Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

chamber is incorporated as part of the siloxane network. There is a significant amount
of dimethylsilyl groups in the polymer, which can be formed only by cleavage of the
Si—C bond. Thus, it can be concluded that the polymerization mechanism
is dominated mainly by the formation and recombination of silyl radicals. The
abstraction of methyl groups is confirmed by the atomic concentrations measured by
XPS. The measured concentrations correspond to Si
2
C
4.1
O
0.9
H
x
compared with
Si
2
C
6
H
18
in the case of the monomer [117]. The oxygen in the polymer is almost
exclusively bound to silicon, as measured by IR spectroscopy and XPS.
Morphology of Ultrathin Plasma Polymer Films
Despite the fact that plasma polymerization leads to rigid and laterally homogeneous
films, a varying number of pinholes is observed in most cases [118]. Pinholes are
crucial defects when the plasma polymer serves as a coating for reactive metals.
In this case, electrolyte can penetrate through these channels to the uncovered
metal surface and give rise to localized forms of corrosion. Not surprisingly,
the ultrathin plasma polymers contain several pinholes or intrinsic channels that
can be located by immersion of the coated sample in a copper sulfate solution
512 Rohwerder et al.
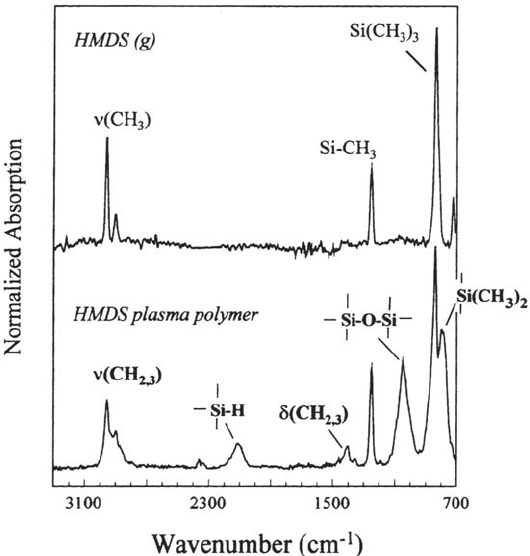
Figure 30 IRRA spectrum of an about 13-nm-thick plasma polymer deposited from a
mixture of argon and hexamethyldisilane (p
Ar
= 25 Pa, p
HMDS
= 10 Pa). For comparison,
the gas-phase spectrum of hexamethyldisilane is also shown. Indicated are the peaks that
are specific for the polymer. All peaks are assigned in Table 3 [115].
Copyright © 2002 Marcel Dekker, Inc.
[c(CuSO
4
) = 10
–3
mol/e] [115]. Copper was deposited in the pinholes while the rest
of the surface was effectively isolated from the electrolyte even by films of a few
nanometers thickness. Van Ooij et al. [104] studied the topology of plasma polymers
deposited on stainless steel substrates from a DC plasma by means of STM. The
topology was dependent on the plasma parameters. Films deposited at low pressure
(6 Pa) were significantly smoother than those deposited at higher pressures (66 Pa).
The lateral homogeneity of ultrathin plasma polymers was confirmed by
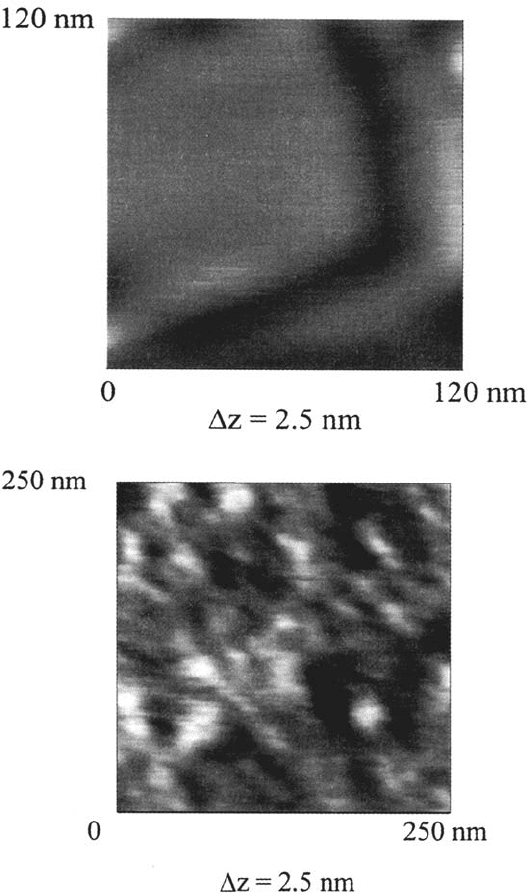
atomic force microscopy measurements on flame-annealed gold substrates [117].
