Martienssen W., Warlimont H. (Eds.). Handbook of Condensed Matter and Materials Data
Подождите немного. Документ загружается.


602 Part 4 Functional Materials
6
5
4
3
2
1
0
α-Sn
hω (eV)
12345
12
10
8
6
4
2
0
K (10
5
cm
–1
)
n
k
n, k
k
Fig. 4.1-47 α-Sn. Index of refraction n, extinction co-
efficient k, and absorption coefficient K vs. photon
energy [1.46]
19
18
17
16
15
14
T (K)
Dielectric constant ε (
∞
)
0 100 200 300 400 500
Ge
ε
1
, ε
2
30
20
10
0
–10
23456
hω (eV)
Ge
ε
2
ε
1
Fig. 4.1-48 Ge. Real and imaginary parts of the dielectric
constant vs. photon energy [1.47]
Fig. 4.1-49 Ge. Temperature dependence of the high-
frequency dielectric constant [1.48]. Experimental data
points [1.49] and ab initio calculations (solid line); the dot-
ted line is the theoretical result without the effect of thermal
expansion, and the dashed line is the solid line shifted to
match the experimental data
Part 4 1.1

Semiconductors 1.1 Group IV Semiconductors and IV–IV Compounds 603
Table 4.1-23 Optical constants of silicon
hν(eV) ε
1
ε
2
n k K (10
3
/cm) R
1.5 13.488 0.038 3.673 0.005 0.78 0.327
2.0 15.254 0.172 3.906 0.022 4.47 0.351
2.5 18.661 0.630 4.320 0.073 18.48 0.390
3.0 27.197 2.807 5.222 0.269 81.73 0.461
3.5 22.394 33.818 5.610 3.014 1069.19 0.575
4.0 12.240 35.939 5.010 3.586 1454.11 0.591
4.5 −19.815 24.919 2.452 5.082 2317.99 0.740
5.0 −10.242 11.195 1.570 3.565 1806.67 0.675
5.5 −9.106 8.846 1.340 3.302 1840.59 0.673
6.0 −7.443 5.877 1.010 2.909 1769.27 0.677
Table 4.1-24 Optical constants of germanium
hν(eV) ε
1
ε
2
n k K (10
3
/cm) R
1.5 21.560 2.772 4.653 0.298 45.30 0.419
2.0 30.361 10.427 5.588 0.933 189.12 0.495
2.5 13.153 20.695 4.340 2.384 604.15 0.492
3.0 12.065 17.514 4.082 2.145 652.25 0.463
3.5 9.052 21.442 4.020 2.667 946.01 0.502
4.0 4.123 26.056 3.905 3.336 1352.55 0.556
4.5 −14.655 16.782 1.953 4.297 1960.14 0.713
5.0 −8.277 8.911 1.394 3.197 1620.15 0.650
5.5 −6.176 7.842 1.380 2.842 1584.57 0.598
6.0 −6.648 5.672 1.023 2.774 1686.84 0.653
Table 4.1-25 Optical constants of silicon carbide
Crystal Refractive index Wavelength
range (nm)
3C-SiC n(λ) = 2.55378 +3.417 × 10
4
/λ
2
467–691
6H-SiC n
o
(λ) = 2.5531 +3.34 × 10
4
/λ
2
n
e
(λ) = 2.5852 +3.68 × 10
4
/λ
2
Part 4 1.1

604 Part 4 Functional Materials
4.1.2 III–V Compounds
4.1.2.1 Boron Compounds
A. Crystal Structure, Mechanical and Thermal Properties
Tables 4.1-26 – 4.1-32.
