Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

Formation of Corrosion Products
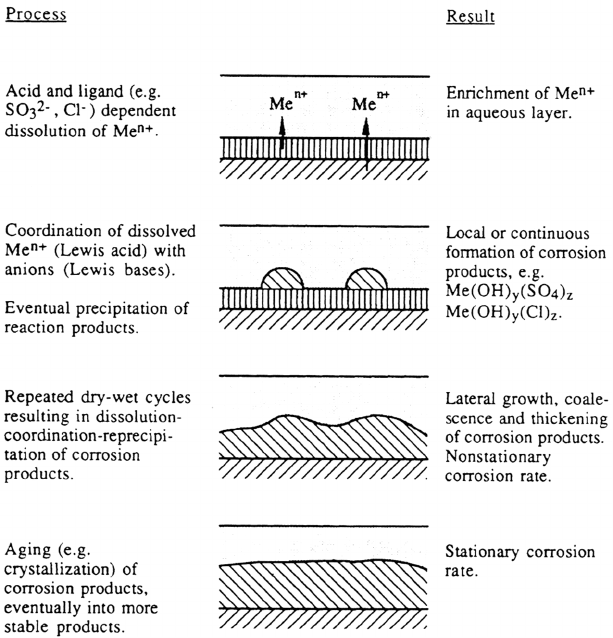
The atmospheric corrosion rate is influenced by many parameters, one of the most
important being the formation and protective ability of the corrosion products
formed. The composition of the corrosion products depends on the participating
dissolved metal ions and the access to anions dissolved in the aqueous layer. The
eventual thickening of the film of corrosion products can be described in a
sequence of consecutive steps—dissolution–coordination–reprecipitation—where
the dissolution step is acid dependent, coordination is based on the HSAB principle,
and reprecipitation depends on the activities of the species involved.
Depending on the rate of crystallization and the rate of formation, the corrosion
products may be amorphous or crystalline. If the former is rate determining, one
expects amorphous phases to form. From colloid chemistry it is known that aging
or slow growth of amorphous phases may result in a transition from the amorphous
to the crystalline state, a process that may occur through slow transformation in
the solid state or through dissolution–reprecipitation processes [60]. This seems to
be in agreement with findings of a transition from amorphous to crystalline basic
nickel sulfates, the former being less corrosion resistant than the latter [61a].
Figure 5 is a schematic illustration of important processes occurring in or at the
solid phase.
Atmospheric Corrosion of Selected Metals
Access to new and more sensitive analytical techniques has resulted in substantial
progress in the characterization of corrosion products formed under both laboratory
and field exposure conditions. These techniques permit the determination of, e.g.,
thickness, chemical composition, and atomic structure of corrosion products formed
at both early and later stages of exposure. When combined with environmental data,
such as deposition rates of corrosion-stimulating atmospheric constituents, relative
humidity, temperature, and sunshine hours, the new techniques have resulted in a
more comprehensive understanding of the complex processes that govern atmospheric
corrosion. In a series of papers, Graedel has summarized the corrosion mechanisms
of zinc [62], aluminum [63], copper [18], iron and low-alloy steel [64], and silver
[19]. It is beyond the scope of this chapter to provide a detailed description of the
specific atmospheric corrosion behavior of each individual metal. Nevertheless, a
summary of atmospheric corrosion behavior of selected metals will be given in light
of the general processes that have been discussed. The summary is based on the
papers by Graedel, unless otherwise stated.
The atmospheric corrosion of zinc starts with the instant formation of a thin film
of zinc hydroxide, which seems to occur in different crystal structures, and the
subsequent formation of a protective layer of basic zinc carbonate, Zn
5
(CO
3
)
2
(OH)
6
.
The pH of the aqueous layer controls the stability of initial corrosion products and
results in the dissolution of Zn
2+
. From the HSAB principle one expects Zn
2+
,
classified as an intermediate acid, to coordinate with a number of different bases. In
accordance with this, the prolonged exposure of zinc can proceed along a variety of
different paths of reaction sequences depending on the actual deposition rates of
atmospheric constituents. Among these Cl
–
and SO
2
seem to be the most important.
