Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

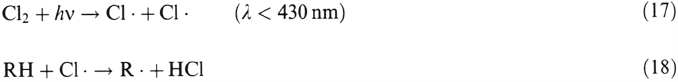
Several of the reactions described have been identified in so-called photochemical
smog formation [16]. This complex set of reactions involves an initial mixture of
nonmethane organic compounds, NO, and NO
2
, which is photochemically
transformed into a final mixture including HNO
3
and O
3
, aldehydes, and
peroxyacetylnitrate.
Principal sources of emission of NH
3
are animal shelters, fertilizer production,
and cleaning detergents. In the aqueous phase, NH
3
establishes equilibrium with
NH
4
+
, which results in increased pH. An important role of NH
3
in atmospheric
corrosion chemistry is to partly neutralize acidifying pollutants by forming particulate
(NH
4
)
2
SO
4
and acid ammonium sulfates, such as NH
4
HSO
4
and(NH
4
)
3
H(SO
4
)
2
. By
increasing the pH of the aqueous phase, NH
3
also increases the oxidation rate of
S(IV) to S(VI), as discussed earlier.
Chlorine-Containing Compounds
Chlorides participate in atmospheric corrosion reactions mainly as aerosols through
transport from marine atmospheres. Other important sources are road deicers and
dust binders on roads, coal burning, municipal incinerators, and fingerprints.
Burning of high-chlorine coals may also result in emission of HCl, which is highly
soluble in water and strongly acidifies the aqueous phase. Cl
2
is emitted from
industrial processes, such as bleaching plants in pulp and paper industries and certain
metal production industries, and from cleaning detergents. Cl
2
can also photo-
dissociate into chlorine radicals, which react with organic compounds (RH) and
form HC1 according to:
Atmospheric Corrosion533
Other Atmospheric Compounds
In addition to the gaseous species already commented on, Table 1 includes HCHO
and HCOOH, which are important indoor corrosion stimulants (as discussed later)
and which can originate from adhesives, tobacco smoke, combustion of biomass, and
plastics, for example. A comparison between typical outdoor and indoor concentrations
of the most important gaseous corrosion stimulants (Table 1) reveals, in general, lower
levels indoors than outdoors. This is mostly due to enhanced indoor absorption of gases
and particulates and also to the retardation and damping of outdoor variations by
ventilating systems and air filtration. Exceptions are NH
3
and the organic species,
which, as a rule, show higher levels indoors than outdoors as a result of anthropogenic
activity.
Of utmost importance in atmospheric corrosion is the presence of particles and
aerosols (an ensemble of particles suspended in the air) of mostly chlorides, sulfates,
and nitrates. The size, shape, and chemical and physical properties of these particles
and aerosols can vary widely. A more detailed description of particles and their role
in (indoor) atmospheric corrosion is given elsewhere [20].
Copyright © 2002 Marcel Dekker, Inc.
THE AQUEOUS LAYER
Formation of the Aqueous Layer
The reaction of water vapor with a metal surface is of paramount importance to
atmospheric corrosion. A large number of studies of initial water–metal interaction
have been made on well-defined single-crystal surfaces of pure or oxidized metals
(for a review, see Ref. 21). Water may bond in molecular form to most clean and
well-characterized metal surfaces. Through the oxygen atom it bonds to the metal
surface or to metal clusters and acts as a Lewis base, as bonding is connected with
a net charge transfer from the water molecule to the surface. Simple models of
preferred adsorption sites are based on Lewis acid-base chemistry, in which water
adsorbs on electron-deficient adsorption sites [22]. Water may also bond in dissociated
form, in which case the driving force is the formation of metal-oxygen or metal-
hydroxyl bonds. The end products resulting from water adsorption are then adsorbed
hydroxyl, atomic oxygen, and atomic hydrogen [21]. On metal oxides, water may
also adsorb in either molecular or dissociative form. The tendency to dissociate seems
to be facilitated by lattice defect sites, as observed, for instance, on monocrystalline
TiO
2
[23], NiO [24], and a-Fe
2
O
3
[25]. The monomolecular thick film of surface
hydroxyl groups formed from dissociation of water is relatively protective and
reduces the subsequent reaction rate of water [26]. The first monolayer of water
adsorbed to the hydroxylated oxide substrate appears to be highly immobile, whereas
the second and third monolayers are more randomly oriented and less immobile
[27]. The water layer adjacent to the gas phase appears to have an icelike structure
[28]. The adsorption characteristics of water are strikingly similar for many different
metals, and the exact nature of the metal oxyhydroxide seems to have only a minor
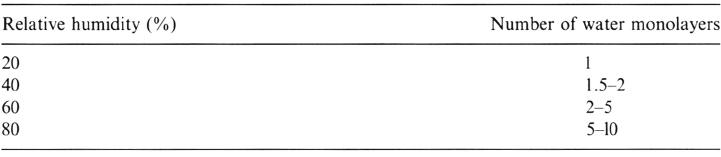
influence on water adsorption phenomena. The quantity of reversibly adsorbed
water increases with the relative humidity and with time. Table 2 presents the
approximate number of water monolayers at 25°C and steady-state conditions,
which have been experimentally determined by the quartz crystal microbalance
method on a variety of metals [28,29].
