Middleton W.M. (ed.) Reference Data for Engineers: Radio, Electronics, Computer and Communications
Подождите немного. Документ загружается.

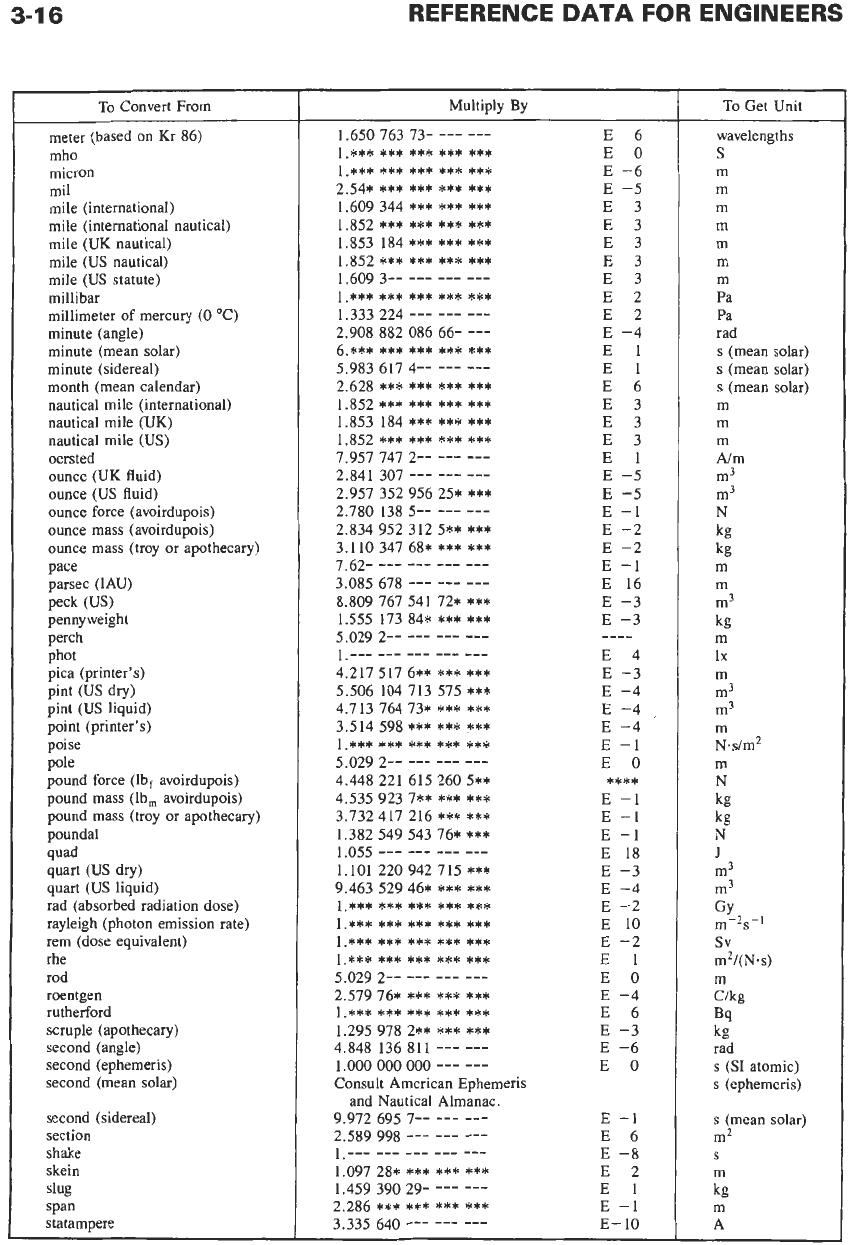
To
Convert From
I
Multiply
By
meter (based on Kr
86)
mho
micron
mil
mile (international)
mile (international nautical)
mile
(UK
nautical)
mile
(US
nautical)
mile
(US
statute)
millibar
millimeter
of
mercury
(0
"C)
minute (angle)
minute (mean solar)
minute (sidereal)
month (mean calendar)
nautical mile (international)
nautical mile (UK)
nautical mile
(US)
oersted
ounce
(UK
fluid)
ounce (US fluid)
ounce force (avoirdupois)
ounce mass (avoirdupois)
ounce mass (troy or apothecary)
pace
parsec (IAU)
peck
(US)
pennyweight
perch
phot
pica (printer's)
pint
(US
dry)
pint
(US
liquid)
point (printer's)
poise
pole
pound force (lb, avoirdupois)
pound mass (lb, avoirdupois)
pound mass (troy or apothecary)
poundal
quad
quart (US dry)
quart
(US
liquid)
rad (absorbed radiation dose)
rayleigh (photon emission rate)
rem (dose equivalent)
rhe
rod
roentgen
rutherford
scruple (apothecary)
second (angle)
second (ephemeris)
second (mean solar)
second (sidereal)
section
shake
skein
span
s tatampere
slug
1.650 763 73-
---
---
I.***
***
***
***
***
I.***
***
***
***
***
2.54*
***
***
***
***
1.609 344
***
***
***
1.852
***
***
***
***
1.853 184
***
***
***
1.852
***
*e*
***
***
I.***
***
***
***
***
1.333 224
--- ---
---
2.908 882 086 66-
---
6.***
*** *** ***
***
5.983 617 4-
---
---
2.628
***
***
***
***
1.852
*** ***
***
***
1.853 184