Atmospheric Corrosion 543
Copyright © 2002 Marcel Dekker, Inc.
In a relatively benign rural atmosphere, the basic zinc carbonate may continue to
grow slowly or may be followed by the formation of a protective basic zinc sulfate,
e.g., Zn
4
SO
4
(OH)
6
· 4H
2
O. In a typical marine atmosphere, characterized by higher
amounts of deposited Cl
–
than of SO
2
, islands of a less protective basic zinc chloride,
Zn
5
Cl
2
(OH)
8
· H
2
O, are formed within days of exposure. These islands grow
laterally and coalesce. Within weeks of exposure, a more protective basic zinc
chlorosulfate, NaZn
4
Cl(OH)
6
SO
4
· 6H
2
O, may be observed [65]. In a typical
urban environment, characterized by higher amounts of deposited SO
2
than of Cl
–
,
the basic zinc sulfate, Zn
4
SO
4
(OH)
6
· 4H
2
O, is observed within, typically, weeks of
exposure. In highly polluted industrial environments, it is eventually followed by
another basic zinc chlorosulfate, Zn
4
Cl
2
(OH)
4
SO
4
· 5H
2
O. Afeature common to most
zinc-containing corrosion products observed is their strong structural resemblance,
with sheets of Zn
2+
in octahedral and tetrahedral coordination and with the main
difference between the structures being the content and bonding between the sheets
[66]. Ageneralized reaction sequence has been proposed that considers the evolution
of corrosion products on sheltered zinc in a variety of atmospheric environments,
including rural, marine, urban, and industrial [66]. Figure 6 displays schematically the
544Leygraf
Figure 5Schematic illustration of processes occurring in or at the solid phase.
Copyright © 2002 Marcel Dekker, Inc.
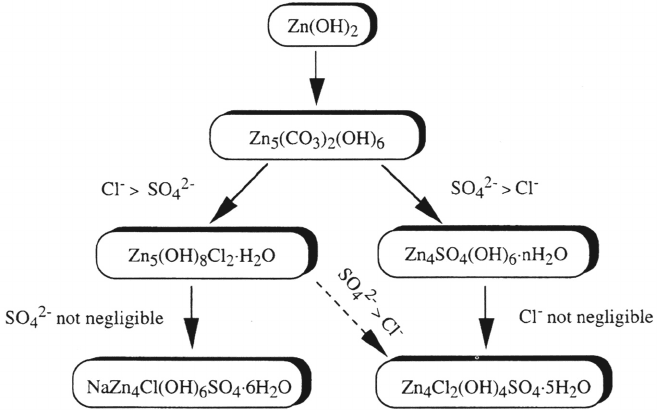
reaction sequence in which sulfate deposition dominates over chloride deposition
(right part) and the reaction sequence in which chloride deposition dominates over
sulfate deposition (left part). Reported corrosion rates (in μm/year) of zinc in outdoor
atmospheres range from 0.2 to 0.3 (rural), from 0.5 to 8 (marine), and from 2 to 16
(urban, industrial).
Aluminum forms initially a few-nm-thick layer of aluminum oxide, γ-Al
2
O
3
,
which after prolonged exposure in humidified air is covered by aluminum
oxyhydroxide, γ-AlOOH, and subsequently by various hydrated aluminum oxides
and aluminum hydroxides. The stability of the compounds decreases with acidity and
results in the dissolution of Al
3+
. The ability of aluminum to form various oxygen-
containing corrosion products is in agreement with the HSAB principle; this is also
the case with the general observation of frequent formation of basic or hydrated
aluminum sulfates and no detection of aluminum sulfides. The sulfates most
frequently found on aluminum are poorly soluble, amorphous, and highly protective.
As with other passivating metals, atmospheric corrosion rates of aluminum increase
readily in the presence of Cl
–
, resulting in local rather than uniform corrosion. Rates
of uniform corrosion (in μm/year) of aluminum outdoors are substantially lower than
for most other structural metals and are from 0.0 to 0.1 (rural), from 0.4 to 0.6
(marine), and ≈1 (urban).