Flame annealed Au was chosen because of its large (about 200 × 200 nm)
monoatomic flat terraces. The measurement was done in the tapping mode to protect
the plasma polymer surface from damage. Figure 31b shows, as an example, the
structure of an about 3-nm-thick hexamethyldisilazane (HMDSZ) plasma polymer.
The film structure can be revealed by a comparison with the bare flame-annealed Au
surface (Fig. 31a). The shallow features of the plasma polymer show an average
diameter of about 20 nm and a height of about 0.5 nm. The structure of the
HMDS-PP resembles that of HMDSZ-PP and is published elsewhere [117]. By
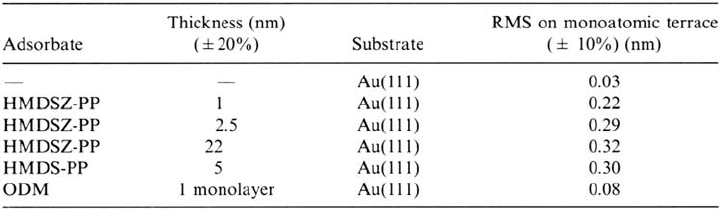
calculating the roughness (RMS value) of the plasma polymers on monoatomic flat
terraces, the microstructure of the plasma polymers can be revealed as a function of
the nature of the monomer and the film thickness (see Table 2). For comparison, a
self-assembling monolayer of octadecylmercaptan (ODM) on flame-annealed
gold was prepared (adsorption from ethanolic solution at open circuit potential,
Δt
ads
= 12 h) and measured in the same way.
The plasma polymer–coated surface shows increased roughness in comparison
with the bare gold surface, whereas the ODM monolayer leads only to a slight
increase in roughness. The lower RMS value of the 1-nm-thick HMDSZ-plasma
polymer in comparison with the thicker films might be due to incomplete coverage
of the measured area so that the bare gold surface contributes to the overall value.
However, it appears that a constant roughness value is measured for a thickness
above 3 nm.
Corrosion Prevention by Adsorbed Monolayers 513
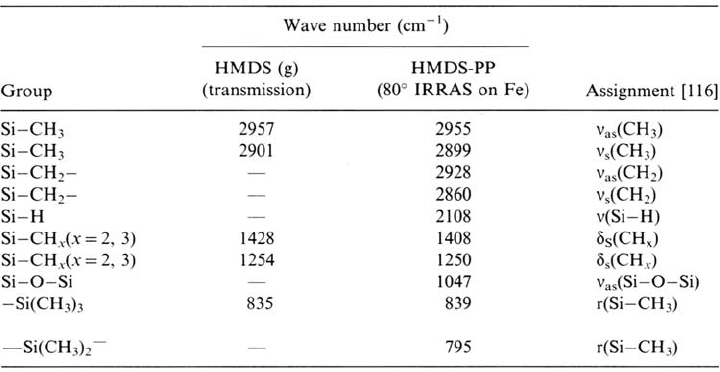
Table 1 Assignment of the IR Peaks of Gaseous HMDS and the HMDS Plasma Polymer
Copyright © 2002 Marcel Dekker, Inc.
514 Rohwerder et al.
Figure 31 (a)AFM picture of a flame-annealed Au surface. The measurement was done
in the tapping mode. The brightness increases with the height. (b) AFM picture of a
flame-annealed Au surface coated with an about 3-nm-thick hexamethyldisilazane
[(CH
3
)
3
Si—NH—Si(CH
3
)
3
, HMDSZ] plasma polymer that was deposited at p
HMDSZ
= 5 Pa.
The brightness increases with the height [105].
Copyright © 2002 Marcel Dekker, Inc.