The bond strength between water and the hydroxylated metal surface is similar to
the bond strength between neighboring, hydrogen-bonded, water molecules [21]. From
this follows the possibility of water clusters on relatively homogeneous surfaces, which
is further promoted on less well-defined surfaces with highly reactive sites, such as kinks
or steps, thereby increasing the probability of anode-cathode area formation.
The aqueous phase formed acts as a solvent for gaseous constituents of the
atmosphere. Preferred sites for corrosion attack may be related to sites where water
534 Leygraf
Table 2 Approximate Number of Water Monolayers on Different Metals Versus Relative
Humidity
Copyright © 2002 Marcel Dekker, Inc.
adsorption is favored and gaseous molecules, such as SO
2
and NO
2
, are easily
dissolved. At aqueous films thicker than about three monolayers, the properties
approach those of bulk water [30]. The relative humidity when this occurs is close
to the “critical relative humidity” [31,32], above which atmospheric corrosion rates
increase substantially and below which atmospheric corrosion is insignificant. The
critical relative humidity for different metals in the presence of SO
2
has been
reported to be between 50 and 90% [28].
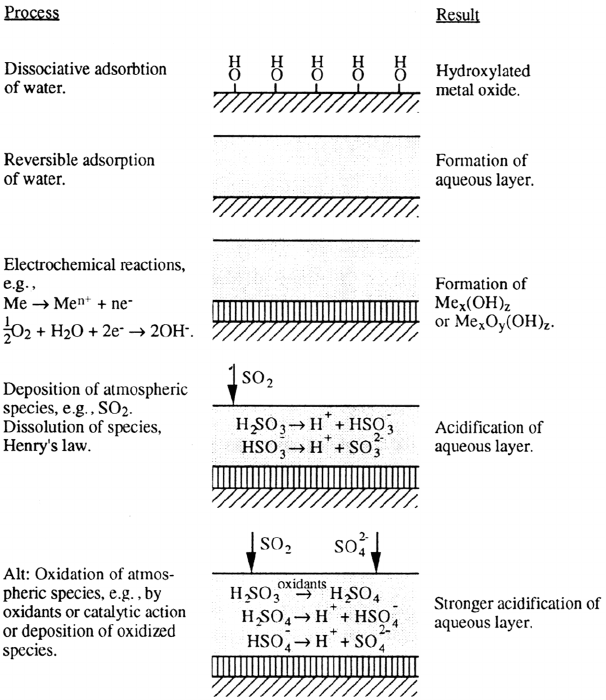
Electrochemical Reactions
The aqueous phase also acts as a conductive medium for electrochemical reactions.
Although atmospheric corrosion is largely dependent on electrochemical reactions,
relatively few electrochemical studies have been focused on the elucidation of
these basic mechanisms. The reason seems to be obvious if one considers the dif-
ficulties in reproducing a thin aqueous film in an electrochemical experiment. The
thickness of such a film may vary with different outdoor exposure conditions,
involving the complex chemistry and photochemistry produced by atmospheric
pollutants together with solar light and resulting in the precipitation of represen-
tative corrosion products and changed transport properties. Nevertheless, systematic
electrochemical experiments have been conducted with special emphasis on
atmospheric corrosion (see, e.g., Refs. 5,33–37). In the absence of pollutants, the
most common anodic and cathodic processes in corrosion of metals exposed to a thin,
neutral, aqueous phase are:
Atmospheric Corrosion 535
Under most atmospheric corrosion conditions, the anode reaction rather than the
cathode reaction is observed to be the rate-limiting step [2]. Upon evaporation of
the aqueous layer, a film of corrosion products—consisting of metal hydroxides or
metal oxyhydroxides—may precipitate. With repeated condensation–evaporation
cycles, this film usually hinders the transport of ions through the corrosion product
or the transport of Me
n+
from the anodic site. Hence, the anodic reaction rate is
lowered and, thereby, the atmospheric corrosion rate.
