Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
400 Other Fuel Cells
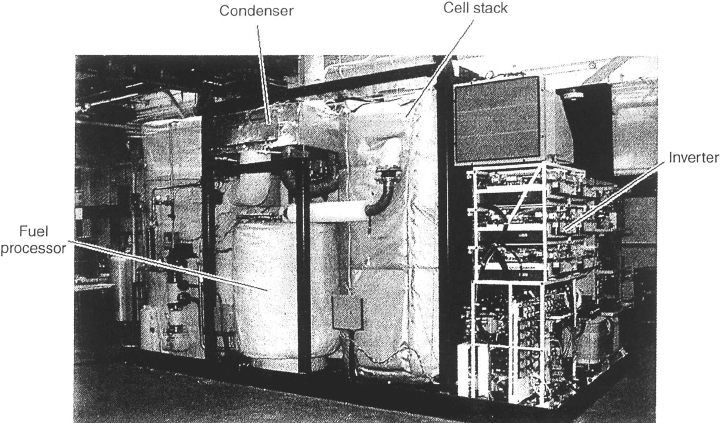
Figure 7.16 PC25C 200-kWe PAFC power plant and accesories. (Reproduced with permission
from Ref. [38].)
advances in durability and lifetime were also achieved during operation of these units.
In 2002, operation of over 245 units all over the world had accumulated 5 million hours
of operation, with durability between major system overhauls exceeding 40,000 h [38].
Worldwide, over 500 PAFC systems had been installed by 2004 [39]. Shown in Figure
7.16, like many fuel cell power plants, the fuel cell itself is actually a smaller fraction of the
overall size and weight of the system compared to the ancillary components. In the case of
the 200-kWe PC25 PAFC, the stack constitutes only about 25% of the system volume and
cost [40], which is a reasonable estimate for most nonportable fuel cell systems. Ancillary
systems provide the following:
1. Fuel Management: The PC25 reforms natural gas to hydrogen fuel.
2. Heat Management: The fuel cell stack must be cooled to avoid electrolyte loss. To
increase the overall efficiency, the waste heat can be used as cogenerated power
through a hot-water heat exchanger system.
3. Water Management: The stack product water is rejected to the atmosphere and used
for steam reforming of the natural gas.
4. Flow and Power Management: The air and hydrogen flow into the stack must be
controlled, and the direct current (DC) produced must be conditioned to produce
the voltage and current levels and phases desired.
5. Sensing and Control: The entire system is operated and controlled as a turnkey,
with no direct operator input in normal operation [41].
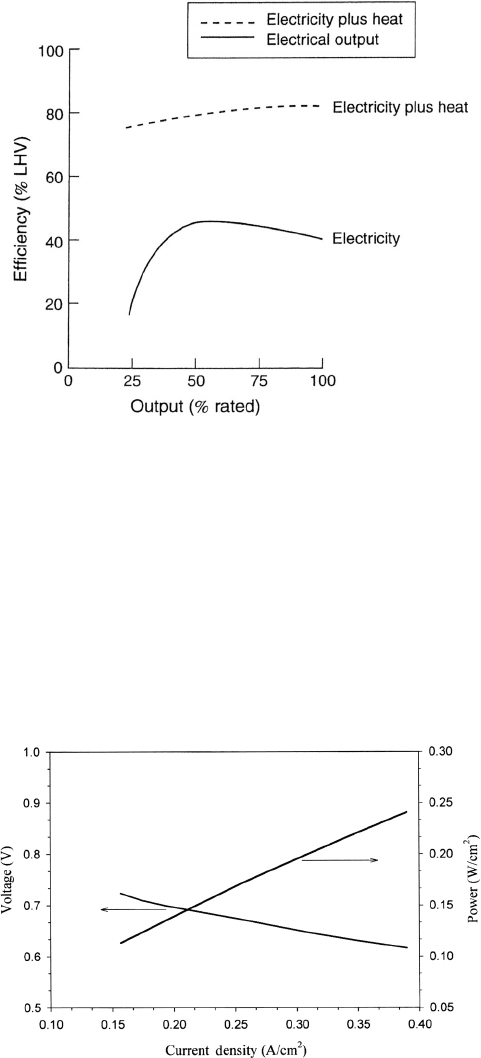
The total electrical efficiency of the PC25 200-kWe unit is shown in Figure 7.17.