Interaction between the Polymerizing Plasma and the Oxide Surface
By using an in situ FTIR cell, Grundmeier and Stratmann [117] could reveal
interactions at the iron oxide/plasma polymer interface. Prior to the plasma polymer
deposition, the iron surface was plasma oxidized. The in situ IRRA spectrum of the
plasma-oxidized surface now served as reference spectrum for the subsequent
measurements. A very short (1–2s) plasma polymer deposition was then performed
and the in situ IRRAspectra were measured again. This procedure was repeated twice
on the same sample, resulting in three layers. The first absorption spectrum was
subtracted from the second one and the second from the third. This procedure leads
to three spectra that represent the structure of each of the three plasma polymer
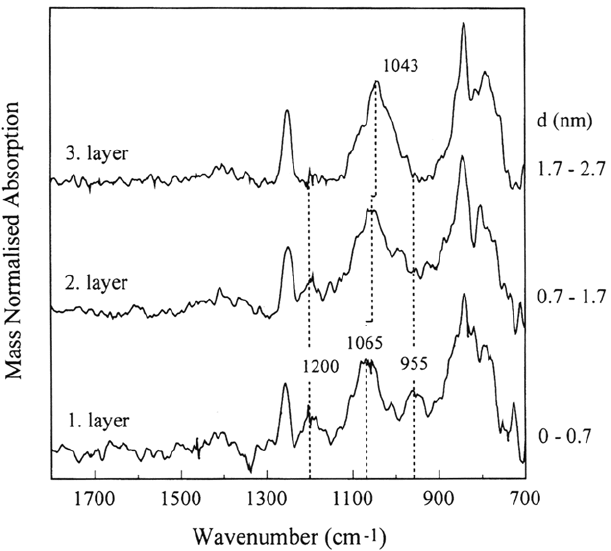
layers (see Fig. 32). All spectra were normalized by the thickness of the respective
layer, d, as measured by the QCM. The IRRA spectrum of the first layer (thickness
~ 0.7 nm) shows two peaks at 1200 and 955 cm
–1
, which are not found in the spec-
trum of the third layer. The spectrum of the second layer (thickness ~ 1nm) contains
the same two peaks but the intensities are much lower than in the first layer. In addition,
the Si—O—Si peak occurs at 1065 cm
–1
in the first layer and shifts to 1056 cm
–1
in
the second and to 1042 cm
–1
in the third layer. The higher frequency of the Si—O—
Si peak at the interface might be a consequence of the change in the angle of the
siloxane group induced by polar interactions between the oxygen of the polymer and
hydrogen on the oxide surface. Any change in the bonding angle means that a
higher energy of the system leads to an increase in the vibration frequency of the
Si—O—Si asymmetric stretching, as observed for an SiO
2
layer on silicon [119].
A comparison of the thickness of the layers with the results concerning the
microstructure of the films leads to the conclusion that almost complete coverage
of the surface by the plasma polymer is reached when the film thickness is about
2 nm. Thus, the special peaks in the first and second layers might be assigned to
the interaction of the deposited layer with the oxidized iron surface. The third
layer is deposited onto a surface that is almost completely covered by the
HMDS-plasma polymer film and therefore cannot interact with the oxide.
According to the literature, the peak at about 955cm
–1
can be assigned to a
metal-oxygen-silicon bond and the peak at 1200 cm
–1
is most probably due to a
Si—O—CH
3
or a metal—O—CH
3
group [116]. Silanol groups would also lead
to absorption in this wavelength region. The IR peak of silanols, however, was
observed at 920 cm
–1
for the chemisorption of polysiloxanes on iron using the
Corrosion Prevention by Adsorbed Monolayers 515
Table 2 Calculated RMS Values for Different Plasma Polymer Layers and a Self-
Assembled octadecylmercaptan (ODM) Film of Flame-Annealed Gold [105]
Copyright © 2002 Marcel Dekker, Inc.
same reflection assembly [120]. This value is about 35 cm
–1
lower in wave number
than the peak observed in Figure 32. Van Ooij et al. [104] studied the influence of
the substrate on the plasma polymer formation using stainless steel as substrate.
Films were deposited from a DC plasma. The authors observed especially for films
deposited at high pressures a dependence of the film structure on the film thickness
and thus proposed a two-stage mechanism. While the film adjacent to the substrate
resembles polydimethylsiloxane, it becomes more random for higher film thickness.
Applications of Plasma Polymers as Corrosion-Resistant Layers
on Reactive Metals
Delamination of Ultrathin Plasma Polymers
Plasma polymers may be applied without subsequent painting. In this case, corrosion
may start in local defects in the plasma polymer and spreading of these defects is a
critical degradation process. Moreover, plasma polymers may be used as model
ultrathin polymers coatings. Grundmeier et al. [121] studied the delamination of
ultrathin plasma polymers by using a scanning Kelvin probe and surface analytical
methods.