Acidifying Pollutants
In the presence of atmospheric acidifying pollutants, such as SO
2
, the anode reaction
is facilitated and, consequently, the total corrosion rate as well. Upon deposition of
SO
2
, interaction with the aqueous phase proceeds with the following reactions:
Despite numerous studies of the important influence and role of SO
2
, there is as yet
no complete description of the interaction, on a molecular level, of SO
2
with an
Copyright © 2002 Marcel Dekker, Inc.

oxidized metal substrate in the presence of an aqueous phase. Recent in situ studies
by means of infrared reflection-absorption spectroscopy have provided evidence
that HSO
–
3
is coordinated with the hydroxylated metal oxide surface through a fast
ligand exchange mechanism, in which the surface OH
–
group is replaced by SO
3
2–
[38]; see Figure 1. Surface-bound H
+
released according to, e.g., reactions (8) and
(21), may easily move from one functional group to another or from one surface site
to another. In accordance with earlier proposed mechanisms for mineral dissolution
in aquatic environments [39], the SO
2–
3
-coordinated metal surface center may be
detached from the oxide lattice when surrounded by two or more H
+
-bonded
functional groups. This results in the release of H
+
ions, which can participate in the
detachment of another SO
2–
3
-coordinated metal surface center. The net effects represent
an acid-dependent dissolution rate (R
diss
) of the oxide that has been written as:
536 Leygraf
where [a
H
+
] is the activity of the protons, and C and n are constants [40]. When
further elaborated, Eq. (22) should include the influence of surface coordinated
ligands.
If S(IV) is oxidized to S(VI), this will result in the formation of H
2
SO
4
and the
release of considerably more H
+
than if H
2
SO
3
is involved. It follows that possible
reaction paths for the oxidation of S(IV) to S(VI) are of crucial importance to
atmospheric corrosion rates, and this has also been the subject of many investigations.
Examples of reaction paths have been summarized in the preceding section.
The acid-dependent dissolution rate of the initially formed oxide or oxyhydroxide
requires that all of the most important acidifying atmospheric pollutants be considered.
Besides SO
2
, these pollutants include CO
2
, NO
2
, and carbonyl compounds, such
as HCHO and CH
3
CHO, which can contribute to the total acidity in the aqueous film
by forming H
2
CO
3
, HNO
3
, HCOOH, and CH
3
COOH.
At equilibrium between the water film and the atmosphere, the concentration
of dissolved atmospheric gases in the film is expressed according to Henry’s law:
where
[X] = concentration in aqueous phase
H(X) = Henry’s law constant for species X
p(X) = partial pressure of X in atmosphere
For many gases, reversible ionization reactions occur and the ordinary Henry’s
law constant, H, is replaced by an effective Henry’s law constant, H
e
. In the case of
SO
2
this can be written as:
Less dissociation takes place if the pH is decreased, which also results in a decrease of
the effective Henry’s law constant and a further slowdown of the dissolution of SO
2
in
the aqueous layer [41].
The pH of the aqueous layer can be determined by formulating the general
requirement equation that electrical neutrality must be maintained within the layer.
Copyright © 2002 Marcel Dekker, Inc.
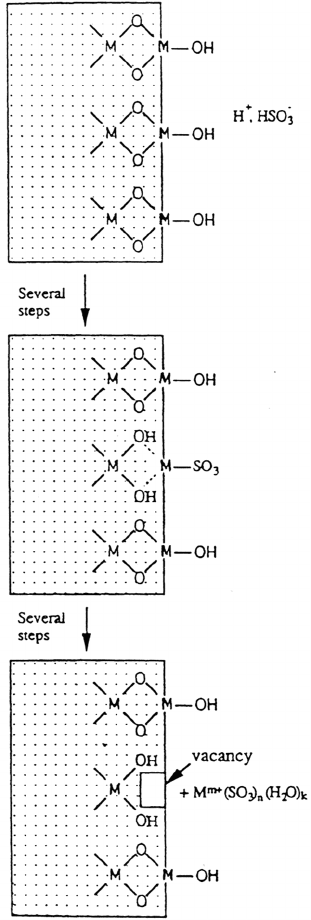
Atmospheric Corrosion 537
Figure 1 Fundamental processes in the initial stages of SO
2
-induced atmospheric
corrosion, including coordination of HSO
3
–
with the hydroxylated metal oxide surface,
replacement of surface OH
–
group by SO
3
2–
and detachment of the SO
3
2–
-coordinated
metal surface center when surrounded by two or more H
+
-bonded functional groups.