Table 4.1-26 Crystal structures of boron compounds
Crystal Space group Crystal Structure Remarks Figure
system type
Boron nitride, hexagonal BN
hex
P6
3
/mmc hex h-BN Stable under normal conditions Fig. 4.1-50
Boron nitride, cubic BN
cub
T
2
d
F43m fcc Zinc blende Metastable under normal conditions Fig.4.1-2
Boron nitride, wurtzite BN
w
C
4
6v
P6
3
mc hex Wurtzite Metastable under all conditions Fig. 4.1-3
Boron phosphide BP T
2
d
F43m fcc Zinc blende Stable under normal conditions Fig. 4.1-2
Boron arsenide BAs T
2
d
F43m fcc Zinc blende Stable in presence of As vapor Fig. 4.1-2
up to 920
◦
C
Boron antimonide BSb T
2
d
F43m fcc Zinc blende Stable under normal conditions Fig. 4.1-2
Table 4.1-27 Lattice parameters of boron compounds
Crystal Lattice parameters (nm) Temperature (K) Remarks
Boron nitride, cubic BN
cub
a =0.36160 (3) RT X-ray diffraction
Boron nitride, hexagonal BN
hex
a =0.25072 (1) 297 Prepared from amorphous BN in
c =0.687 (1) 297 N
2
atmosphere at room temperature
Boron phosphide BP a = 0.45383 (4) 297
Boron arsenide BAs a = 0.4777
Boron antimonide BSb a = 0.512 Ab initio pseudopotential calculation
Fig. 4.1-50 The hexagonal lattice of h-BN (BN
hex
)
Table 4.1-28 Densities of boron compounds
Crystal Density Remarks
(g/cm
3
)
Boron nitride, cubic BN
cub
3.4863 X-ray
diffraction
Boron nitride, hexagonal BN
hex
2.279 X-ray density
Boron phosphide BP
Boron arsenide BAs 5.22
Part 4 1.2

Semiconductors 1.2 III–V Compounds 605
Table 4.1-29 Melting point T
m
or decomposition temperature of boron compounds
Crystal Melting point T
m
(K) Remarks
Boron nitride, cubic BN
cub
> 3246
Boron nitride, hexagonal BN
hex
3200–3400 N
2
ambient; pyrometric measurement
Boron phosphide BP 1400 Decomposition temperature
Table 4.1-30 Linear thermal expansion coefficient α of boron compounds
Crystal Expansion coefficient α(K
−1
) Remarks
Boron nitride, cubic BN
cub
1.15× 10
−6
X-ray measurement
Boron nitride, hexagonal BN
hex
40.6 (4) ×10
−6
Boron phosphide BP 3.65 × 10
−6
At 400 K
Boron arsenide BAs 4.1×10
−6
Calculated
Table 4.1-31 Heat capacities and Debye temperatures of boron compounds
Crystal Debye temperature Θ
D
(K) Remarks
Boron nitride, cubic BN
cub
1730 (70) See Fig. 4.1-51 for temp. dependence of c
p
Boron nitride, hexagonal BN
hex
598 (7) Calorimetry
Boron phosphide BP 985 See Fig. 4.1-52 for temp. dependence of c
p
Boron arsenide BAs 800
Table 4.1-32 Phonon wavenumbers/frequencies of boron compounds. Values for hexagonal boron nitride (BN
hex
)
obtained from infrared reflectivity; values for boron phosphide (BP) from ab initio pseudopotential calculations
Crystal
Phonon wavenumbers (cm
−1
)
Remarks
Boron nitride, cubic BN
cub
˜
ν
LO
1305 (1)
˜
ν
TO
1054.7 (6) RT; Raman scattering
Boron arsenide BAs
˜
ν
LO
(Γ) 714
˜
ν
TO
(Γ) 695 Raman scattering
Table 4.1-32 Phonon wavenumbers/frequencies, cont.
BN
hex
Phonon branch Wavenumber (cm
−1
)
˜
ν
TO1
783
˜
ν
LO1
828
˜
ν
TO2
1510
˜
ν
LO2
1595
˜
ν
TO1
767
˜
ν
LO1
778
˜
ν
TO2
1367
˜
ν
LO2
1610
Table 4.1-32 Phonon wavenumbers/frequencies, cont.
BP
Phonon branch Frequency (THz)
ν
TO
(Γ) 24.25
ν
LO
(X) 24.00
ν
TO
(X) 15.81
ν
LO
(X) 21.04
ν
TO
(X) 9.20
ν
LO
(L) 22.91
ν
TO
(L) 15.18
Part 4 1.2

606 Part 4 Functional Materials
Heat capacity c
p
(J K
–1
mol
–1
)
24
20
16
12
8
4
0
300
Temperature T (K)
0 50 100 150 200 250
h-BN
r-BN
c-BN
w-BN
T (K)
c
p
(J K
–1
mol
–1
)
10020304050
1.0
0.8
0.6
0.4
0.2
0
Fig. 4.1-51 BN. Heat capacities for four polymorphic
boron nitride modifications. Data taken from [1.50]
B. Electronic Properties
Tables 4.1-33 – 4.1-35.