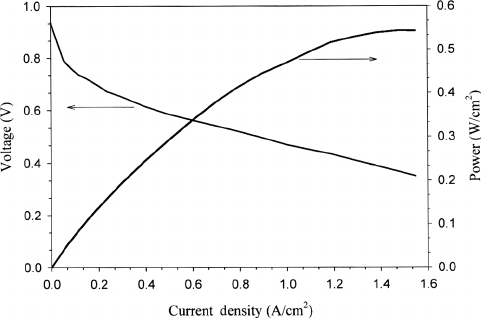
A polarization curve from a cell in an early PC25 unit is given in Figure 7.18. Modern
PAFCs can be higher than this, as shown in Figure 7.19. For operation in the electric mode
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
7.3 Phosphoric Acid Fuel Cells 401
Figure 7.17 Measured electrical and overall (combined heat and power) efficiency for United
Technologies Fuel Cell, PC25C 200 kWe PAFC power plant. (Reproduced with permission from Ref.
[38].)
only, around 40% chemical-to-electrical conversion is realized, which is better than many
combustion systems for submegawatt power application. When the waste heat recovery to
the hot water is included (the system puts out about 200–225 kW of recoverable heat),
the system efficiency reaches 80%, an outstanding figure that compares very well with
combustion-based technologies. Overall combined efficiency can now reach 87% in the
cogeneration mode [42].
Overall, the PC25 unit represents the first real fuel cell product to be commercially
sold. The system size, weight, and reliability have been continuously improved, and
Figure 7.18 Polarization curve from 200-kWe PC25 PAFC system operating at atmospheric pres-
sure. (Adapted from Ref. [43].)
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
402 Other Fuel Cells
Figure 7.19 Polarization curve from 200 kWe PC25 PAFC system operating at 180
◦
C and atmo-
spheric pressure. Pt-CO/C supported cathode catalyst, and SiC:ZrSiO
4
electrolyte matrix. (Adapted
from Ref. [43].)
operation in all environments around the world has been technically quite successful.
However, the overall system cost remains at $3000–4000 per kWe, which is about three
times higher than needed for deep stationary power market penetration [45]. The reliable
off-grid power and quiet, low emission output have found a niche in premium power
market venues such as banks, hospitals, or government buildings, however. In these
applications, a combustion-based generator may be undesired or comparably unattractive,
and the PAFC cost is acceptable. In the case of the Police Barracks in Central Park, New
York, the cost of bringing in additional electrical capacity to the barracks ($1.2 million)
was greater than the PC25 system ($800,000) [46]. A gas turbine generator would have
been less expensive but would have been much louder in the middle of the park. In special
applications such as these, the PAFC and its reliable power (>95% service reliability has
been observed) are ideal. An added benefit is the heat cogeneration, which can be used
for a variety of purposes, including space heating, water heating, or even absorption cycle
air conditioning. Future research and development of these systems will revolve around
achieving a product more cost-competitive with other stationary power systems.
7.3.1 Operation and Configurations
The PAFC is an acid-based electrolyte technology that is in many ways quite similar to the
PEFC, and much of the technology learned during early PAFC development is now being
applied (or relearned!) to PEFC systems. The PAFC operates at an elevated temperature
compared to the PEFC, at 160–210
◦
C. The higher temperature PAFC was chosen for
development for terrestrial applications after the failure of the (AFC) to demonstrate suitable
performance with CO
2
. The operating pressure of PAFCs ranges from 1 to 9 atm, and the
flow stoichiometries are similar to other fuel cells. Higher operating pressures require larger
parasitic losses and ancillary component costs, so most installed systems now operate at
near atmospheric pressure. The observed performance increase observed for an increase in
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
7.3 Phosphoric Acid Fuel Cells 403
Hydrogen oxidation reaction (HOR)
H
2
2H
+
+ 2e
–
Anode
cathode
matrix
Oxidizer gas
Fuel gas
2H
+
+ O
2
+ 2e
–
1
—
2
H
2
O
Separator plate
Oxidizer reduction reaction (ORR)
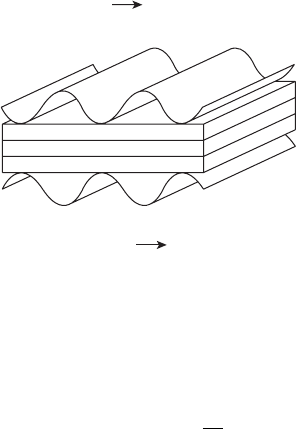
Figure 7.20 Illustration of PAFC electrode and components.