***
***
***
1.852
***
***
***
***
7.957 747 2--
---
---
2.841 307
---
---
---
2.957 352 956 25*
***
2.780 138
5--
---
---
2.834 952 312 5**
***
3.110 347 6%
***
***
,
1
609 3--
___
___
___
7.62-
--_
--_
___
___
3.085 678
---
---
---
8.809 767 541 72*
***
1.555
173 84*
***
***
5.029 2--
---
-__
___
1
- --
- - - - - -
---
-
- -
4.217 517 6**
***
***
5.506 104 713 575
***
4.713 764 73*
***
***
3.514 598
***
***
***
1.***
***
***
***
***
5 029 2--
___
___
___
4.448 221 615 260 5**
4.535 923
7**
***
***
3.732 417 216
***
***
1.382 549 543 76*
***
1,055
--- -__
___
___
1.101 220 942 715
***
9.463 529 46*
***
***
1.***
***
***
***
***
I.***
***
***
***
***
1.***
***
***
***
***
I.***
*** ***
***
***
5.029 2--
--_
___
___
2.579 76*
***
***
***
1.***
***
***
***
***
1.295 978 2**
***
***
4.848 136 811
--- ---
1.000
000
000
---
---
Consult American Ephemeris
9.972 695
I--
---
---
2.589 998
---
---
---
1,
- -- -
-
-
-
-
- -
--
-
-
-
1.097 28*
***
***
***
1.459 390 29-
---
---
2.286
***
***
***
***
3.335 640
---
--- ---
and Nautical Almanac.
E6
EO
E -6
E -5
E3
E3
E3
E3
E3
E2
E2
E -4
El
El
E6
E3
E3
E3
El
E
-5
E
-5
E
-1
E -2
E -2
E -1
E 16
E -3
E
-3
E4
E -3
E -4
E
-4
E -4
E
-1
----
EO
****
E -1
E -1
E
-1
E
18
E -3
E -4
E -2
E 10
E
-2
El
EO
E
-4
E6
E
-3
E -6
EO
E
-1
E6
E
-8
E2
El
E
-1
E-
IO
To
Get Unit
lm
m
rn
m
m
m
m
Pa
Pa
rad
s
(mean solar)
s
(mean solar)
s
(mean
solar)
m
m
m
Ah
m3
m3
N
kg
kg
m
m
m3
kg
m
Ix
m
m3
m3
m
N.s/m2
m
N
kg
kg
m3
m3
GY
m-2s-l
N
J
sv
m*/(N.s)
m
Clkg
Bq
kg
rad
