Lin S.D. Water and Wastewater Calculations Manual
Подождите немного. Документ загружается.


Step 5. Convert the values of K
L
a at 20ºC using ⫽ 1.024
Using Eq. (5.26)
For tap water
K
L
a
(T)
⫽ K
L
a
(20ºC)
20⫺T
or
For wastewater
Step 6. Compute the ␣ value using Eq. (5.27)
6.2 Diffused aeration
Diffused aeration systems distribute the gas uniformly through the
water or wastewater, in such processes as ozonation, absorption, activated-
sludge process, THM removal, and river or lake reaeration, etc. It is more
costly using diffused aeration for VOC removal than the air stripping
column.
The two-resistance layer theory is also applied to diffused aeration.
The model proposed by Mattee-Müller et al. (1981) is based on mass
transfer flux derived from the assumption of diffused bubbles rising
in a completely mixed container. The mass transfer rate for diffused
aeration is
(5.30)
where F ⫽ mass transfer rate
Q
G
⫽ gas (air) flow rate, m
3
/s or ft
3
/s
Q
L
⫽ flow rate of liquid (water), m
3
/s or ft
3
/s
F 5 Q
G
H
u
C
e
a1 2 exp
K
L
aV
H
u
Q
L
b
5 0.64
5
0.70 h
21
0.10 h
21
a 5
K
L
a for wastewater
K
L
a for tap water
5 0.70 h
21
K
L
a
s20d
5 0.64 h
21
s1.024d
20216
5 1.10 h
21
5 1.00 h
21
s1.024d
20216
K
L
a
s20d
5 K
L
a
sT d
u
202T
Public Water Supply 355
H
u
⫽ unitless Henry’s constant (see, Section of Design of Packed
Tower)
C
e
⫽ effluent (exit) gas concentration, µg/L
K
L
a ⫽ overall mass transfer coefficient, per time
V ⫽ reaction volume (water), m
3
or ft
3
Assuming that the liquid volume in the reactor is completely mixed
and the air rises as a steady state plug flow, the mass balance equation
can be expressed as
(5.31a)
or
(5.31b)
where C
i
⫽ initial concentration, µg/L
If >> 1, the transfer of a compound is with very low Henry’s constant
such as ammonia. Air bubbles exiting from the top of the liquid surface
is saturated with ammonia in the stripping process. Ammonia removal
could be further enhanced by increasing the air flow. Until < 4, the
exponent term becomes essentially zero. When the exponent term is
zero, the air and water have reached an equilibrium condition and the
driving force has decreased to zero at some point within the reactor
vessel. The vessel is not fully used. Thus the air-to-water ratio could be
increased to gain more removal.
On the other hand, if << 1, the mass transfer efficiency could be
improved by increasing overall mass transfer coefficient by either
increasing the mixing intensity in the tank or by using a finer diffuser.
In the case for oxygenation, < 0.1, the improvements are required.
Example: A groundwater treatment plant has a capacity of 0.0438 m
3
/s
(1 MGD) and is aerated with diffused air to remove trichloroethylene with
90% design efficiency. The detention time of the tank is 30 min. Evaluate the
diffused aeration system with the following given information.
T ⫽ 20ºC
C
i
⫽131 µg/L , (expected C
e
⫽ 13.1 µg/L)
H
u
⫽ 0.412
K
L
a ⫽ 44 h
⫺1
u 5
K
L
aV
H
u
Q
G
C
e
C
i
5
1
1 1 H
u
Q
G
/Q
L
[1 2 exp s2 ud]
C
e
C
i
5
1
1 1 H
u
Q
G
/Q
L
[1 2 exp s2 K
L
aV/H
u
Q
G
]
356 Chapter 5

Public Water Supply 357
solution:
Step 1. Compute volume of reactor V
Step 2. Compute Q
G
, let Q
G
⫽ 30 Q
L
⫽ 30V
Step 3. Compute
Step 4. Compute effluent concentration C
e
with Eq (5.31b)
This exceeds the expected 90%.
6.3 Packed towers
Recently, the water treatment industry used packed towers for stripping
highly volatile chemicals, such as hydrogen sulfide and VOCs from
water and wastewater. It consists of a cylindrical shell containing a
support plate for the packing material. Although many materials can
be used as the packing material, plastic products with various shapes
and design are most commonly used due to less weight and lower cost.
Packing material can be individually dumped randomly into the cylin-
der tower or fixed packing. The packed tower or columns are used for
mass transfer from the liquid to gas phase.
Figure 5.4 illustrates a liquid-gas contacting system with a down-
ward water velocity L containing c
1
concentration of gas. The flows are
counter current. The air velocity G passes upward through the packed
material containing influent p
1
and effluent p
2
. There are a variety of
mixing patterns, each with a different rate of mass transfer. Removal
of an undesirable gas in the liquid phase needs a system height of z and
a selected gas flow rate to reduce the mole fraction of dissolved gas from
c
1
to c
2
. If there is no chemical reaction that takes place in the packed
% removal 5
s131 2 10d 3 100
131
5 92.4
5 10.0 mg/L
5
131
mg/L
1 1 0.412 3 30[1 2 exps 2 3.56d]
C
e
5
C
i
1 1 H
u
Q
G
/Q
L
[1 2 exps 2 ud]
u 5
K
L
aV
H
u
Q
G
5
44 3 79
0.412 3 2370
5 3.56
Q
G
5 30 3 79 m
3
5 2370 m
3
5 79 m
3
V 5 0.0438 m
3
/s 3 60 s/min 3 30 min