Band Structures of Boron Compounds.
Boron Nitride, Cubic (BN
cub
). All band structure calcu-
lations lead to an indirect-gap structure (Fig. 4.1-53).
Boron Nitride, Hexagonal (BN
hex
). All recent
band structure calculations yield an indirect gap
(Fig. 4.1-54). The conduction band minimum is located
at P.
Boron Phosphide (BP). BP is an indirect-gap semicon-
ductor (Fig. 4.1-55).
Boron Arsenide (BAs). The calculated band structure
shows an indirect (Γ–X) gap of a few eV. The conduction
Table 4.1-33 First Brillouin zones of boron compounds
Crystal Figure
Boron nitride, cubic BN
cub
Fig. 4.1-23
Boron nitride, hexagonal BN
hex
Fig. 4.1-24
Boron phosphide BP Fig. 4.1-23
Boron arsenide BAs Fig. 4.1-23
BP
1.5
1.4
1.3
1.2
1.1
1.0
0.9
0.8
0.7
Heat capacity c
p
(J g
–1
K
–1
)
T (K)
1100
1000
900
800
700
600
Debye temperature Θ
D
(K)
320 400 500 600 700
Θ
D
c
p
Fig. 4.1-52 BP. Temperature dependence of specific heat
capacity and Debye temperature [1.51]
21
14
7
0
–7
–14
–21
KXL ΓΓ
E (eV)
c-BN
Wave vector k
Fig. 4.1-53 Band structure of cubic boron nitride
Part 4 1.2

Semiconductors 1.2 III–V Compounds 607
Table 4.1-34 Energy gaps of boron compounds
Crystal Quantity Bands Energy (eV) Remarks
Boron nitride, cubic BN
cub
E
g, ind
Γ
15v
to X
1c
6.2 Soft-X-ray emission spectroscopy
E
g, dir
Γ
15v
to Γ
1c
14.5 Reflectivity
Boron nitride, hexagonal BN
hex
E
g
5.9 (1) Inelastic electron scattering
E
g, th
7.1 (1) Temperature dependence of electrical resistivity
Boron phosphide BP E
g, ind
2.1 X-ray emission and absorption
E
g, dir
Γ 4.4
X 6.5
L 6.5
Boron arsenide BAs E
g, ind
0.67 Absorption
E
g, dir
1.46
Boron antimonide BSb E
g
Γ to ∆ 0.527 Calculated
Table 4.1-35 Effective masses of electrons (m
n
) and holes (m
p
) for cubic boron nitride (in units of the electron mass m
0
)
Crystal m
n
m
p, heavy
m
p, light
Remarks
Boron nitride, cubic BN
cub
0.752 Calculated from band structure data
0.375 0.150 Parallel to [100]
0.926 0.108 Parallel to [111]
band minimum is on the ∆ axis close tothe X point. As in
silicon, the Γ
15c
level is below the Γ
1c
level (Fig. 4.1-56).
Boron Antimonide (BSb). Boron antimonide is an
indirect-gap semiconductor (Fig. 4.1-57).
21
14
7
0
–7
–14
–21
KH A LΓΓMA
E (eV)
h-BN
Wave vector k
Fig. 4.1-54 Band structure of hexagonal boron nitride
Fig. 4.1-55 Band structure of boron phosphide
20
15
10
5
0
–5
–10
–15
–20
ΓKΓ LΣ∆X Λ
E (eV)
Wave vector k
BP
K
1
Γ
1
X
1
L
1
Σ
1
∆
1
Γ
1
Λ
1
K
1
K
1
X
3
K
2
L
1
Σ
1
K
1
Σ
1
Λ
1
K
1
L
3
Σ
1
∆
1
Λ
3
K
2
Γ
15
X
5
L
1
Σ
2
∆
5
Γ
15
Λ
1
K
2
Γ
1
X
1
L
3
Σ
1
∆
1
Γ
1
Λ
3
K
1
Γ
15
X
3
L
1
Σ
1
∆
1
Γ
15
Λ
1
K
1
Γ
12
X
5
L
3
Σ
1
∆
5
Γ
12
Λ
3
Σ
2
Σ
2
Part 4 1.2

608 Part 4 Functional Materials
Energy E (eV)
12
9
6
3
0
–3
–6
–9
–12
–15
–18
Wave vector k
XΓ U,K ΓL Λ∆ Σ
L
1c
Γ
1c
BAs
L
3c
L
1c
L
3v
L
1v
Γ
15c
Γ
15v
X
5c
X
1c
X
3c
X
5v
X
3v
X
v
1
K
1c
K
1c
K
1c
K
2v
K
2c
K
1v
K
1v
K
1v
Γ
15v
Γ
1v
Γ
15c
Γ
1c
L
1v
Fig. 4.1-56 Band structure of boron arsenide
C. Transport Properties
Electronic Transport, General Description. Boron Ni-
tride, Cubic (BN
cub
). Owing to the large gap, all
literature data refer to extrinsic conduction. For
the temperature dependence of the conductivity, see
Fig. 4.1-58. Hall measurements on nominally undoped
thin films yield values of the carrier mobility of around
500 cm
2
/Vs.