pressure when the cell operates at 190
◦
C and 323 mA/cm
2
has been correlated as [47]:
V (mV) = 146 log
P
2
P
1
(7.9)
As discussed in Chapter 4, this is higher than the Nernst voltage increase due to increased
cathode exchange current density.
Both systems have an acid-based electrolyte (PEFC is sulfuric acid based), although the
PAFC is a liquid electrolyte solution system and the PEFC electrolyte exists as a partially
bound solution in a solid polymer matrix. Between the PEFC and PAFC, the anode HOR
and cathode ORR are the same. A schematic of the materials and electrochemical reactions
in the PAFC system is shown in Figure 7.20. Both systems use a noble metal catalyst or
alloy with noble metals on the electrodes, and both suffer from poor ORR kinetics relative
to alkaline-based systems. Ironically, since operation of the PEFC at 80
◦
C results in catalyst
poisoning from CO as well as water management issues that the PAFC avoids, developers
seek higher temperature PEFC membranes that can operate at 120–200
◦
C like the PAFC
but maintain the high power density advantage of the PEFC.
Similar to a MCFC, in the PAFC, the electrolyte is stored between the electrodes within
athin∼50% porosity, 50–200-µm-thick porous matrix. The purpose for the silicon carbide
(SiC) porous matrix used in PAFCs is to retain the acid electrolyte by capillary forces. If
there were no matrix, the acid could simply drain into the flow channels or bulge according to
gravimetric forces. It is critical that the bubble pressure of the matrix exceed 35 kPa, or elec-
trolyte blow-through and excessive crossover from internal pressure differentials would oc-
cur. The maximum pressure differential between the anode and cathode is limited to around
20 kPa to avoid this problem.
Like all components, the electrode structure has evolved over time, and now a carbon-
supported heterogeneous catalyst of platinum or platinum alloys with other metals such
as chromium, vanadium, or cobalt is commonly used [40]. Similar to PEFC electrodes,
precious metal catalyst loading of around 0.25 on the anode to 0.5 mg/cm
2
are used.
Although platinum is the base catalyst material, it is not a major contributor to the cost
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
404 Other Fuel Cells
of the system and no longer impedes market implementation, since other components
dominate PAFC system cost [40].
The electrode is sprayed or cast onto a porous electrode substrate which is similar in
some ways to the DM used in polymer electrolytes and AFCs. The purposes of this porous
substrate are multiple:
1. To enable transport of reactants, products, electrons, and heat to and from the
electrode,
2. to act as electrolyte reservoirs for phosphoric acid, and
3. to serve as the flow channels.
In the PEFC, only the first purpose is relevant. The eventual depletion of electrolyte from the
PAFC matrix by evaporation results in gas crossover losses and low bulk ionic conductivity
that must be avoided or the system will reach end of life. Therefore, a resevior of electrolyte
is needed to replace lost electrolyte in service.
It is difficult to refill the electrolyte in the PAFC system while in operation, so a
lifetime of acid should be available at the beginning of life. In older designs, the electrolyte
storage matrix would be thicker or include an internal reservoir to accommodate additional
electrolyte. However, this has the detrimental effect of increasing system bulk and ohmic
losses through the electrolyte. In order to reduce the size of the SiC matrix needed for
prolonged operation as well as simplify stack design, some of the storage of the electrolyte
has been moved out of the electrolyte matrix and into the porous and wettable carbon
paper substrate material. Electrolyte storage in the carbon paper substrate is accomplished
in hydrophilic, small pores. In order to promote gas flux, however, there is typically
some wet proofing of the overall substrate with PTFE. The catalyst also shares a dual
hydrophobic–hydrophilic nature. The dual hydrophobic–hydrophilic nature of the substrate
and catalyst layer is similar to that in PEFCs, except that in the PEFCs the hydrophilic pores
in the catalyst layer and DM are filled with water, not electrolyte, which is not desired. In
the PAFC, the storage of electrolyte, which is in liquid form at operating conditions in the
substrate greatly extends operation life.