516 Rohwerder et al.
Figure 32 In situ IRRA difference spectra revealing the structure of the subsequently
deposited three layers of HMDS plasma polymer on plasma-oxidized iron. The polymer
was deposited at a partial pressure of p
HMDS
= 5Pa. The value d means the thickness of
each deposited plasma polymer layer [117].
Copyright © 2002 Marcel Dekker, Inc.
The delamination rate was determined with a scanning Kelvin probe. A small
amount of fine sodium chloride was introduced into a circular deepening in the
middle of the polished and ethanol-cleaned iron sample. After the sample was
introduced into the plasma reactor, it was cleaned and activated in one step by an
oxygen plasma, leading to a carbon-free and highly oxidized iron surface. In the
next step an ultrathin plasma polymer of hexamethyldisilane was deposited on the
cleaned substrate, leading to a well-defined metal-polymer interface. The thickness
of the deposited polymer was controlled by the in situ measurement of the resonance
frequency of the quartz crystal and was about 5 nm, so that the film thickness is
in the range of the escape depth of the photoelectrons.
This special kind of defect preparation has the advantage that water
condenses slowly after the insertion of the sample into the scanning Kelvin probe
at 93% relative humidity. The growing droplet is fixed in the deepening due to the
hydrophobic nature of the plasma film (water contact angle 92 ± 2°). Thus, the
electrolyte does not spread over the delamination zone. Because a saturated
sodium chloride solution is formed within the deepening, no drying of the defect
is observed. A Cr/Ni wire was used as the vibrating reference electrode and
provided a local resolution of about 100 μm. X-ray photoelectron spectra were
obtained using a Physical Electronics ESCA 5600 XP spectrometer.
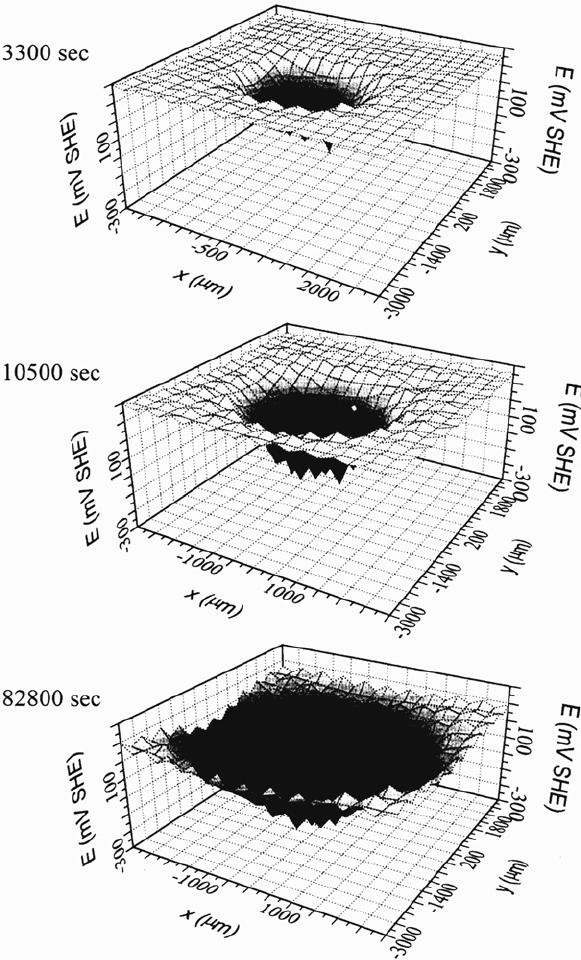
In Figure 33 the potential distribution over the circular defect and the
surrounding intact plasma polymer–coated area as measured with the scanning
Kelvin probe is shown with increasing corrosion time. Obviously, negative
potentials are measured within the defect and very positive potentials are observed
in the intact region. With increasing corrosion time the delamination front marked
by the sharp decrease of the corrosion potential shifts into the former intact area
and reveals that undermining occurs. Line scans shown in Figure 34 reveal the
activation of the defect and the progress of the delamination front.