Boron Nitride, hexagonal (BN
hex
). Figure 4.1-59 shows
the temperature dependence ofthe electrical resistivity at
high temperatures. In an undoped nanocrystalline film,
resistivity values of the order of 2 ×10
11
Ω cm have been
found.
Boron Phosphide (BP). Boron phosphide is extrinsic at
room temperature, and the transport is limited by im-
purity scattering. Figure 4.1-60 shows the temperature
dependence of the conductivity, carrier concentration,
and Hall mobility of several samples. In n-doped
single crystals, electron mobilities of the order of
30–40cm
2
/V s have been found at 300 K; in p-doped
single crystals, mobilities around 500 cm
2
/Vs have
been measured at 300 K.
Boron Arsenide (BAs). There is almost no information
about the semiconducting properties of BAs.
Energy E (eV)
12
9
6
3
0
–3
–6
–9
–12
–15
–18
Wave vector k
XΓL ∆V
L
1c
Γ
1c
BSb
L
3c
L
1c
L
3v
L
1v
L
1v
Γ
15c
Γ
15v
Γ
1v
X
5c
X
1c
X
3c
X
5v
X
3v
X
1v
Fig. 4.1-57 Band structure of boron antimonide
123
T
–1
(10
–3
K
–1
)
σ (Ω
–1
cm
–1
)
10
–3
10
–4
10
–5
10
–6
10
–7
BN
cub
Fig. 4.1-58 c-BN (BN
cub
). Electrical conductivity vs. re-
ciprocal temperature for several different polycrystalline
samples above RT [1.52, 53]
Part 4 1.2

Semiconductors 1.2 III–V Compounds 609
T
–1
(×10
–4
K
–1
)
ρ (Ω cm)
10
14
10
13
10
12
10
11
10
10
10
9
10
8
10
7
10
6
10
5
10
4
78956 1011
BN
hex
A
B
C
D
E
Fig. 4.1-59 h-BN (BN
hex
). Resistivity vs. temperature
above 700
◦
C. A, from bulk resistance of a disk meas-
ured in the c direction; B – E, earlier literature data for
comparison [1.54]
2×10
2
10
18
10
17
6
4
8
2
3
5
7
2×10
16
9
6
4
8
3
5
7
9
6
8
3
5
7
9
4×10
–1
10
1
2
6
4
5
7
9
8
1.0 1.5 2.0 2.5 3.0 3.5
2×10
18
10
10
2
6
8
3
5
7
9
2
4
6
5
7
9
4
8
800 400 200 100 25
T (°C)
T
–1
(10
–3
Κ
–1
)
Concentration n (cm
–3
)
Conductivity σ (Ω
–1
cm
–1
)
Hall mobility µ
H,n
(cm
2
(V s)
–1
)
σ
No. 1
µ
H,n
No. 1
No. 3
σ
No. 3
Fig. 4.1-60 Temperature dependence of the conductivity
σ
i
, the carrier concentration n, and the mobility µ
H,n
of n-type BP(100) wafers [1.55]. Sample No. 1 contains
autodoped Si with a concentration of 5 ×10
18
atoms/cm
3
and sample No. 3 contains 5 × 10
19
atoms/cm
3
Part 4 1.2
610 Part 4 Functional Materials
D. Electromagnetic and Optical Properties
Table 4.1-36.