As discussed in Chapter 5, the pore size and hydrophobicity control the capillary
pressure in the liquid. In a hydrophylic media, the smaller pore sizes have a liquid suction
pressure, drawing liquid in. In the PAFC, the SiC matrix has uniformly small pores and is
more hydrophilic than the catalyst layer or substrate reservoir, so that losses in electrolyte
are readily replaced by suction from stored electrolyte these locations. The pressure and
pore size distribution set up the electrolyte–catalyst–reactant interface area and thus are
critical factors to control the performance of the electrode. The uniformity and control of the
pore size in the matrix are extremely important to prevent local drainage spots with severe
crossover. Control of the pore size distribution and hydrophobicity of the catalyst layer,
substrate, and storage matrix are critical to ensure long life and maximized triple-phase
boundary area for reaction in the catalyst layer.
There have been many internal configurations of the PAFC stack plates, the most
recent involving the use of carbon paper substrate as an electrolyte reservoir and a flow
distributor, as discussed. The flow field design in a PAFC is similar to a PEFC, but there is
no special provision for flooding, since this is not an issue with the medium-temperature
PAFC. Typically, the flow fields are aligned in plane and perpendicular to one another
(cross-flow), and external manifolding is used, as shown in Figures 7.21 and 7.22. By
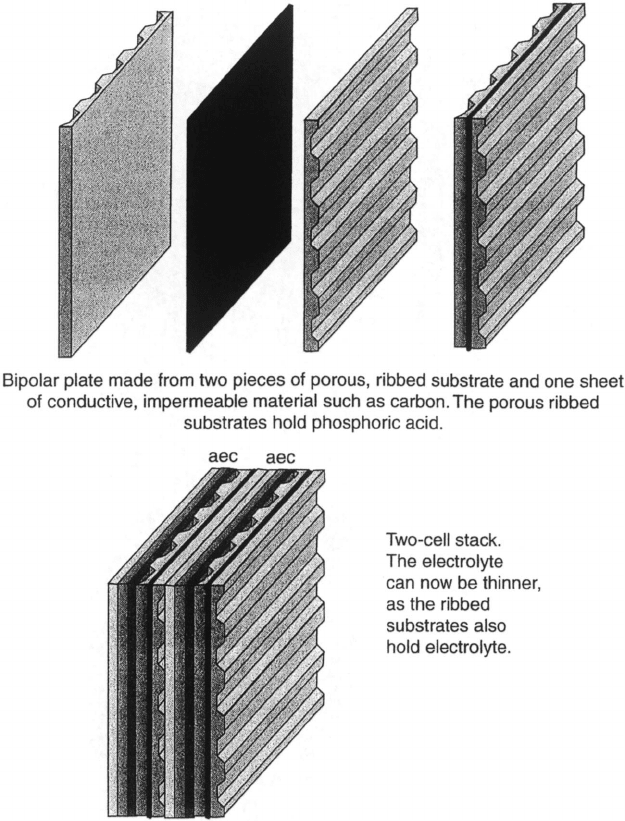
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
7.3 Phosphoric Acid Fuel Cells 405
Figure 7.21 Illustration of PAFC stack flow field assembly with stored electrolyte. (Reproduced
with permission from Ref. [26].)
shaping the carbon paper in parallel channels, there is no need to separately machine a
bipolar plate with flow fields, and system size and cost savings are realized.