In ambient air copper initially forms a film with a total thickness of around
3 nm. It seems to contain at least two layers, both of which contain copper as Cu
+
.
The inner layer close to the metal consists of Cu
2
O and the outer layer of bound
hydroxyl, water, and adventitious hydrocarbon [67]. Cu dissolves into the aqueous
layer as Cu
+
, which readily oxidizes to Cu
2+
. From the presence of both Cu
+
(soft
acid) and Cu
2+
(intermediate acid) a variety of corrosion products are expected.
Atmospheric Corrosion 545
Figure 6 Formation sequences for compounds in zinc corrosion products formed under
sheltered conditions dominated by chloride deposition (left sequence) and sulfate deposition
(right sequence). (From Ref. 66. Courtesy of the American Society for Testing and
Materials.)
Copyright © 2002 Marcel Dekker, Inc.
With reduced sulfur compounds present in significant concentrations, copper mainly
forms Cu
2
S. Without reduced sulfur compounds, the corrosion products are frequently
complex mixtures of basic copper sulfates, chlorides, nitrates, and carbonates, with a
composition that depends on the actual deposition rates of the corresponding air
constituents. Abundant phases are Cu
4
SO
4
(OH)
6
and Cu
3
SO
4
(OH)
4
in urban areas
and Cu
2
Cl(OH)
3
and possibly basic sulfates in marine atmospheres. Other phases
observed are Cu
4
SO
4
(OH)
6
· H
2
O, Cu
2
NO
3
(OH)
3
, and Cu
2
CO
3
(OH)
2
. Similar to that
for zinc, a generalized reaction sequence has been proposed for sheltered copper,
which integrates all existing knowledge of phases formed in copper patina during
exposure in atmospheric environments with varying degree of sulfur and chloride
pollution [67]. The phases observed bear structural resemblance and are mostly
layered, a common observation in basic salts of divalent cations such as Cu
2+
and
Zn
2+
. Representative corrosion rates (in μm/year) of copper exposed outdoors are
≈ 0.5 (rural), ≈ 1 (marine), 1–2 (urban), and <_ 2.5 (industrial).
Initial stages of atmospheric corrosion of iron involve the incorporation of
oxygen and water into a rich variety of different iron oxides or iron oxyhydroxides as
rust layers. These processes are preceded by initial dissolution of Fe
2+
, which only
slowly oxidizes to Fe
3+
. From this follows that corrosion products formed at earlier
stages of exposure contain iron in both valence states but only as Fe
3+
at later stages.
A complex chemistry is anticipated with the participation of an intermediate acid
slowly transforming into a hard acid. The oxidation of Fe
2+
in the aqueous layer may
involve several possible oxidants, such as the hydroxyl radical (OH·), the
hydroperoxyl radical (HO
2
·), and hydrogen peroxide (H
2
O
2
). Detailed discussions
of the interaction between SO
2
and iron have been presented elsewhere [68]. It results
in the formation of iron(II) sulfates, subsequent oxidation of Fe
2+
, and concomitant
formation of iron(III) oxyhydroxide, the release of SO
4
2–
, the dissolution of more Fe
2+
formation of new iron(II) sulfate, etc. [69]. This acid regeneration cycle continues until
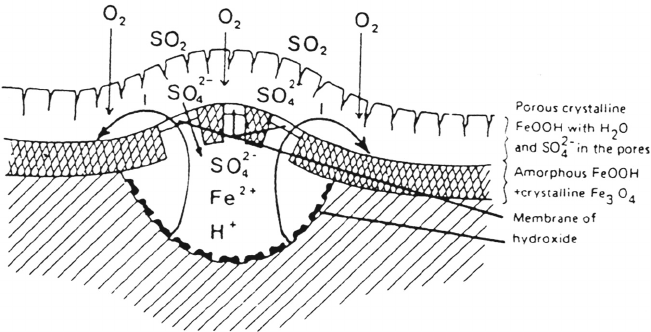
basic iron(III) sulfate is formed as an end product. The complex interaction of
SO
2
and iron is believed to take place in locally distributed sulfate “nests,”
546 Leygraf
Figure 7 Sketch of sulfate “nest” on steel. (From Ref. 6.)