An interesting question refers to the comparability of the delamination of
ultrathin polymers to that of macroscopic thick coatings. Therefore the sample was
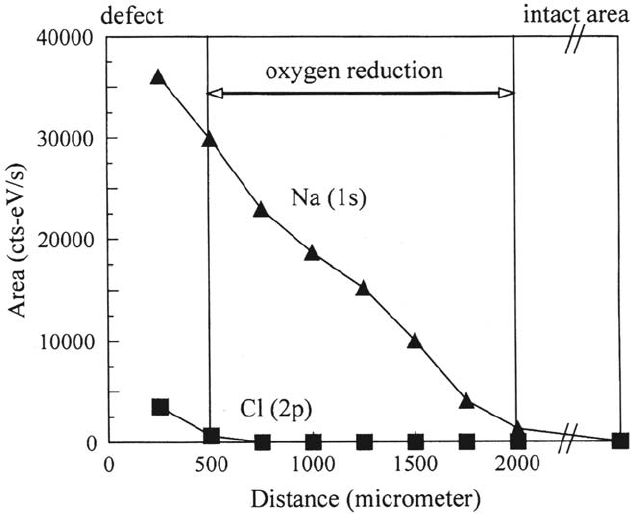
inserted into the XP spectrometer and the delaminated area was analyzed locally
with high-energy resolution (0.6 eV). Figure 35 presents the line scans of the Na 1s
and the Cl 2p peaks starting at the border of the defect and moving toward the intact
area. The local resolution was 400 μm and the total length of the line scan was 2000 μm.
The line scan reveals that no chloride is found in the delaminated area and that the
concentration of sodium ions decreases toward the delamination frontier. The final
length of the undermined region measured by the scanning Kelvin probe and the
distribution of sodium ions as measured by means of electron spectroscopy for
chemical analysis (ESCA) are in good accordance.
It can be concluded from the former results that the delamination of the
ultrathin polymers behaves similarly to that of the model coating. It is now an
advantage of the very small thickness that the metal-polymer interface can be
characterized by XP spectroscopy without the separation between polymer and metal
substrate, which otherwise leads to significant distortions caused by organic
contamination, water absorption, and oxidation at ambient atmosphere. It was found
that the ultrathin solid films are oxidized and transformed to a gel-like structure due
to the osmotic swelling of the film in the delaminated region [121].
Corrosion Prevention by Adsorbed Monolayers 517
Copyright © 2002 Marcel Dekker, Inc.
518 Rohwerder et al.
Figure 33 Potential distribution over the circular defect and the surrounding intact
plasma polymer–coated area as measured with the scanning Kelvin probe is shown with
increasing corrosion time [121].
Copyright © 2002 Marcel Dekker, Inc.
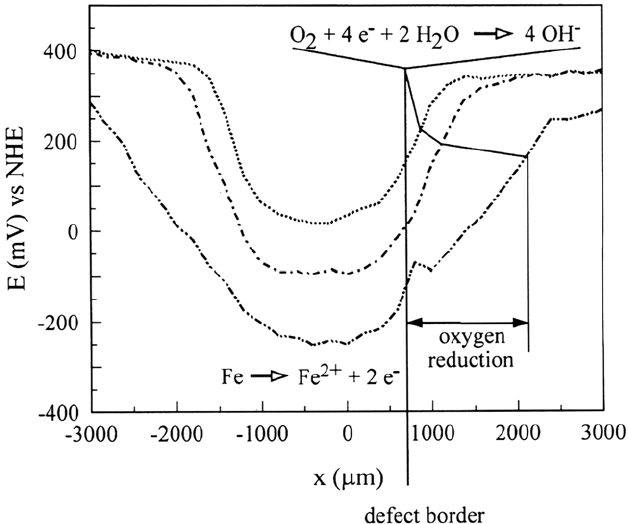
Corrosion Prevention by Adsorbed Monolayers 519
Figure 34 Scanning Kelvin probe line scans shown across the circular defect in Figure 33,
revealing the activation of the defect and the progress of the delamination front [121].
Influence of Ultrathin Plasma Polymers on Delamination Kinetics
of Polymer-Coated Steel and Galvanized Steel
As stated before, an ultrathin plasma polymer usually requires a stabilizing top coat.
