(5.52a)
For plug flow
(5.52b)t 5
V
Q
5
L
v
5
1
K
aln
C
i
C
e
b
t 5
V
Q
5
1
K
a
C
i
2 C
e
C
e
b
5 0.20 mL
z 5
200 mg
x
5
200 mg 3 1 mL
1000 mg
y 5 200 mg sof polymer in 100 mL distilled waterd
y mg
100 mL
5
2000 mg
1000 mL
Public Water Supply 377
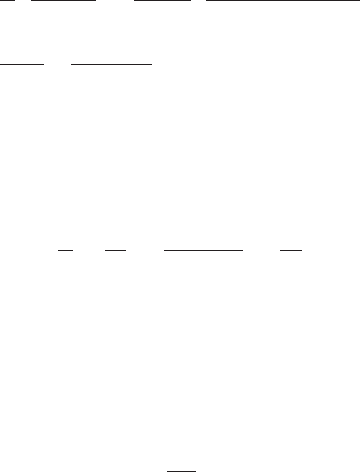
where t ⫽ detention time of the basin, min
V ⫽ volume of basin, m
3
or ft
3
Q ⫽ flow rate, m
3
Ⲑs or cfs
K ⫽ rate constant
C
i
⫽ influent reactant concentration, mgⲐL
C
e
⫽ effluent reactant concentration, mgⲐL
L ⫽ length of rectangular basin, m or ft
v ⫽ horizontal velocity of flow, m/s or ftⲐs
Example 1: Alum dosage is 50 mgⲐL, 379 ⫽ 90 per day based on laboratory
tests. Compute the detention times for complete mixing and plug flow reac-
tor for 90% reduction.
solution:
Step 1. Find C
e
Step 2. Calculate t for complete mixing
Using Eq. (5.52a)
Step 3. Calculate t for plug flow
Using Eq. (5.52b)
Power requirements.
Power required for turbulent mixing is tradition-
ally based on the velocity gradient or G values proposed by Camp and
Stein (1943). The mean velocity gradient G for mechanical mixing is
(5.53)G 5 a
P
V
b
1/2
5 36.8 min
t 5
1
K
aln
C
i
C
e
b 5
1440 min
90
aln
50
5
b
5 144 min
5
1day
90
3
1440 min
1 day
3 9
t 5
1
K
a
C
i
2 C
e
C
e
b 5
1
90/day
a
50 mg/L 2 5 mg/L
5 mg/L
b
5 5 mg/L
C
e
5 s1 2 0.9dC
i
5 0.1 3 C
i
5 0.1 3 50 mg/L
378 Chapter 5

where G ⫽ mean velocity gradient; velocity (ft/s)/distance (ft) is equal
to per second
P ⫽ power dissipated, ft ⭈ lb/s or N ⭈ m/s (W)
m ⫽ absolute viscosity, lb ⭈ s/ft
2
or N ⭈ s/m
2
V ⫽ volume of basin, ft
3
or m
3
The equation is used to calculate the mechanical power required to
facilitate rapid mixing. If a chemical is injected through orifices with
mixing times of approximately 1.0 s, the G value is in the range of 700
to 1000/s. In practice, G values of 3000 to 5000/s are preferable for rapid
mixing (ASCE and AWWA, 1990).
Eq. (5.53) can be expressed in terms of horsepower (hp) as
(5.54a)
For SI units the velocity gradient per second is:
(5.54b)
where kW ⫽ energy input, kW
V ⫽ effective volume, m
3
m ⫽ absolute viscosity, centipoise, cp
⫽ 1 cp at 20°C (see Table 4.1a, 1cp ⫽ 0.001 N ⋅ s/m
2
)
The equation is the standard design guideline used to calculate the
mechanical power required to facilitate rapid mixing. Camp (1968)
claimed that rapid mixing at G values of 500 to 1000/s for 1 to 2 min pro-
duced essentially complete flocculation and no further benefit for pro-
longed rapid mixing. For rapid mixing the product of Gt should be 30,000
to 60,000 with t (time) generally 60 to 120 s.