358 Chapter 5
tower, the gas lost by water should be equal to the gas gained by air. If
the gas concentration is very dilute, then
(5.32)
where L ⫽ liquid velocity, m/s, m
3
/(m
2
⋅ s), or mol/(m
2
⋅ s)
G ⫽ gas velocity, same as above
⫽ change of gas concentration in water
⫽ change in gas fraction in air
From Eq. (5.23d), for stripping (c and c
s
will be reversed):
(5.23e)
then
(5.33)
Multiplying each side by L, we obtain
(5.34)
where dz is the differential of height.
The term (c ⫺ c
s
) is the driving force (DF) of the reaction and is constantly
changing with the depth of the column due to the change of c with time.
Integrating the above equation yields
(5.35)
z 5
L
K
L
a
3
c
2
c
1
3
DF
dc
dsDFd
3
dz 5
L
K
L
a
3
c
2
c
1
dc
dsc 2 c
s
d
Ldt 5 dz 5
Ldc
K
L
asc 2 c
s
d
dt 5
dc
K
L
asc 2 c
s
d
dc
dt
5 K
L
asc 2 c
s
d
⌬p
⌬c
L⌬c 5 G⌬p
G
p
2
L
c
1
dz
Partial
z
p
1
Pressure
p
L
G
p
2
c
2
p
1
c
2
c
c
1
Concentration
Figure 5.4 Schematic diagram of packed column.

Public Water Supply 359
The integral of DF is the same as the log mean (DF
lm
) of the influent
(DF
i
) and effluent driving forces (DF
e
). Therefore
(5.36)
and
(5.37)
where z ⫽ height of column, m
L ⫽ liquid velocity, m
3
/m
2
⋅ h
c
i
, c
e
⫽ gas concentration in water at influent and effluent,
respectively, mg/L
K
L
a ⫽ overall mass transfer coefficient for liquid, h
⫺1
DF
i
, DF
e
⫽ driving force at influent and effluent, respectively, mg/L
DF
lm
⫽ log mean of DF
i
and DF
e
, mg/L
Example 1: Given
T ⫽ 20ºC ⫽ 293 K
L ⫽ 80 m
3
water (m
2
⋅ h)
G ⫽ 2400 m
3
air/(m
2
⋅ h)
c
i
⫽ 131 g/L trichloroethylene concentration in water at
entrance
c
e
⫽ 13.1 g/L CCHCl
3
concentration at exit
K
L
a ⫽ 44 h
⫺1
Determine the packed tower height to remove 90% of trichloroethylene
by an air stripping tower.
solution:
Step 1. Compute the molar fraction of CCHCl
3
in air p
2
Assuming no CCHCl
3
present in the air the entrance, i.e.
p
i
⫽ 0
MW of CCHCl
3
⫽ 131, 1 mole ⫽131g of CCHCl
3
per liter.
c
e
5 13.1 mg/L 5 0.1 3 10
23
mol/m
3
swith 90% removald
5 1 3 10
23
mol/m
3
c
i
5 131 mg/L 5 131
mg
L
3
1 mol/g
131 3 10
6
mg/g
3
10
3
L
1 m
3
DF
lm
5
DF
e
2 DF
i
ln sDF
e
>DF
i
z 5
Lsc
i
2 c
e
d
K
L
aDF
lm