Iron substrates were plasma modified by an oxygen plasma treatment and by the
deposition of an ultrathin plasma polymer as described before. Grundmeier and
Stratmann [115] investigated the influence of an interfacial plasma polymer on the
delamination kinetics of a clear coat on iron. The sample was coated with a
water-based, one-component, polyurethane acrylate primer. An artificial defect with
a reservoir for the electrolyte (0.5 M NaCl) and a linear defect border was prepared
within the coated substrate. To reveal the influence of the plasma deposition on the
delamination kinetics the sample was prepared with one half of the substrate not
modified and the other half plasma modified. Masking of the unmodified region was
achieved using a thin aluminum foil placed on the desired area. This technique
guarantees that both areas have the same properties before the plasma treatment and
are coated with the primer in the same way. Any observed differences should,
therefore, be due only to the plasma deposition.
Figure 36 shows a 3D plot of a scanning Kelvin probe measurement of an iron
sample that was coated with a 5-nm-thick HMDS plasma polymer on one half of the
sample surface prior to the deposition of the primer [115]. The water contact angle
Copyright © 2002 Marcel Dekker, Inc.
on the HMDS plasma polymer was 92 ± 2°. This means that the surface energy
decreased with respect to a plasma-oxidized surface, which shows a water contact
angle of less than 10°. Nevertheless, wetting of the plasma polymer surface by the
primer was sufficient to lead to homogeneous spreading and adhesion of the primer.
The area modified by the plasma polymer shows little undermining even after
1265 min, whereas the area coated only by the primer shows fast delamination.
A comparison of the delamination kinetics is shown in Figure 37. The progress of the
steepest slope of the potential profile is plotted versus the time after the activation of
the defect. Comparison of the corresponding kinetics leads to the conclusion that the
ultrathin plasma polymer gives rise to a significant decrease in the delamination rate.
Plasma polymers with a special surface structure suitable to bond to an epoxy
amine primer were used as interfacial coupling layers on iron and galvanized steel
and led to even better results [122]. However, in all cases the system always
showed cathodic delamination at the polymer/metal oxide interface, indicating
the importance of oxygen reduction on the oxide surface. For verification, after the
delamination of the sample, the delaminated polymer was pulled off and the
underside of the polymer and the iron surface were investigated by XPS to reveal
whether the system delaminated at the plasma polymer/metal interface or at the
520 Rohwerder et al.
Figure 35 Small spot ESCA line scans of the Na
1s
and the Cl
2p
peaks starting at the
border of the defect (see Figs. 33 and 34) and moving toward the intact area (spatial
resolution 400 μm, total length of the line scan 2000 μm) [121].
Copyright © 2002 Marcel Dekker, Inc.
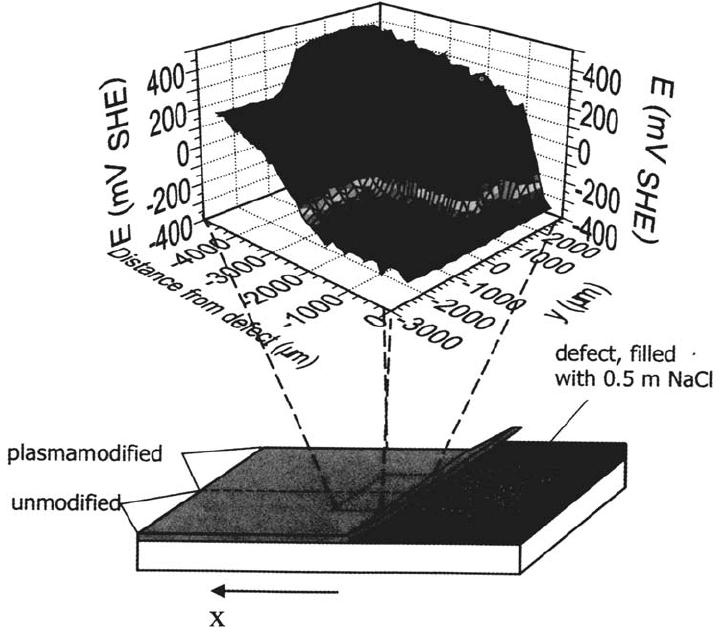
Corrosion Prevention by Adsorbed Monolayers 521
Figure 36 Potential profile of a delaminated primer-coated iron specimen that was half
side plasma polymerized and afterward coated by a 100-μm-thick primer (x = 0: defect
border, y < 0: not plasma modified, y > 0: plasma plasma polymerized). The thickness of
the HMDS plasma polymer was 5 nm [105].
Copyright © 2002 Marcel Dekker, Inc.