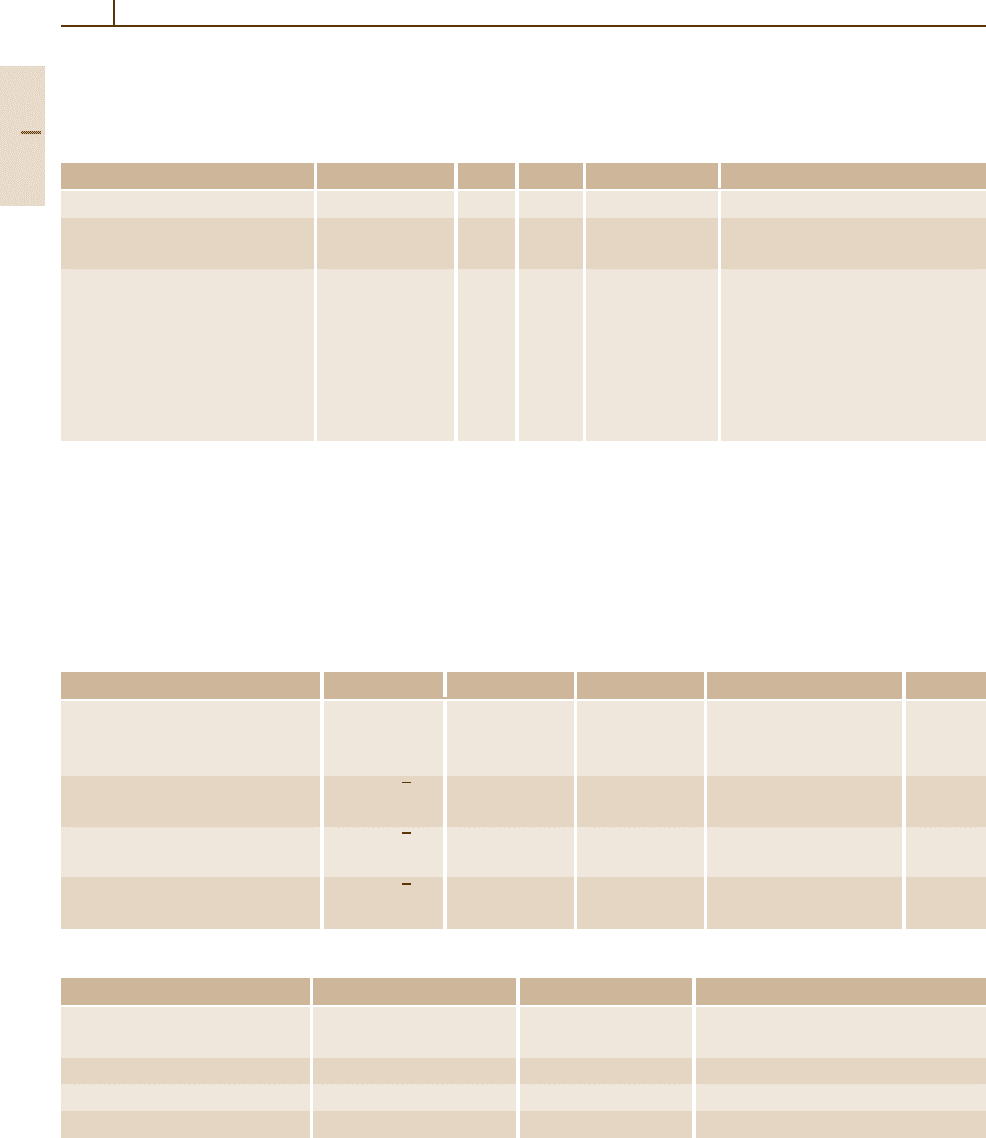
Table 4.1-36 Dielectric constant ε and refractive index n of boron compounds
Crystal Temperature (K) ε(0) ε(∞) n at λ(nm) Remarks
Boron nitride, cubic BN
cub
300 7.1 2.097 712.5 IR reflectivity
Boron nitride, hexagonal BN
hex
300 5.09 4.10 2.13 Parallel to c axis, IR reflectivity
7.04 4.95 1.65 Perpendicular to c axis, IR reflectivity
Boron phosphide BP 300 11 7.8 Schottky barrier reflectance
RT 3.34 (5) 454.5 Brewster angle method
3.34 (5) 458
3.32 (5) 488
3.30 (5) 496
3.26 (5) 514.5
3.00 (5) 632.8
4.1.2.2 Aluminium Compounds
A. Crystal Structure, Mechanical and Thermal Properties
Tables 4.1-37 – 4.1-44.