s (SI atomic)
s
(ephemeris)
s (mean solar)
mz
m
kg
m
A
S
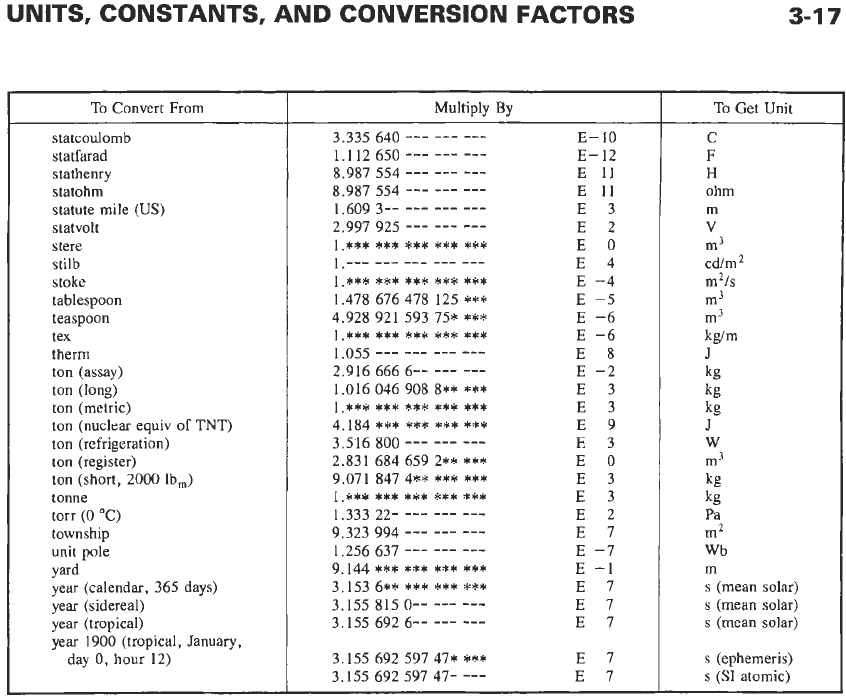
UNITS, CONSTANTS, AND CONVERSION FACTORS
3-1
7
To
Convert From
statcoulomb
statfarad
stathenry
statohm
statute
mile
(US)
statvolt
stere
stilb
stoke
tablespoon
teaspoon
tex
therm
ton (assay)
ton (long)
ton (metric)
ton (nuclear equiv of
TNT)
ton (refrigeration)
ton (register)
ton (short,
2000
Ib,)
tonne
torr
(0
"C)
township
unit pole
Yard
year (calendar,
365
days)
year
(sidereal)
year (tropical)
year
1900
(tropical, January,
day
0,
hour
12)
Multiply
By
3.335 640
---
---
---
1.1 12 650
---
---
---
8.987 554
---
---
---
8.987 554
---
---
---
2.997 925
---
---
---
I.***
***
***
***
***
1,609 3-- ---
--- ---
I.***
***
***
***
***
1.478 676 478 125
***
4.928 921 593 75*
***
I.***
***
*** ***
***
1,055
___
___
___
---
2.916 666 6--
---
---
1.016 046 908
8**
***
I.***
***
***
***
***
4.184
***
***
***
***
3.516
800
--- ---
---
2.831 684 659 2**
***
9.071 847 4**
***
***
I.***
***
*** ***
***
1,333 22- ---
---
---
9.323 994 ---
---
---
1.256 637
---
---
---
9.144
w*
*** ***
***
3.153 6**
***
***
***
3.155 815
0--
---
---
3.155 692 6--
---
---
3.155 692 597 47*
***
3.155 692 597 47- ---
E- 10
E-
12
E 11
E 11
E3
E2
EO
E4
E -4
E -5
E -6
E -6
E8
E
-2
E3
E3
E9
E3
EO
E3
E3
E2
E7
E -7
E -1
E7
E7
E7
E7
E7
To
Get Unit
C
F
H
ohm
m
V
m3
cd/m2
m2/s
m3
m3
kg/m
J
kg
kg
kg
m3
kg
kg
Pa
m2
Wb
m
s (mean solar)
s
(mean solar)
s
(mean solar)
s
(ephemeris)
s
(SI
atomic)
J
W

4
Properties
of
Materials
Revised
by
Eugene
A.