360 Chapter 5
Applying Eq. (5.32)
80 m
3
air/(m
2
⋅ h)(1 ⫺ 0.1) ⫻ 10
⫺3
mol gas/m
3
air ⫽ 2400 m
3
/(m
2
⋅ h)( p
e
⫺ 0)
p
e
⫽ 3.0 ⫻ 10
⫺5
mol gas/m
3
air
Step 2. Convert p
e
in terms of mol gas/mol air
Let V ⫽ volume of air per mole of air
From Step 1
Step 3. Compute DF for gas entrance and exit
At gas influent (bottom)
p
i
⫽ 0
c
e
⫽ 13.1 g/L ⫽ 0.0131 mg/L
c
s
⫽ 0
DF
i
⫽ c
e
⫺ c
s
⫽ 0.0131 mg/L
At gas effluent (top)
From Table 5.3, Henry’s constant H at 20ºC
H ⫽ 550 atm
Convert atm to atm L/mg, H
d
5 7.55 3 10
25
atm
#
L/mg
H
d
5
H
55,600 3 MW
5
550 atm
55,600 3 131 mg/L
p
e
5 7.2 3 10
27
mol gas/mol air
c
i
5 131 mg>L 5 0.131 mg/L
c
s
5 to be determined
p
e
5 3.0 3 10
25
mol gas/m
3
air
5 3.0 3 10
25
mol gas
m
3
air
3
0.024 m
3
air
1 mol air
5 7.2 3 10
27
mol gas/mol air
V 5
nRT
P
5
s1 moleds0.08206 L atm/mol Kd 3 s293 Kd
1 atm
5 24.0 L
5 0.024 m
3
L⌬c 5 G⌬p

Public Water Supply 361
Step 4. Compute DF
lm
From Eq. (5.37)
Step 5. Compute the height of the tower z
From Eq. (5.36)
Example 2 In a groundwater remediation study, volatile organic carbon
removal through the vapor phase, the total hydrocarbons analyzer measured
1,1,1,-trichloroethane (Jones et al., 2000). The extraction pump and air strip-
per combined air flow averaged 0.40m
3
/min (14ft
3
/min). The concentration of
1,1,1,-trichloroethane averaged 25 parts per million by volume (ppmv) of air.
Determine the amount of 1,1,1,-trichloroethane removed daily. Temperature
is 20ºC.
solution
Step 1. Calculate 1,1,1,-trichloroethane concentration using the ideal gas law
T ⫽ 20°C ⫽ (20 ⫹ 273)K ⫽ 293 K
⫽ 41.6 mol/m
3
This means 41.6 mole of total gases in lm
3
of the air.
The MW of 1,1,1,-trichloroethane (CH
3
CC1
3
) ⫽ 133
One ppmv of 1,1,1,-trichloroethane ⫽ 41.6 ⫻ 10
⫺6
mol/m
3
⫻ 133g/mol
⫻ 1000 mg/g
⫽ 5.53 mg/m
3
25 ppmv of 1,1,1,-trichloroethane ⫽ 5.53 mg/m
3
⫻ 25
⫽ 138 mg/m
3
n 5
PV
RT
5
s1 atmd s1000 L/mg
3
d
0.082sL
#
atm/mol-Kd 3 293 K
z 5
Lsc
i
2 c
e
d
K
L
aDF
lm
5
80 m/h 3 s0.131 2 0.0131d mg/L
44 h
21
3 0.0487 mg/L
5 4.4 m
DF
lm
5
DF
e
2 DF
i
ln sDF
e
/DF
i
d
5
0.1215 mg/L 2 0.0131 mg/L
ln s0.1215/0.0131d
5 0.0487 mg/L
5 0.1215 mg/L
DF
e
5 c
i
2 c
s
5 0.131 mg/L 2 0.0095 mg/L
5 0.0095 mg/L
c
s
5
p
e
p
t
H
d
5
7.2 3 10
27
mol gas/mol air 3 1 atm
7.55 3 10
25
atm
#
L/mg
Step 2. Calculate the daily removal
Daily removal ⫽ 138 mg/m
3
⫻ 0.40 m
3
/min ⫻ l440 min/d
⫽ 79500 mg/d
⫽ 79.5 g/d
Design of packed tower. Process
design of packed towers or columns is
based on two quantities: the height of the packed column, z, to achieve
the designed removal of solute is the product of the height of a transfer
unit (HTU) and the number of transfer unit (NTU). It can be expressed
as (Treybal, 1968):
z ⫽ (HTU)(NTU) (5.38)
The HTU refers the rate of mass transfer for the particular packing
materials used. The NTU is a measure of the mass transfer driving
force and is determined by the difference between actual and equilib-
rium phase concentrations. The height of a transfer unit is the constant
portion of Eq. (5.35):
(5.39)
The number of transfer units is the integral portion of Eq. (5.35). For
diluted solutions, Henry’s law holds. Substituting the integral expres-
sion for NTU with p
1
⫽ 0, the NTU is
(5.40)
where
(5.41)
⫽ stripping factor, unitless when H
u
is unitless
c
1
, c
2
⫽ mole fraction for gas entrance and exit, respectively
Convert Henry’s constant from terms of atm to unitless:
(5.42)
⫽ H/4.56T
a
1 L of water
55.6 mole
b
H
u
5 cH
atmsmol gas/mol aird
mol gas/mol water
d a
1 mol air
0.082T atm L of air
b
R 5
H
u
G
L
NTU 5
R
R 2 1
ln
sc
1
/c
2
dsR 2 1d 1 1
R
HTU 5
L
K
L
a
362 Chapter 5