Example 2: A rapid mixing tank is l m ⫻ l m ⫻ 1.2 m. The power input is
746 W (1 hp). Find the G value at a temperature of 15°C.
solution: At 10°C, m ⫽ 0.00113 N ⭈ s/m
2
(from Table 4.1a)
V ⫽ 1m ⫻ 1m ⫻ 1.2 m ⫽ 1.2 m
3
P ⫽ 746 W ⫽ 746 N ⋅ m/s
G 5 a
kW 3 10
mV
b
1/2
G 5 a
550 hp
mV
b
1/2
Public Water Supply 379
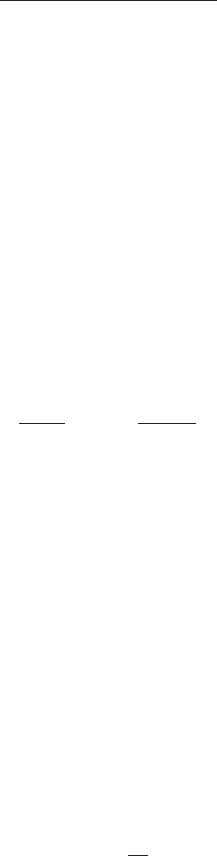
Using Eq. (5.53):
9 Flocculation
After rapid mixing, the water is passed through the flocculation basin.
It is intended to mix the water to permit agglomeration of turbidity set-
tled particles (solid capture) into larger floes which would have a mean
velocity gradient ranging 20 to 70 s
⫺1
for a contact time of 20 to 30 min
taking place in the flocculation basin. A basin is usually designed in four
compartments (ASCE and AWWA 1990).
The conduits between the rapid mixing tank and the flocculation
basin should maintain G values of 100 to 150 s
⫺1
before entering the
basin.
For baffled basin, the G value is
(5.55)
where G ⫽ mean velocity gradient, s
⫺1
Q ⫽ flow rate, ft
3
/s
␥ ⫽ specific weight of water, 62.4 lb/ft
3
H ⫽ head loss due to friction, ft
⫽ absolute viscosity, lb ⋅ s/ft
2
V ⫽ volume of flocculator, ft
3
t ⫽ detention time, s
For paddle flocculators, the useful power input of an impeller is
directly related to the drag force of the paddles (F). The drag force is the
product of the coefficient of drag (C
d
) and the impeller force (F
i
). The drag
force can be expressed as (Fair et al., 1968):
(5.56a)
(5.57)
then
(5.56b)F 5 0.5 C
d
rAv
2
F
i
5 rA
v
2
2
F 5 C
d
F
i
G 5 a
QgH
mV
b
0.5
5 a
62.4H
mt
b
0.5
5 742 s
21
5 a
746 N
#
m/s
0.00113 N
#
s/m
2
3 1.2 m
3
b
0.5
G 5 sP/mVd
0.5
380 Chapter 5
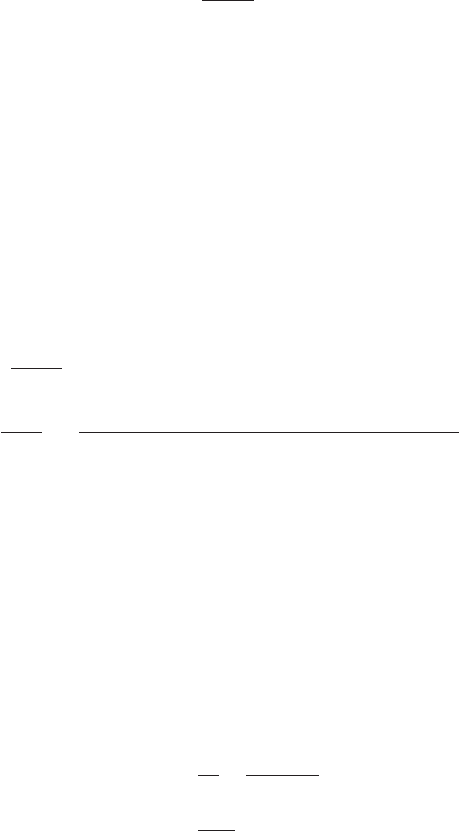
where F ⫽ drag force, lb
C
d
⫽ dimensionless coeffiicient of drag
r ⫽ mass density, lb ⋅ s
2
/ft
4
A ⫽ area of the paddles, ft
2
v ⫽ velocity difference between paddles and water, fps
The velocity of paddle blades (v
p
) can be determined by
(5.58)
where v
p
⫽ velocity of paddles, fps
n ⫽ number of revolutions per minute, rpm
r ⫽ distance from shaft to center line of the paddle, ft
The useful power input is computed as the product of drag force and
velocity difference as below:
(5.59)
Example 1: In a baffled basin with detention time of 25 min. Estimate head
loss if G is 30/s, m ⫽ 2.359 ⫻ 10
⫺5
lb ⭈ s/ft
2
at T ⫽ 60°F (Table 4.1b).