Copyright © 2002 Marcel Dekker, Inc.
which involve the assumed existence of semipermeable membranes of iron(III)
oxyhydroxide maintaining a high activity of species involved at localized parts of the
electrolyte (Fig. 7). Evidence of nitrate- or carbonate-containing corrosion products
is sparse, although coordination of these anions with Fe
3+
is expected according to
the HSAB principle. There is abundant evidence of accelerated atmospheric corrosion
rates for iron caused by chlorides, which may result in the formation of basic
iron(II,III) chlorides and b-FeOOH. Atmospheric corrosion rates for iron are relatively
high and exceed those of other structural metals. They range (in μm/year) from 4 to
65 (rural), from 26 to 104 (marine), from 23 to 71 (urban), and from 26 to 175
(industrial).
In addition to the structural metals already discussed, results have been reported
from studies of the atmospheric corrosion behavior of other metals, many of which
are used as materials in electronic or electric equipment.
The atmospheric corrosion of nickel is similar to that of zinc. Nickel exists
solely as Ni
2+
and instantaneously forms nickel hydroxide and, subsequently,
NiSO
4
· 6H
2
O, has been observed [61b]. After prolonged exposure, an amorphous
basic nickel sulfate is formed, frequently mixed with small amounts of carbonate,
with less protective ability. This phase can crystallize and form another basic nickel
sulfate, with higher stability and protective ability [61a]. No evidence of other anions
in the corrosion products has been found so far. The corrosion rates of nickel are
comparable to those of copper [70].
Silver exhibits a corrosion behavior which hardly resembles that of any of the
other metals described. Its unique behavior is to a large extent governed by the
existence of Ag
+
upon dissolution of silver into the aqueous layer. Ag
2
S is the most
abundant component of the corrosion products formed. AgCl can form in
environments with high chloride content, whereas no oxides, nitrates, sulfates, or
carbonates have been reported in connection with atmospheric exposure. Silver
exhibits corrosion rates comparable to those of aluminum, lower than those of zinc,
and much lower than those of iron.
Tin forms both SnO
2
and SnO as dominating corrosion products in a variety of
environments, resulting in a relatively high protective ability. Laboratory exposures
involving mixtures of SO
2
, NO
2
, and H
2
S suggest that tin is relatively unaffected by
these pollutants [71]. Accordingly, efforts to correlate corrosion effects of tin in
natural atmospheric environments with concentrations of SO
2
and NO
2
have so far
been unsuccessful [72]. Tin exhibits corrosion rates comparable to those of silver [72].
ATMOSPHERIC CORROSION OUTDOORS
Early field exposure programs with selected metals were implemented in the United
States and the United Kingdom in the beginning of the 20th century. The environments
were classified as “rural,” “urban,” “marine,” and “industrial,” and it was soon
recognized that corrosion rates vary considerably between different types of
environment. Over the decades a large number of outdoor corrosion test programs
have been implemented with the general aim of identifying and, possibly, quantifying
the most important environmental parameters involved in atmospheric
corrosion processes. The large body of data available has provided evidence that
corrosion rates under outdoor exposure conditions are strongly influenced by SO
2
Atmospheric Corrosion 547
Copyright © 2002 Marcel Dekker, Inc.
and Cl
–
as well as by climatic factors (humidity and temperature). Despite this, the
general goal of predicting the performance of a given metal in a given environment
is far from being attained [73]. The reason is that many other factors influence
corrosion rates: initial exposure conditions, sample mass and orientation, extent of
sheltering, wind velocity, the nature of corrosion products formed, and pollutants that
are not measured. The difficulties in making accurate predictions of corrosivity can
be exemplified by the variations in corrosion rates between steel and zinc in a world-
wide ASTM Site Calibration at 45 outdoor locations [74]. The mass loss between
different sites for both steel and zinc after 2 years of exposure varied two orders of
magnitude or more. Moreover, the ranking of test sites with respect to mass loss of
zinc was considerably different from that of iron. The ratio of steel to zinc mass loss
varied from around 10 to more than 350. Significant variations in mass loss between
different exposure periods were also observed for both metals. Similar experience has
been gained from several other exposure programs [75,76].