Public Water Supply 363
When T ⫽ 20ºC ⫽ 293 K
H
u
⫽ H/4.56 ⫻ 293 ⫽ 7.49 ⫻ 10
⫺4
H, unitless (5.42a)
G ⫽ superficial molar air flow rate (k mol/s ⋅ m
2
)
L ⫽ superficial molar water flow rate (k mol/s ⋅ m
2
)
The NTU depends upon the designed gas removal efficiency, the air-
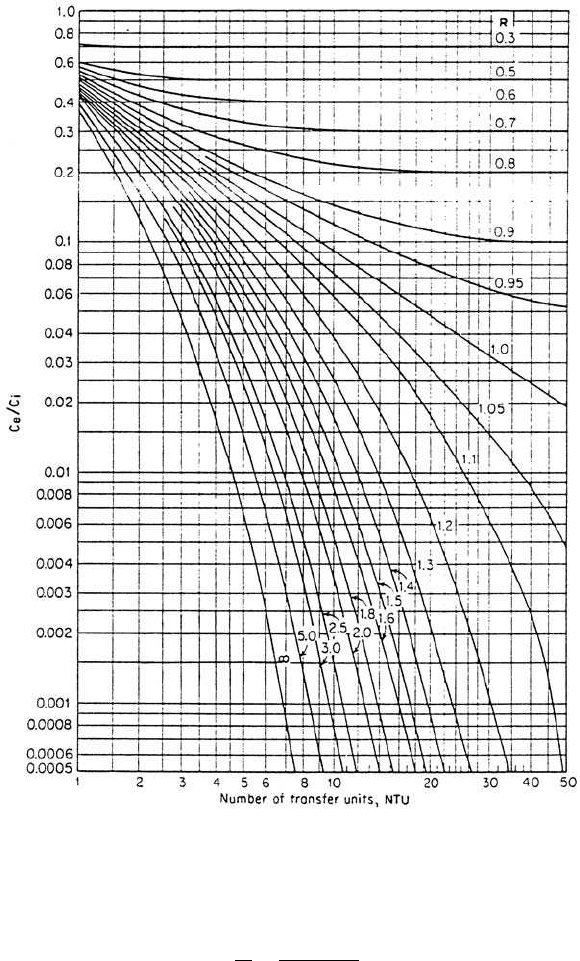
water velocity ratio, and Henry’s constant. Treybal (1968) plotted the
integral part (NTU) of Eq. (5.35) in Fig. 5.5. By knowing the desired
removal efficiency, the stripping factor, and Henry’s constant, the
NTU in a packed column can be determined for any given stripping
factor of the air to water flow rate ratios. It can be seen from Fig. 5.5
that when the stripping factor, R, is greater than 3, little improvement
for the NTU occurs.
Example: Using the graph of Fig. 5.5 to solve Example 1 above. Given:
T ⫽ 20°C ⫽ 293 K
L ⫽ 80 m
3
water/(m
2
column cross section h)
G ⫽ 2400 m
3
air/(m
2
column cross section h)
c
1
⫽ 131 g/L of CCHCl
3
c
2
⫽ 13.1 g/L of CCHCl
3
K
L
a ⫽ 44 h
⫺1
solution:
Step 1. Compute HTU with Eq. (5.39)
Step 2. Compute H
u
, the unitless Henry’s constant at 20°C
From Table 5.3
H ⫽ 550 atm
Using Eq. (5.42a)
H
u
⫽ 7.49 ⫻ 10
⫺4
H ⫽ 7.49 ⫻ 10
⫺4
⫻ 550
⫽ 0.412
Step 3. Compute the stripping factor R using Eq. (5.41)
⫽ 12.36
R 5
H
u
G
L
5
0.412 3 2400 m/h
80 m/h
HTU 5
L
K
L
a
5
80 m/h
44 h
21
5 1.82 m
364 Chapter 5
Figure 5.5 Number of transfer units for absorbers or strippers with constant
absorption or stripping factors (D. A. Cornwell, Air Stripping and aeration. In:
AWWA, Water Quality and Treatment. Copyright 1990, McGraw-Hill, New York,
reprinted with permission of McGraw-Hill).
Step 4. Find NTU from Fig. 5.5
Since
Using R ⫽ 12.36 and c
2
Ⲑc
1
⫽ 0.1, from the graph in Fig. 5.5, we obtain
NTU ⫽ 2.42
c
2
c
1
5
13.1 mg/L
131 mg/L
5 0.1