Copyright © 2002 Marcel Dekker, Inc.
This means that the sum of products of molar concentration and charge of cationic
species must equal the sum of products of molar concentration and charge of anionic
species. If we consider only the interaction of SO
2
with the aqueous layer, the pH is
determined by
538Leygraf
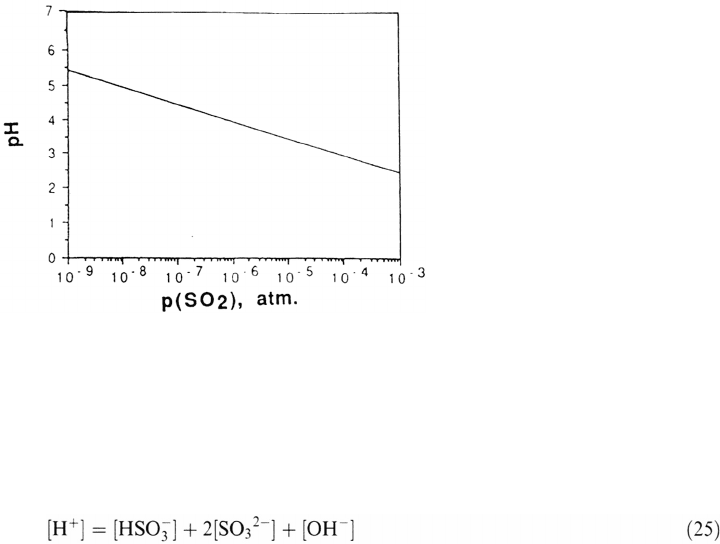
Figure 2Variation of pH of the aqueous layer as a function of p(SO
2
).
The calculated pH of the aqueous layer as a function of p(SO
2
), according to (24)
and (25), is shown in Figure 2. Amore exact estimate of the aqueous layer pH
must involve the contribution of other acidifying pollutants, such as CO
2
, NO
2
,
and HCHO, as well as the properties of the solid surface film, such as its coordination
properties and dissociation product. Acomplicating factor is the high ionic strength of
many species involved, especially if the aqueous film undergoes wetting and drying
cycles. If so, the concentrations of species are replaced by their activities. Henry’s
law constants for different gases are given in Table 3. It should be noted that
Henry’s law applies only if equilibrium is maintained between the gas in the
atmosphere and that in the aqueous phase. Consequently, it does not apply under
nonequilibrium conditions, caused by mass transport restrictions in the atmosphere,
in the aqueous layer, or across the gas–liquid interface.
Deposition of Pollutants
The incorporation of atmospheric species into the aqueous layer may occur
through either dry or wet deposition. In dry deposition there is no involvement of
any precipitation, whereas wet deposition requires, e.g., rain, dew, fog, or snow for
atmospheric pollutants to deposit. Indoors or in highly polluted areas close to
emission sources, dry deposition is considered to be dominating but the relative
importance of wet deposition may be difficult to establish because of the incidental
nature of precipitation. Controlled field studies combined with extensive laboratory
exposures have been undertaken within the National Acid Precipitation Assessment
Program (NAPAP) to explore the relative contribution of wet and dry deposition to
increased corrosion rates of a number of metals [45].
Copyright © 2002 Marcel Dekker, Inc.
Auseful parameter is the dry deposition velocity, which is defined as the ratio
of deposition rate or surface flux per time unit of any gaseous compound and the
concentration of the same compound in the atmosphere [46]. The concept of dry
deposition velocity of SO
2
and its relevance to atmospheric corrosion rates is well
established [47]. By examining data based on both field and laboratory exposures.
it can be concluded that the factors controlling dry deposition fall into aerodynamic
processes and surface processes. Aerodynamic processes are connected with the
actual depletion of the gaseous constituent (e.g., SO
2
) in the atmospheric region
next to the aqueous phase and the ability of the system to mix new SO
2
into this
region. This ability depends on, for instance, the actual wind speed, type of wind
flow, and shape of sample. Surface processes, on the other hand, are connected with
the ability of the aqueous layer to accommodate SO
2
. This ability increases with the
thickness of the aqueous layer and, hence, with the relative humidity, the pH of the
solution (as discussed earlier), and the alkalinity of the solid surface.