Table 4.1-37 Crystal structures of aluminium compounds
Crystal Space group Crystal system Structure type Remarks Figure
Aluminium nitride AlN I C
4
6v
P6
3
mc hex Wurtzite At normal pressure Fig. 4.1-3
AlN II
O
5
h
Fm3m fcc NaCl structure At 21 GPa (shock
compression)
Aluminium phosphide AlP I T
2
d
F
4
3m
fcc Zinc blende At normal pressure Fig. 4.1-2
AlP II O
5
h
Fm3m fcc NaCl structure High-pressure phase
Aluminium arsenide AlAs T
2
d
F
4
3m
fcc Zinc blende Transition to NiAs structure Fig. 4.1-2
at high pressure
Aluminium antimonide AlSb I T
2
d
F
4
3m
fcc Zinc blende At normal pressure Fig. 4.1-2
AlSb II Cmcm Orthorhombic At high pressure
Table 4.1-38 Lattice parameters of aluminium compounds
Crystal Lattice parameters (nm) Temperature Remarks
Aluminium nitride AlN a = 0.31111 300 K X-ray diffraction on ultrafine powder
c =0.49788
Aluminium phosphide AlP a = 0.54635 (4) 25
◦
C Epitaxial film on GaP
Aluminium arsenide AlAs a = 0.566139 291 K High-resolution X-ray diffraction
Aluminium antimonide AlSb a = 0.61355 (1) 291.15 K Powder, X-ray measurement
Part 4 1.2

Semiconductors 1.2 III–V Compounds 611
Table 4.1-39 Densities of aluminium compounds
Crystal Density (g/cm
3
) Temperature (K) Remarks
Aluminium nitride AlN 3.255 X-ray diffraction
Aluminium phosphide AlP 2.40 (1)
Aluminium arsenide AlAs 3.760 Calculated from lattice parameter
Aluminium antimonide AlSb 4.26 293
Table 4.1-40 Elastic constants c
ik
of aluminium compounds
Crystal c
11
(GPa) c
12
(GPa) c
13
(GPa) c
33
(GPa) c
44
(GPa) Remarks
Aluminium nitride AlN 296 (18) 130 (11) 158 (06) 267 (18) 241 (20)
a
Aluminium phosphide AlP 140.50 (28) 62.03 (24) 70.33 (7) Ultrasound ( f = 10 and 30 MHz)
Aluminium arsenide AlAs 190 (1) 53.8 (1) 59.5 (1) T = 300 K
122.1 (3) 56.6 (3) 59.9(1) T = 77 K
112.6 57.1 60.0 T = 0 K; extrapolated data
Aluminium antimonide AlSb 88.34 40.23 43.22 T = 296 K; ultrasound
a
Calculated from the mean square displacement of the lattice atoms measured by X-ray diffraction.
Table 4.1-41 Melting point T
m
of aluminium compounds
Crystal Melting point T
m
(K)
Aluminium nitride AlN 3025
Aluminium phosphide AlP 2823
Aluminium arsenide AlAs 2013 (20)
Aluminium antimonide AlSb 1327(1)
Table 4.1-42 Linear thermal expansion coefficient α of aluminium compounds
Crystal Expansion coefficient α(K
−1
) Temperature Remarks
Aluminium nitride AlN α
perpend
4.35× 10
−6
300 K Powder; X-ray diffraction
α
parallel
3.48× 10
−6
Aluminium phosphide AlP
Aluminium arsenide AlAs 5.20 (5) ×10
−6
15–840
◦
C X-ray diffraction
Aluminium antimonide AlSb Fig. 4.1-61
6
4
2
0
–2
0 40 80 120 160 200 240 280 320 360
T (K)
α (10
–6
K
–1
)
AlSb
Fig. 4.1-61 AlSb. Coefficient of linear thermal expansion
vs. temperature [1.56,57]
Part 4 1.2