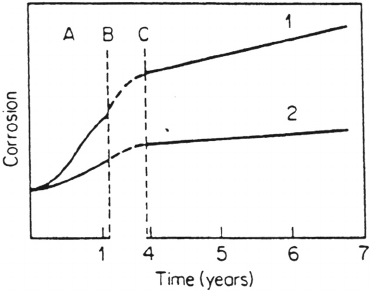
According to Barton [2], a generalized description of corrosion rate versus time
includes an induction period, a period for establishing stationary conditions, and a
stationary period; see Figure 8. These periods can be associated with the present
description of the aqueous layer and formation of corrosion products. During the
induction period, the metal is covered with a spontaneously formed oxide and
the aqueous layer, which affords a certain protection depending on the metal and the
aggressiveness of the atmosphere. The transition period involves the transformation
from the oxide layer into a fully developed layer of corrosion products via local
formation and coalescence of corrosion products. The stationary period, finally, is
characterized by full coverage of the surface by corrosion products, eventually reaching
constant properties with respect to chemical composition and atomic structure, and
stationary corrosion rates. The two initial periods are shorter the more aggressive the
exposure conditions are. For steel they typically last for years in very benign (indoor)
atmospheres but only a few months in highly polluted industrial areas [2].
548 Leygraf
Figure 8 Schematic division of atmospheric corrosion into different periods, namely an
induction period (A), a period for establishing stationary conditions (B to C), and a stationary
period (from C). (From Ref. 2. Courtesy of John Wiley & Sons.)
Copyright © 2002 Marcel Dekker, Inc.
Atmospheric Corrosion 549
A frequent observation during the two initial periods is the marked influence of
initial exposure conditions on the subsequent corrosion rate [77,78]. Wet
conditions—caused by rainfall or high relative humidity—are more severe than dry
conditions during the first days of exposure; they result in higher corrosion rates
when the exposure time extends to several months. In this respect zinc seems to be
more sensitive than steel. These observations are, at least partly, explained by the
formation of corrosion products possessing different protective abilities. Especially
on zinc, a diversity of structurally related corrosion products can be formed, the
nature of which depends on initial exposure conditions [66]. An additional cause may
be the seasonal dependence of the concentrations of H
2
O
2
and O
3
[62].
During the third period of exposure, characterized by stationary corrosion rates,
the interpretation of corrosion rates in terms of environmental data is more easily
accomplished than during the two initial periods with varying corrosion rates. In
reality, atmospheric corrosion is only quasi-stationary and regarded as a series of
processes, when the surface is wet enough to allow rapid corrosion rates, and
interrupted by periods of dryer conditions with negligible corrosion rates. An
important concept here is the so-called time of wetness based on devices that
monitor the actual time during which the surface is wet or the critical relative
humidity exceeded [79]. In many cases, time of wetness estimates have been based
on measurements of temperature and relative humidity. A common definition of time
of wetness is the time during which, simultaneously, the temperature is above 0°C
and the relative humidity ≥80%. However, this definition should not be taken too
literally because the actual time when the surface is wet enough for rapid corrosion
to occur depends on many other parameters, such as type, mass, orientation, and
degree of sheltering of the metal; hygroscopic properties of corrosion products
and of surface contaminants; type and level of pollutants; air velocity; and solar
flux. An alternative way to estimate the time of wetness is by means of electrochemical
cells (Fig. 9), which consist of thin electrically separated metal electrodes and involve
the detection of a current or an electromotive force when the thickness of the aqueous
layer is sufficient to accelerate atmospheric corrosion [79–82]. In this case, too, the
result will depend on many parameters, such as cell design, formation of
corrosion products on metal electrodes, and criteria for defining time of wetness
based on current or electromotive force responses.
Figure 9 An electrochemical cell for determining time of wetness. (A) Material containing
the embedded electrodes. (B) Noble metal electrodes (e.g., Pt or Cu). (C) Base metal
electrodes (e.g., Fe or Zn). (From Ref. 2. Courtesy of John Wiley & Sons.)