Mechtly
General Properties of the Elements 4-3
Periodic Classification
of
the Elements
4-3
Physical Properties of the Elements 4-3
Galvanic Series in Sea Water 4-3
Temperature-EMF Characteristics of Thermocouples 4-3
Electromotive Force and Other Properties
Conducting Materials
4-3
Semiconducting Materials 4-3
Insulating Materials 4-12
Magnetic Materials 4-1
7
Soft Magnetic Metals
Permanent-Magnet Materials
Ferrites
4-
1
4-2
REFERENCE
DATA
FOR ENGINEERS
Magnetostriction
4-27
Piezoelectricity
4-28
Acoustic Properties
of
Some Materials
4-32

PROPERTIES OF MATERIALS
4-3
GENERAL PROPERTIES
OF
THE ELEMENTS
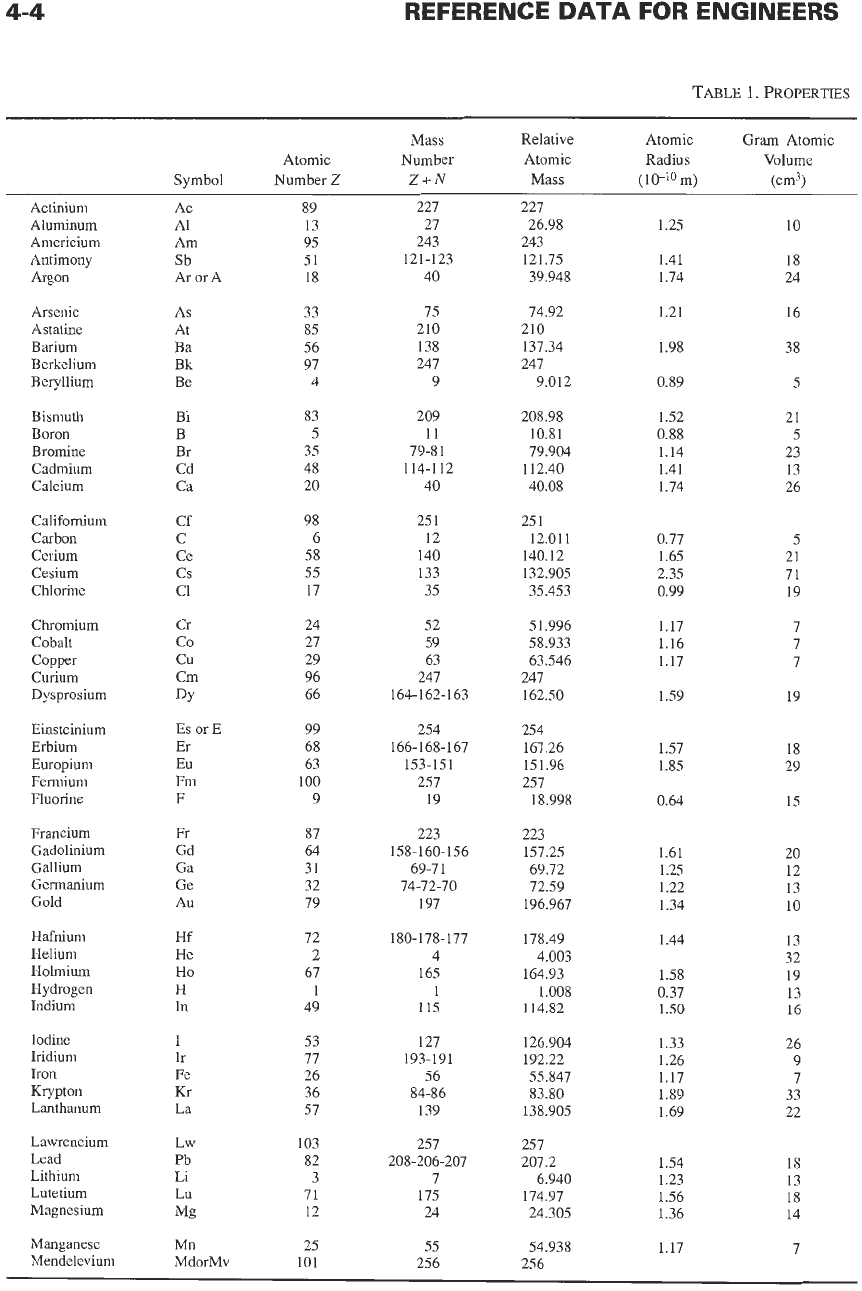
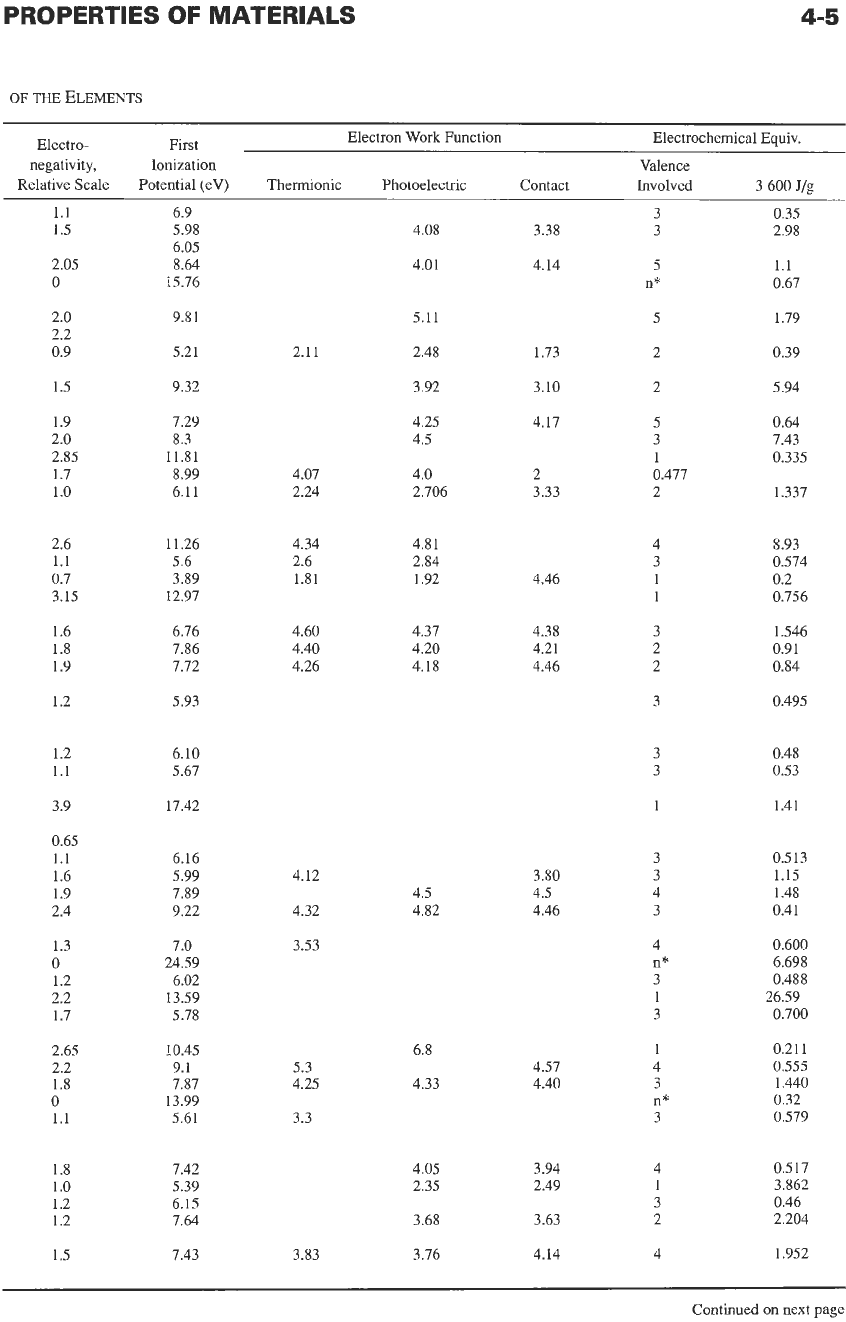
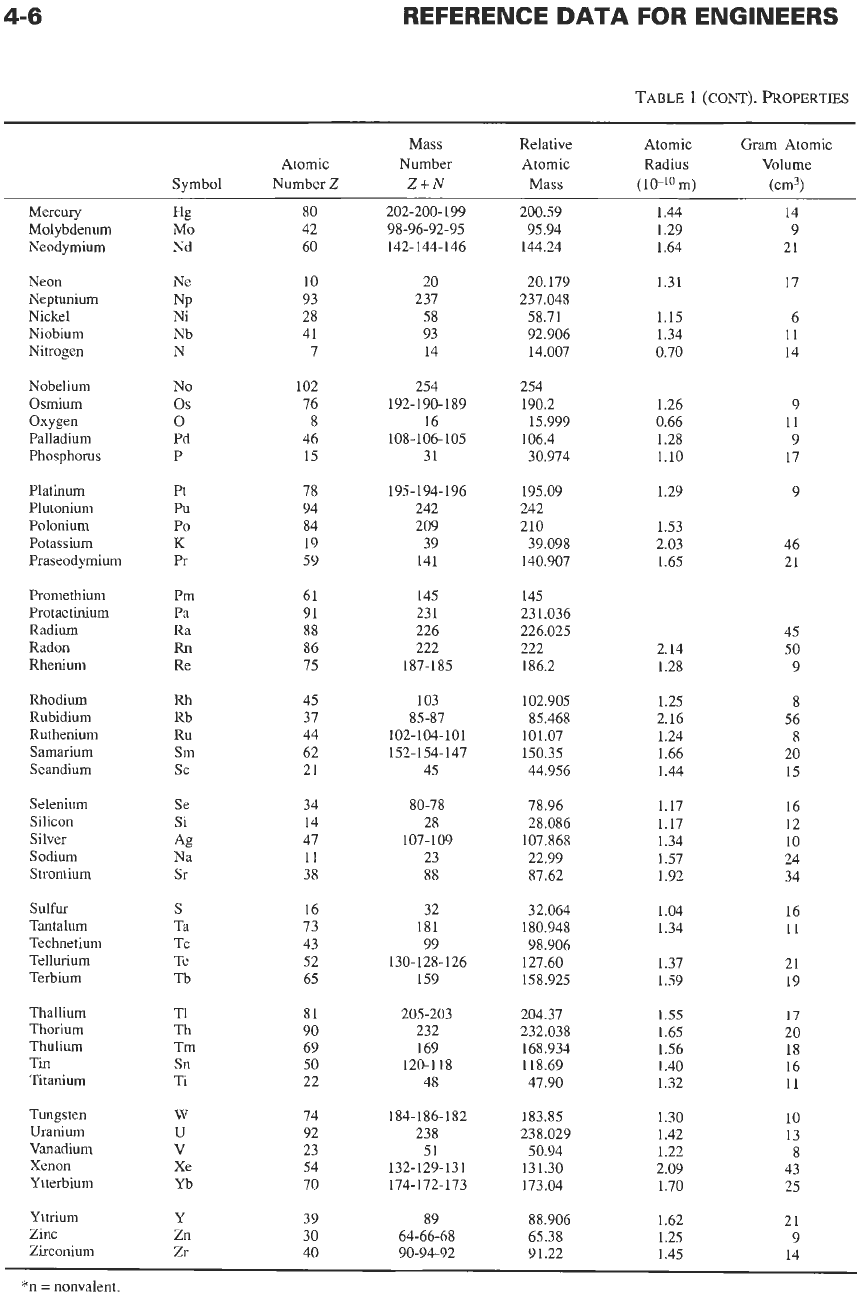
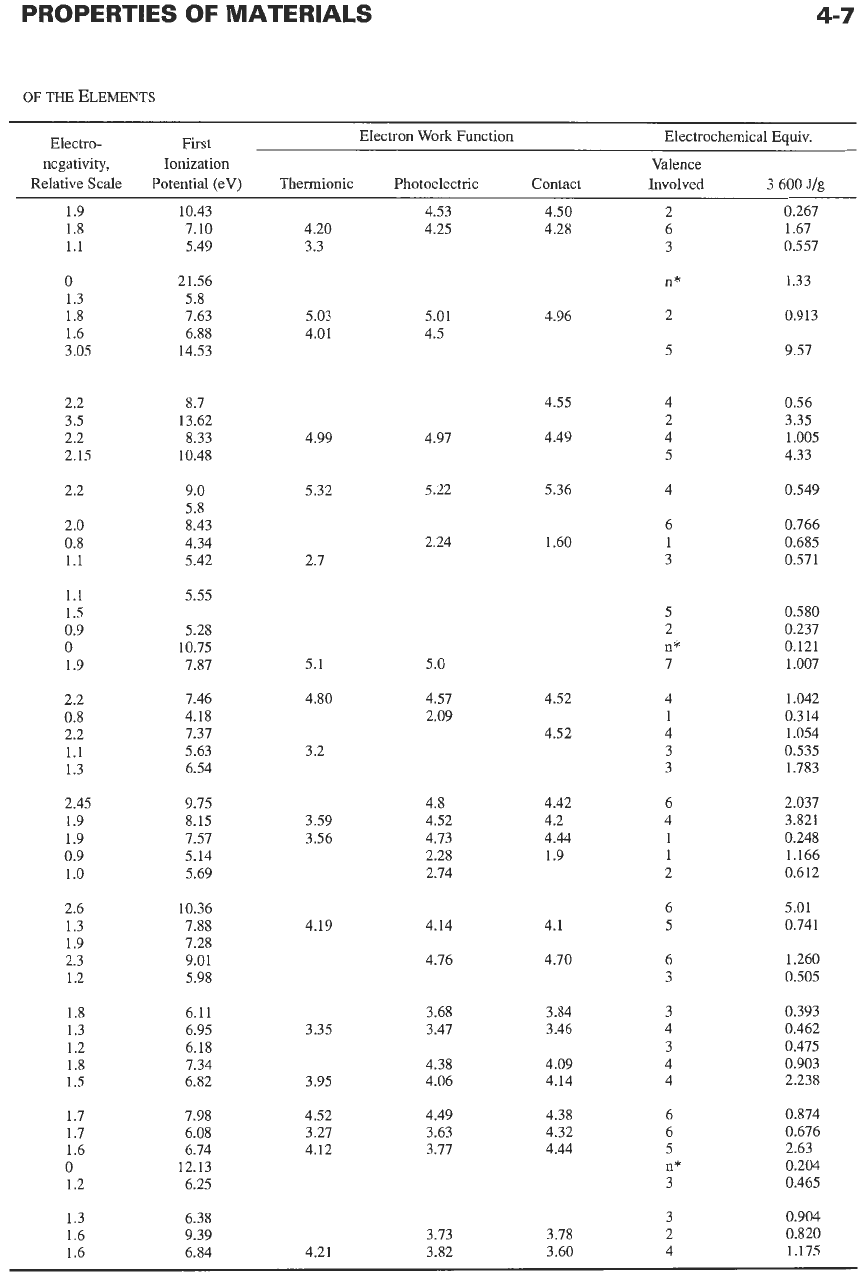
Some properties of the elements are listed in Table
l.* Some of the listed quantities are defined
as
follows.
Atomic number
Z represents the number of protons
per
atom.
Mass number
Z
+
N
is equal to the number of pro-
tons
Z
plus the number of neutrons
N
present in the
nuclei.
Mass
numbers of the most abundant isotopes
are given in order of decreasing abundance. For exm-
ple, Cadmium Cd-48 mass numbers 114-112 means
that cadmium atoms of greater abundance (28.86%)
have
a
mass
number of 114; that is, the nucleus of
Cd1I4 has 114
-
48
=
66 neutrons while isotope Cdl”
of lower abundance (24.07%) has 112
-
48
=
64 neu-
trons.
Atomic radius
values listed provide
a
comparison of
sizes (deduced from interatomic spacing of bound
atoms).
Gram atomic volume
in cubic centimeters gives the
volume occupied in the solid state by an atom at its
melting point. The gram atomic volume contains the
Avogadro number of at0ms.t
Electronegativity
represents the relative tendency of
an
atom
to
attract shared electron pairs. The highest
electronegativity is assigned to fluorine with the value
3.90.
First ionization potential
is the work in electron
volts required to pull
1
electron
off
an
isolated neutral
atom.
1 electron volt
=
1.602
x
joule?
Electron
work
function,
expressed
in
electronvolts,
represents the energy that must be supplied to an elec-
tron to cross over the surface barrier of a metal. That
energy may be supplied by heat (thermionic work
function), by light (photoelectric work function), or by
contact with a dissimilar metal (contact potential).