solution: Using Eq. (5.55)
Example 2: A baffled flocculation basin is divided into 16 channels by 15
around-the-end baffles. The velocities at the channels and at the slots are
0.6 and 2.0 fps (0.18 and 0.6 m/s), respectively. The flow rate is 12.0 cfs (0.34 m
3
/s).
Find (a) the total head loss neglecting channel friction; (b) the power dis-
sipated; (c) the mean velocity gradient at 60°F (15.6°C); the basin size is
16 ⫻ 15 ⫻ 80 ft
3
; (d) the Gt value, if the detention (displacement) time is
20 min, and (e) loading rate in gpd/ft
3
.
solution:
Step 1. Estimate loss of head H
Loss of head in a slot 5
2
2
64.4
5 0.0621 sftd
Loss of head in a channel 5
v
2
1
2g
5
0.6
2
2 3 32.2
5 0.00559 sftd
5 0.51 ft
H 5
G
2
mt
62.4
5
s30s
21
d
2
s2.359 3 10
25
lb
#
s/ft
2
d 3 s25 3 60 sd
62.4 lb/ft
3
G 5 a
62.4H
mt
b
1/2
P 5 Fv 5 0.5 C
d
r Av
3
v
p
5
2p r n
60
Public Water Supply 381

(a)
Step 2. Compute power input P
(b)
Note: g ⫽ 62.37 lb/ft
3
at 60°F (Table 5.1b)
Step 3. Compute G
From Table 4.1b
At 60°F,
Using Eq. (5.53)
(c)
Step 4. Compute Gt
(d)
Step 5. Compute loading rate
Q ⫽ 12 cfs ⫽ 12 cfs ⫻ 0.646 MGD/cfs
⫽ 7.75 MGD
⫽ 7.75 ⫻ 10
6
gpd
(e) Loading rate ⫽ Q/V ⫽ 7.76 ⫻ 10
6
gpd/19,200 ft
3
⫽ 404 gpd/ft
3
Example 3: A flocculator is 16 ft (4.88 m) deep, 40 ft (12.2 m) wide, and 80 ft
(24.4 m) long. The flow of the water plant is 13 MGD (20 cfs, 0.57 m
3
/s).
Rotating paddles are supported parallel to four horizontal shafts. The rotat-
ing speed is 2.0 rpm. The center line of the paddles is 5.5 ft (1.68 m) from the
shaft (mid-depth of the basin). Each shaft equipped with six paddles. Each
paddle blade is 10 in (25 cm) wide and 38 ft (11.6 m) long. Assume the mean
velocity of the water is 28% of the velocity of the paddles and their drag coef-
ficient is 1.9. Estimate:
5 49.200
Gt 5 41 s
21
3 20 min 3 60 s/min
5 41.0 s
21
G 5 a
P
mV
b
1/2
5 a
763 f t”
#
lb/s
2.359 3 10
25
lb
#
s/f t
2
3 19,200 f t
3
b
1/2
V 5 16 ft 3 15 ft 3 80 ft 5 19,200 ft
3
m 5 2.359 3 10
25
lb
#
s/ft
2
5 763 ft
#
lb/s
P 5 Q
g
H 5 12 ft
3
/s 3 62.37 lb/ft
3
3 1.02 ft
5 1.02 sftd
H 5 16 3 0.00559 1 15 3 0.0621
382 Chapter 5
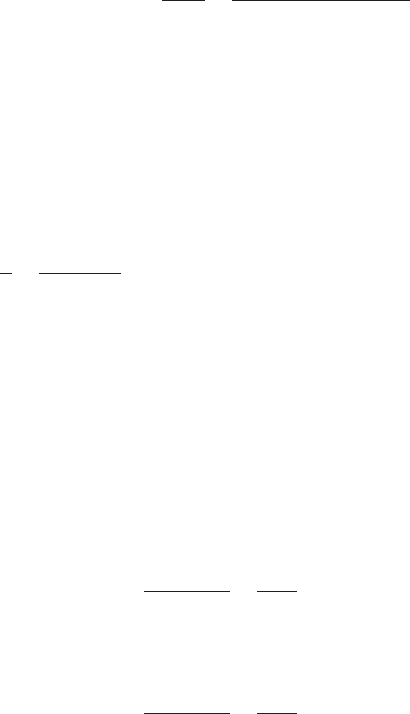