The dry deposition velocity (V
d
) can be expressed as the inverse of the sum of
two resistances, namely the aerodynamic resistance (R
a
)and the surface resistance
(R
s
):
Atmospheric Corrosion539
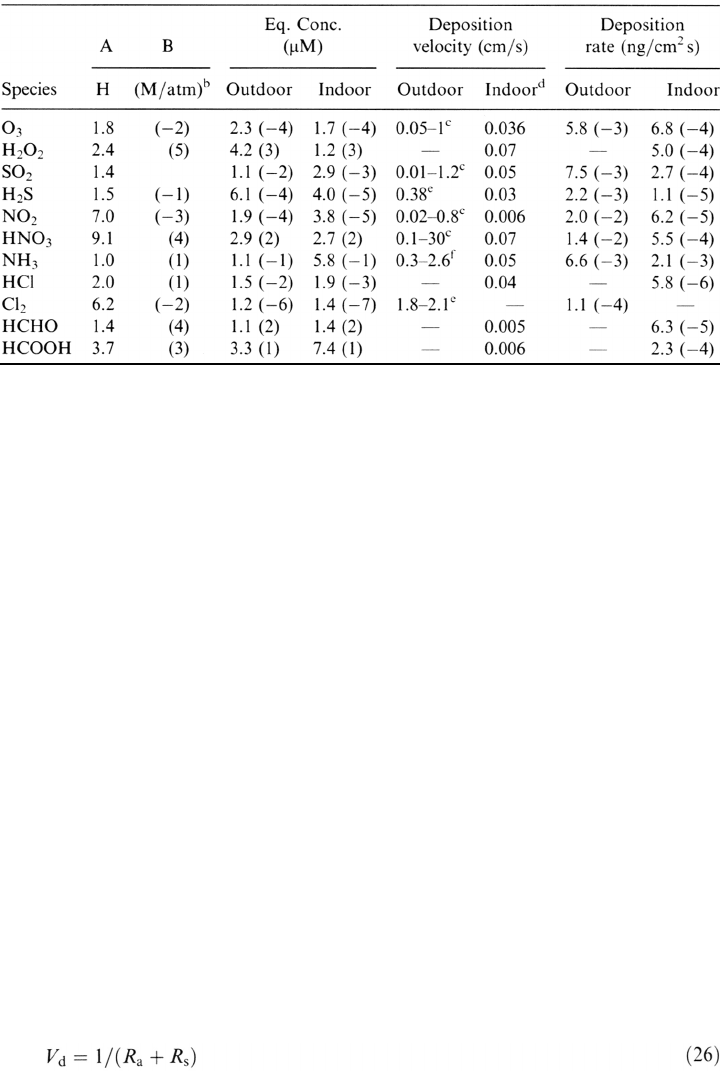
Table 3Characteristics of Selected Gaseous Air Constituents
a
a
The equilibrium solution concentration and deposition rate values were based on concentrations
from Table 1 and using geometric mean values for the intervals. 1.8 (–2) means 1.8 ˘ 10
–2
.
b
Henry’s law constant, from Ref. 19.
c
Ref. 42.
d
Ref. 19.
e
Ref. 43.
f
Ref. 44.
Under most exposure conditions the dry deposition velocity will be the combined
effect of both resistances [46]. At well-stirred and highly turbulent airflow conditions,
however, R
d
» 0 and the dry deposition velocity solely depends on surface
Copyright © 2002 Marcel Dekker, Inc.
processes. Ideal absorbers of SO
2
are alkaline surfaces such as lead peroxide or
triethanolamine, for which R
s
» 0. The dry deposition velocity in this case is
determined mainly by aerodynamic processes. Typical ranges for dry deposition
velocities onto various materials under outdoor and indoor conditions are summarized
in Table 3.
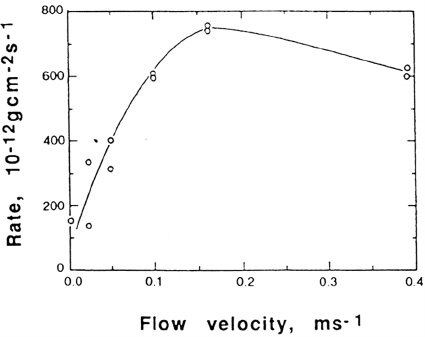
Acombined experimental and theoretical approach to mass transport limitations
in atmospheric corrosion has been performed by Volpe [48,49] to obtain dry
deposition velocities and probabilities of H
2
S, NO
2
, and O
3
reacting with Ag. This
approach clearly shows the effect of airflow velocity on the corrosion rate and the
significance of hydrodynamic and concentration boundary layers next to the metal
surface, characterized by deviating tangential airflow velocity and concentration
of air constituents; see Figure 3. An application of boundary layer theory to buildings
and other objects has been reported aimed at postulating the economic assessment of
corrosion damage [50].