Copyright © 2002 Marcel Dekker, Inc.
550 Leygraf
It is also possible to measure quantitatively the amount of water on metal
surfaces under in situ conditions by means of the quartz crystal microbalance [83].
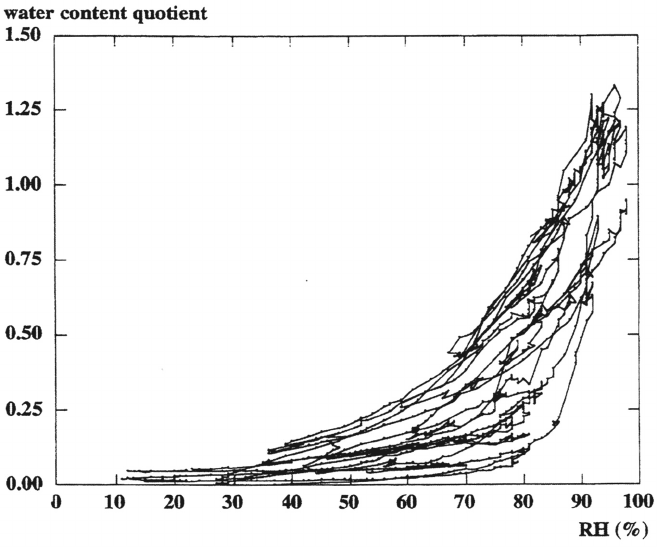
This is illustrated in Figure 10, where the water content quotient on a gold surface,
given as the mass of water in the aqueous adlayer divided by the mass of other
deposited species on gold, is plotted as a function of relative humidity during
consecutive 24-h cycles in an outdoor environment. Each cycle is represented by one
increase (during nighttime) and one decrease (during daytime) in water mass and
relative humidity, respectively. From the figure it is evident that significant amounts
of water are present at relative humidity far below 80%. Analysis of the data shows
that the actual time when the surface is wetted is significantly longer than the time
when the temperature is above 0°C and the relative humidity ≥ 80%. Hence, this
definition of time of wetness may be erroneous [83]. Despite these uncertainties in
definition and experimental accuracy, the time of wetness exhibits a surprisingly
good correlation with outdoor corrosion rates of steel in many types of environ-
ment [84].
Because of the many parameters capable of influencing atmospheric corrosion
rates, exposure programs have placed increased emphasis on standardized test
procedures. The International Organization for Standardization (ISO) has
formulated several corrosion testing standards [85–88] and also implemented a
Figure 10 The water content quotient on gold, given as the mass of water in the aqueous
adlayer divided by the mass of other deposits, as a function of relative humidity (RH) during
14 consecutive days of outdoor exposure in an urban environment. (From Ref. 83. Courtesy
of The Electrochemical Society.)
Copyright © 2002 Marcel Dekker, Inc.
worldwide atmospheric exposure program known as ISOCORRAG [89]. This
program aims at generating a basis for the procedures used in the ISO classification
system. The ISO classification system presents two different approaches to assessing
the corrosivity of any environment. One approach is based on the exposure of
standard specimens of aluminum, steel, copper, and zinc for 1 year and determining
a measured corrosivity class from mass loss data. The other approach is based on SO
2
concentration, deposition of Cl
–
, and time of wetness at the site, which results in an
estimated corrosivity class.Having determined a corrosivity class with one of these
methods, it is possible to estimate the extent of corrosion damage to aluminum, steel,
copper, and zinc for either short-term or long-term exposures. The ISO classification
system provides adequate data for many practical purposes, including prediction of
long-term corrosion behavior in different environments and the need for protective
coatings. However, experience has shown that certain observations need further
clarification. These observations include the frequent difference in corrosion rate
between the top side and bottom side of flat standard specimens and between
flat standard specimens and open helix standard specimens [90]. They also include
the possible role of pollutants other than those measured. Future work within
ISOCORRAG and other exposure programs should shed more light on these and
other not yet fully elucidated issues.