The parallel and straight nature of the flow fields cannot be easily fed fuel in a stack
using internal manifolding, and an external manifold system like that illustrated in Figure
7.22 is commonly used. This leads to sealing problems along the sides of the porous substrate
not used for gas flow, where the edge pores must be closed by dipping or sealing the substrate
edges. The entire assembly is then completed with a flat, thin separation plate that serves
to isolate the reactants and electrolyte and transport current between cells in series.
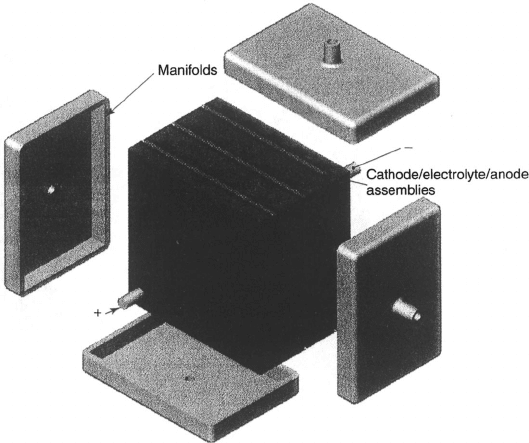
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
406 Other Fuel Cells
Figure 7.22 PAFC stack external manifolding concept. (Reproduced with permission from Ref.
[26].)
The main advantage of the PAFC is that the higher temperature eliminates or reduces
two major problems with the PEFC, CO poisoning sensitivity and water management. The
PAFC cannot accomplish internal reformation like the MCFC or SOFC, but because of the
elevated temperature of 200
◦
C compared to 80
◦
C for the PEFC, the anode in the PAFC can
tolerate a 1–2% CO in the feed stream. This allows operation on reformed natural gas and
other fuel feedstocks with minimal CO filtering, greatly reducing reformer size and control
requirements.
Due to the high-temperature operation and electrolyte behavior, water management in
the PAFC is not a major concern. The electrolyte is a highly concentrated acid solution
that is conductive without water and has a very low vapor pressure at high concentration.
The electrolyte concentration varies between 90 and 100% during operation depending
on the flow rate, current density, and operating temperature. During operation, water is
generated at the cathode, which is readily evaporated into the flow stream or absorbed into
the electrolyte. If the water is absorbed into the electrolyte, dilution increases the vapor
pressure and drives off the water at a faster rate. Therefore, water management in the
electrolyte is self-regulating. The generated water leaves the system as steam, which can
be used for the steam reformation process in the fuel-processing subsystem or to provide
thermal energy for cogeneration application.
Another major advantage of the electrolyte system is that control over freeze damage
can be accomplished by dilution of the electrolyte to lower concentrations, which can reduce
the freeze point of the electrolyte to below −40
◦
C [45] before shipping. Once in operation,
the PAFC will self-regulate the acid concentration when the operating temperature reaches
a high normal value through the vapor pressure dependency discussed. Compared to the
SOFC, the PAFC does not suffer the major material compatibility or manufacturing diffi-
culties. Neither the SOFC nor the PAFC is a rapid-startup system, but operation at 200
◦
C
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
7.3 Phosphoric Acid Fuel Cells 407
does not present the major thermal stress and seal leakage issues the SOFC suffers from.
Provision for removal of the waste heat is accomplished through cooling pipes embedded
between every 5–10 cells in the stack.
Electrode and Electrolyte System Phosphoric acid, in high concentrations (90–100%),
was chosen as the electrolyte in PAFC systems because of its low vapor pressure in high
concentrations, which permits long-term operation with a fixed electrolyte system. Below
160
◦
C, phosphoric acid is a poor ionic conductor, and problems of carbon monoxide
poisoning that plague PEFCs become problematic. Therefore, operation between 160 and
below 210
◦
C is typical. Above this temperature severe electrolyte loss becomes a limiting
factor. The electrolyte inventory in a PAFC is critical to maintain performance over time
and protect downstream piping from severe corrosion from high-acid effluent. Therefore,
there must be an electrolyte reservoir to allow changes in the electrolyte volume with time.
Electrolyte volume can be retained if the cell temperature or cathode flow stoichiometry is
decreased, and more water remains in the electrolyte, diluting the solution. The electrolyte
inventory can be lost over time due to three reasons:
1. Evaporation of Electrolyte The vapor pressure of phosphoric acid is extremely low
(a few ppm at 150–200
◦
C [45]) but finite. Over time, electrolyte loss is substantial.