(a) the difference in velocity between the paddles and water
(b) the useful power input
(c) the energy consumption per million gallons (Mgal)
(d) the detention time
(e) the value of G and Gt at 60°F
(f) the loading rate of the flocculator
solution:
Step 1. Find velocity differential v
Using Eq. (5.58)
(a)
⫽ 0.83 fps
Step 2. Find P
A ⫽ paddle area ⫽ 4 shaft ⫻ 6 pads/shaft ⫻38 ft ⫻ 10/12 ft/pad ⫽ 760 ft
2
Using Eq. (5.59):
(b) P ⫽ 0.5C
d
A
3
⫽ 0.5 ⫻ 1.9 ⫻ 1.938 lb ⭈ s
2
/ft
4
⫻ 760 ft
2
⫻ (0.83 fps)
3
⫽ 800 ft ⋅ lb/s
or ⫽ (800/550) hp
⫽ 1.45 hp
or ⫽ 1.45 ⫻ 0.746 kW
⫽ 1.08 kW
Step 3. Determine energy consumption E
(c)
or
5 1.99 kW
#
h/Mgal
E 5
1.08
kW
13 Mgal/d
3
24 h
d
5 2.68 hp
#
h/Mgal
E 5
1.45 hp
13 Mgal/d
3
24 h
d
r 5
g
g
5
62.4 lb/f t
3
32.2 f t/s
2
5 1.938 lb
#
s
2
/f t
4
v 5 v
p
s1 2 0.28d 5 1.15 fps 3 0.72
5 1.15 fps
v
p
5
2prn
60
5
2 3 3.14 3 5.5 ft 3 2
60 s
Public Water Supply 383

Step 4. Determine detention time t
Basin volume, V ⫽ 16 ft ⫻ 40 ft ⫻ 80 ft ⫽ 5.12 ⫻ 10
4
ft
3
⫽ 5.12 ⫻ 10
4
ft
3
⫻ 7.48 gal/ft
3
⫽ 3.83 ⫻ 10
5
gal
(d)
⫽ 0.0295 day ⫻ 1440 min/d
⫽ 42.5 min
Step 5. Compute G and Gt
Using Eq. (5.53):
(e) G ⫽ (P/mV)
0.5
⫽ (800/2.359 ⫻ 10
⫺5
⫻ 5.12 ⫻ 10
4
)
0.5
⫽ 25.7 (fps/ft, or s
⫺1
)
Gt ⫽ (25.7s
⫺1
) (42.5 ⫻ 60 s)
⫽ 65,530
Step 6. Compute the loading rate
(f )
⫽ 254 gpd/ft
3
10 Sedimentation
Sedimentation is one of the most basic processes of water treatment.
Plain sedimentation, such as the use of a presedimentation basin (grit
chamber) and sedimentation tank (or basin) following coagulation—
flocculatiaon, is the most commonly used in water treatment facilities.
The grit chamber is generally installed upstream of a raw water pump-
ing station to remove larger particles or objects. It is usually a rectan-
gular horizontal-flow tank with a contracted inlet and the bottom should
have at minimum a 1:100 longitudinal slope for basin draining and
cleaning purposes. A trash screen (about 2-cm opening) is usually
installed at the inlet of the grit chamber.
Loading rate 5
Q
V
5
13 3 10
6
gpd
5.12 3 10
4
ft
3
t 5
V
Q
5
3.83 3 10
5
gal
13 3 10
6
gal/d
5 0.0295 day
384 Chapter 5