With the increasing concern about acidifying pollutants and their influence on
atmospheric corrosion rates, scientists’interest has been focused on NO
2
as an
additional gaseous corrosion stimulant. Whereas the SO
2
concentration has shown a
significant decline over the past decades in many urban and industrial areas,
estimated emission of NO
2
has shown a continuous increase in the same type of
environment [91]. Studies in laboratories using synthetic air have provided
unambiguous evidence of increased corrosion rates when NO
2
is added to air
containing SO
2
(Fig. 11).
The synergistic effects of SO
2
and NO
2
interactions are based on observations
of several metals: copper [92], nickel [93], steel [94], and zinc [95]. With the general
aim of performing a quantitative evaluation of the effect of sulfur pollutants in
combination with NO
x
, other pollutants, and climatic parameters, an international
exposure program within the UN Economic Commission for Europe (UN/ECE) was
implemented in 1987, including 39 test sites in 12 European countries, the United
States, and Canada [96]. The program is based on exposure of structural metals, stone
materials, paint coatings, and electric contact materials at test sites where measure-
ments of environmental parameters are already in progress. Judging from analysis of
results after 8 years of exposure, the influence of SO
2
on the corrosion rate of, e.g.,
carbon steel, weathering steel, zinc, cast bronze, and nickel is significant. No influence
of NO
2
has so far been observed on any of the materials investigated [97]. Hence, the
important role of NO
2
in laboratory exposures is not visible in the present field
exposures—most likeky as a result of catalysts or oxidizers (e.g., O
3
) in field
exposures that aid in promoting the oxidation of S(IV) to S(VI) and hide the effect of
NO
2
. Future work should explore more precisely the reason for this discrepancy.
The conclusions from the UN/ECE program can be compared with those from
another exposure program performed at three test sites in Southern California and
characterized by very low levels (< l0 ppb) of SO
2
. Under these conditions, SO
2
is
Atmospheric Corrosion 551
Copyright © 2002 Marcel Dekker, Inc.
shown to be of little consequence, whereas NO
2
, which is photochemically
converted to HNO
3
, is of significant importance [98].
There is ample evidence that synergistic effects caused by interacting
atmospheric constituents are significant in laboratory exposures. It remains to be
proved to what extent they contribute during field exposures. In addition to SO
2
and
NO
2
, the interaction between SO
2
and O
3
[95] and between H
2
S and Cl
2
[99] is
expected to be important.
ATMOSPHERIC CORROSION INDOORS
Whereas the accumulated knowledge of outdoor exposures has extended over many
decades of corrosion tests, the history of indoor corrosion tests is short and mainly
goes back to the growing interest in corrosion effects on electronic materials during
the last two decades or so [100,101]. There are obvious differences in outdoor and
indoor exposure conditions and, consequently, expected differences between outdoor
and indoor corrosion behavior, some of which are summarized here.
The aqueous layer formed under outdoor exposure conditions is strongly
influenced by seasonal and diurnal variations in humidity and by precipitation, dew,
snow, or fog. Indoors, on the other hand, the aqueous layer is often governed by
relatively constant humidity conditions. This means that there are practically no
wet-dry cycles indoors and that the influence of indoor humidity can hardly be
described by introducing a time of wetness factor.
552 Leygraf
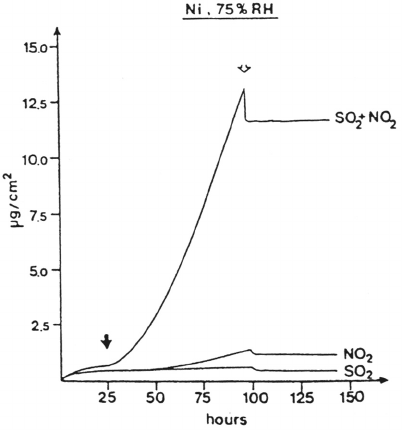
Figure 11 Mass changes of nickel as a function of exposure time in humid (75% relative
humidity) flowing air. The filled arrow indicates introduction of 250 ppb SO
2
and/or 300 ppb
NO
2
, and the open arrow indicates termination of the supply of gaseous pollutants and of high
humidity in the flowing air. (From Ref. 93. Courtesy of The Electrochemical Society.)
Copyright © 2002 Marcel Dekker, Inc.