An immediate consequence of dry deposition velocities is the kinetic constraints
to obtain equilibrium between a gas in the atmosphere and the same gas in the
aqueous layer. Especially in outdoor exposure conditions, characterized by
wet-dry cycles, it is anticipated that the actual concentration of most corrosion-
stimulating gases under many conditions may be far from equilibrium.
Nevertheless, thermodynamic considerations have been most useful for predicting
the formation of different corrosion end products and their stability domains
[51,52]. Ageneral and useful observation made by Graedel is the similarity
between corrosion products found after prolonged exposure of metals and minerals
formed by natural processes and containing the same metals (see, e.g., Ref. 53).
Figure 4 is a schematic illustration of important processes occurring in or at the
aqueous layer.
540Leygraf
Figure 3Influence of airflow velocity on atmospheric corrosion rate of Cu in a
laboratory mixed-gas environment. (From Ref. 49. Courtesy of The Electrochemical Society.)
Copyright © 2002 Marcel Dekker, Inc.
THE SOLID PHASE
Acid-Dependent Dissolution
As discussed earlier, initial electrochemical reactions and repeated wet-dry cycles
may result in precipitation of thin films of metal hydroxide, oxyhydroxide, or
oxide. The precipitation processes of these compounds are complex and pass
through the colloid state before solid films are formed [54]. These films form on
the metal surface normally by fast nucleation and spreading and by slower thickening,
once the film has completely covered the surface. If the film is stable, it thickens
through a high-field ion conduction mechanism; otherwise the film may thicken
through a dissolution-reprecipitation mechanism [55]. One approach to predicting
the ability to form a protective oxygen-containing film is by considering the heat
of the adsorption of oxygen on the metal. Compilations of such data [56] suggest
strong metal-oxygen bond formation and highly protective layers in various
atmospheres in the case of, e.g., Ti, Al, or Cr, but only weak metal-oxygen bonds
Atmospheric Corrosion 541
Figure 4 Schematic illustration of processes occurring in or at the aqueous layer.
Copyright © 2002 Marcel Dekker, Inc.

and practically nonexistent protective oxygen-containing films in the case of, e.g.,
Ag or Zn.
As a consequence of exposure to the aqueous layer, the oxygen-containing film
can dissolve—the corrosion rate being normally controlled by the rate of dissolution
of the film formed [55]. As mentioned in the preceding section, the dissolution rate
of many oxides and other minerals is acid dependent and can be written in the form:
542 Leygraf
Metal-Anion Coordination Based on the Lewis Acid–Base Concept
During prolonged exposure a variety of different corrosion products may form, the
exact nature of which largely depends on the ability of the dissolving metal ion to
coordinate with other ions in the aqueous layer. A general treatment of classification
of participating ions in coordination processes can be based on the Lewis acid–base
concept. So far this theory has found only few applications in corrosion science [57].
It turns out to be most useful in rationalizing the specific behavior of different
metals during atmospheric corrosion and therefore merits a short introduction.
Upon interaction of two species, a pair of electrons from one species can
be used to form a covalent bond to the other one. The species that donates the
electron pair is called a Lewis base, and the species that accepts the electron pair
is called a Lewis acid. Acids or bases with valence electrons that are tightly held
and not easily distorted are hard acids or bases. Acids or bases with valence elec-
trons that are easily polarized or removed are soft. According to the hard and soft
acid-base (HSAB) principle, hard acids are preferably coordinated with hard
bases and soft acids are preferably coordinated with soft bases [58]. The hardness
of an element usually increases with its oxidation state. Selected Lewis acids
and bases and their classification into hard, intermediate, and soft acids and bases
are listed in Table 4 [59].
In full agreement with experience from atmospheric corrosion, Table 4 suggests
that hard acids such as Cr
3+
and Ti
4+
form oxygen-containing films, whereas soft
acids such as Cu
+
and Ag
+
coordinate with reduced sulfur compounds. Intermediate
acids, such as Fe
2+
, Cu
2+
, and Zn
2+
, are expected to coordinate with a broader range
of bases.
Source: From Ref. 59.
Copyright © 2002 Marcel Dekker, Inc.