This loss can be mitigated by reduced-temperature operation to reduce the vapor
pressure, a higher initial inventory of acid, or an acid condensation zone near the
exit. In this approach, an uncatalyzed cold zone near the fuel cell exit is used to
collect condensed phosphoric acid and return it to the matrix inventory [48].
2. Reaction with Poisons or Fuel Cell Components Obviously, the catalysts and other
materials used in the PAFC must be compatible with H
3
PO
4
electrolyte. However,
the activity of a material is related to the surface area, and when the normally
inert SiC electrolyte matrix particles were reduced from 5 to 0.5 µm to decrease
electrolyte thickness and stack power density, acid consumption from an H
3
PO
4
–Si
reaction is observed. The size of the SiC particles used in the electrolyte matrix is
now around 1 µm [48]. Consumption by poisons can be eliminated with filtering of
the reactants.
3. Uptake by Fuel Cell Components The separator plates used in PAFC operation
have a closed porosity of around 15%. During operation, the pores become open,
drawing acid in by capillary action. By the end of lifetime, significant electrolyte
can be stored in the separator plate. Additionally, electrochemical pumping occurs,
in which the acid anion accumulates at the anode during high-current operation.
This increases the pressure at the anode and can result in loss of performance due
to electrolyte excursion into the electrode or channel. Additionally, the electrolyte
pumping can result in cell-to-cell motion through the separator plate. Over time,
the cathode side of the stack can become depleted of electrolyte first, resulting in
excessive crossover and performance decay.
Example 7.2 Loss of PAFC Electrolyte by Vaporization Over Time Given a 0.5-m
2
-
active-area PAFC operating at 150 mA/cm
2
and an anode stoichiometry of 1.4, determine
the amount of electrolyte lost to the anode flow over 40,000 h operation. Assume the
equilibrium concentration of H
3
PO
4
in vapor form is 3 ppm at the operating temperature
of 200
◦
C. The anode flow is pure hydrogen.

c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
408 Other Fuel Cells
SOLUTION We must solve for the total vapor uptake in the flow, assuming it reaches
equilibrium fully saturated conditions at the exit. The relationships are the same as for
moist air mixtures, only now it is the “relative humidity” of H
3
PO
4,v
with which we are
concerned.
From Chapter 3, the definition of mole fraction is
y
H
3
PO
4,v
=
˙
n
v
˙
n
total
=
˙
n
v
˙
n
others
+
˙
n
v
⇒
˙
n
v
≈ y
H
3
PO
4,v
˙
n
others
(small
˙
n
v
)
where
˙
n
others
is the molar flow rate of everything but the acid vapor. Since there is no water
generated at the anode and the input is dry, this is equivalent to the total molar flow rate of
hydrogen out of the fuel cell:
˙
n
H
2
,out
= (λ
a
− 1)
iA
nF
= (0.4)
(0.150 A/cm
2
)(0.5m
2
)(10,000 cm
2
/m
2
)
(2 e
−
eq/mol H
2
)(96,485 C/eq)
= 1.55 × 10
−3
mol H
2
/s
The vapor equilibrium is 3 ppm, a mole fraction of 3.0 × 10
−6
. Therefore
˙
n
v
≈ (3.0 ×10
−6
mol H
3
PO
4
/mol H
2
)(1.55 × 10
−3
mol H
2
/s) (40,000 h) (3,600 s/h)
= 0.669 mol
Since the molecular weight of H
3
PO
4
is 98 g/mol, this is equivalent to losing 65 g of acid
just from the hydrogen side during the 40,000-h lifetime of operation.
COMMENTS: The cathode would also absorb some electrolyte vapor as well and, since
the flow rate and stoichiometry are higher on the cathode, would absorb more. On the
cathode, the solution is slightly more complicated since water vapor generated from reaction
will also be vaporized into the flow. From this calculation, one can see how even a small
decrease in the vapor pressure can greatly extend the operational lifetime of the stack.
Durability Durability of the PAFC system has been demonstrated to be in excess of
40,000 hours between major overhauls of the system and stack components [38]. However,
several durability issues degrade performance with time and must be addressed with system
engineering design and material solutions. Many improvements were made in this regard
between the subsequent generations of PAFC development and have eliminated modes
of edge corrosion, damage during startup and shutdown cycles, or increased carbon and
catalyst support integrity. However, issues that shorten lifeimte in the PAFC system remain
and in many cases are remarkably similar to those observed in the PEFC system. Many of
the lessons learned in PAFC design and operation can be applied to PEFC system operation,
since many of the degradation modes are similar. Continuing durability issues for the PAFC
include the following [38]:
1. Electrolyte acid loss
2. Electrode stability and impurities
3. Carbon support corrosion
4. Anode starvation
5. Load cycling
c07 JWPR067-Mench December 19, 2007 17:46 Char Count=
7.3 Phosphoric Acid Fuel Cells 409
Of these, acid loss is perhaps the most insidious problem, as discussed. Simply reducing
the operating temperature 25–30
◦
C can double the life of the stack by reducing electrolyte
vaporization and slowing other degradation modes [45]. This constant loss prevents the
potential elimination of the electrolyte reservoir storage in the substrate and prohibits
further major improvements in power density. Impurities in the fuel and oxidizer stream
can gradually degrade performance, as in all fuel cells [40]. Hydrogen sulfide (H
2
S) can
enter the anode stream through fuel impurities or be present in the air as impurity from
local industrial processes. At levels greater than 50 ppm, the stack-level damage to the
anode catalyst from H
2
S can be permanent. Chlorides, ammonia, and dust constitute other
potential poisons.
Carbon support corrosion and platinum dissolution at the cathode can occur at high
potentials, and as a result, operation or idling the fuel cell at voltages above 0.8 V is not
normally permitted. During startup, an auxiliary load cuts cell voltage below 0.8 V to prevent
damage. Heat treatment of the carbon support structure and use of graphitized carbon can
reduce this loss, which is an approach that also is successful in PEFCs. Carbon corrosion
is also observed in PEFCs during startup and shutdown and if there are locations of anode
starvation. This mode of irreversible loss is exacerbated in PAFCs, since the operating
temperature is higher. One fundamental disadvantage of higher temperature operation is
that all of the undesired reactions (e.g., corrosion of supports, piping, current collectors,
platinum dissolution) are accelerated by the increased temperature. Of course, the increased
temperature also hastens the kinetics of the desired HOR and ORR, too. This is a common
trade-off in fuel cell systems: At high temperatures, the kinetics are more facile, but the
undesired reactions are also accelerated. At low temperature, the degradation reactions are
slowed, but so are the kinetics of the desired reaction.
Fuel starvation can very rapidly degrade cell performance. If any portion of the anode
is starved of hydrogen, the electrode potential will rise to oxygen evolution, and irreversible
oxidation of the carbon support, substrate, and separator plate will ensue. To eliminate this,
the anode flow field often has several passes, so that the hydrogen concentration is not
reduced to low values in any locations [45].
7.3.2 Summary of Advantages and Disadvantages
The PAFC has some definite technical advantages. In many ways, the research thrusts in
PEFC technology are based on achieving the same midrange temperature of operation as
the PAFC while avoiding the drawbacks. The main advantages of the PAFC system are as
follows:
1. Ease of Water Management The water level in the electrolyte is self-regulating
by changing vapor pressure, and the temperature is high enough that liquid water
flooding is not an issue.
2. Ability to Operate on 1–2% CO in Feed Stream The CO sticking coefficient on
platinum is greatly reduced at 200
◦
C, so that the PAFC can operate on a wide variety
of fuel feeds with a simple steam reformer subsystem and minimal CO cleanup.
3. Demonstrated High Reliability and Developed System The PAFC system is the
first fuel cell to reach the consumer production stage and has millions of operation
hours accumulated with hundreds of 200-kWe units. This system has demonstrated
high service reliability of over 95% as well as combined thermal efficiency of over
80% in the cogeneration mode.