Electrochemical equivalents
are expressed in joules
per gram (J/g) liberated at the electrode.
PERIODIC CLASSIFICATION OF
THE ELEMENTS
Fig.
1
is
a
periodic table of the elements.
Oxidation number
is defined as the charge that an
atom appears to have in a compound when electrons
are counted according to certain rules:
*
Tables 1 and
2,
Chart 1, and Fig.
1
of
this chapter are
partly based on data
from
the following sources:
Handhook
of
Chemistry and Physics,
55th
ed., CRC Press, Inc., 1974;
Fun-
damentals
of
Chemistry,
John
Wiley
&
Sons;
The Encyclope-
dia
of
Electrochemistry,
Reinhold Publishing
Corp.,
1964;
American Znstitute
of
Physics Handbook,
3rd ed., McGraw-
Hill
Book
Co., 1972;
Lunge’s Handbook
of
Chemistry,
11th
ed., McGraw-Hill
Book
Co., 1973.
t
See Chapter
3
for precise valve.
(A)
In the free elements each atom has an oxidation
number
of
0.
(B)
Electrons shared between
two
unlike atoms are
counted with the more electronegative
atom.
(C) Electrons shared between
the
two like atoms are
divided equally between sharing atoms.
PHYSICAL PROPERTIES OF
THE ELEMENTS
Some of the physical properties of the elements are
listed in Table
2.
GALVANIC SERIES
IN
SEA
WATER
In sea water, two dissimilar metals connected by
a
conductor form a galvanic cell. If the two metals are in
different groups of Chart
1
(separated by spaces), the
metal coming first in the series-starting from cor-
roded end to protected end-will be anodic (i.e., cor-
roded by the metal contained in the group farther from
the corroded end). If the two metals are
in
the same
group, no appreciable corrosive action will take place.
TEMPERATURE-EMF
CHARACTERISTICS OF
THERMOCOUPLESS
Fig. 2 shows temperature-emf characteristics of
thermocouples.
Electromotive Force
and
Other
Properties
Electromotive force and other properties of thermo-
couples are listed in Tables
3
and 4.
CONDUCTING MATERIALS
Conducting materials (Tables
5-7)
can be classified
as follows:
Conductors: Resistivities from
lCG
to
lo4
Clan (1
to
100
pClan). Conductivities from
lo4
to
lo6
Sa-’.
Semiconductors: Resistivities from
1@
to
lo9
Clncm. Conductivities from
lC9
to
lo4
Sa-’.
Insulators: Resistivities from
lo9
to
loz5
Clan.
Conductivities from to
lC9
Sa-’.
SEMICONDUCTING
MATERIALS
Some properties of semiconductor materials are
listed in Table
8.
j
R.
L.
Weber,
Temperature Measurement and Control
(Philadelphia:
Blakiston
Co., 1941; pp. 68-71).
4-4
TABLE
1.
PROPERTIES
Mass Relative Atomic Gram Atomic
Atomic Number Atomic Radius Volume
Symbol Number
Z
Z+N
Mass
(1
0-10
m) (cm3)
Actinium
Aluminum
Americium
Antimony
Argon
Arsenic
Astatine
Barium
Berkelium
Beryllium
Bismuth
Boron
Bromine
Cadmium
Calcium
Californium
Carbon
Cerium
Cesium
Chlorine
Chroniium
Cobalt
Copper
Curium
Dysprosium
Einsteinium
Erbium
Europium
Fermium
Fluorine
Francium
Gadolinium
Gallium
Germanium
Gold
Hafnium
Helium
Holmium
Hydrogen
Indium
Iodine
Iridium
Iron
Lanthanum
Lawrencium
Lead
Lithium
Lutetium
Magnesium
Manganese
Mendelevium
Ktypton
Ac
A1
Am
Sb
Ar or A
As
At
Ba
Bk
Be
Bi
B
Br
Cd
Ca
Cf
C
Ce
cs
c1
Cr
co
cu
Cm
DY
Es or E
Er
Eu
Fm
F
Fr
Gd
Ga
Ge
Au
Hf
He
Ho
H
In
I
Ir
Fe
Kr
La
Lw
Pb
Li
Lu
Mg
Mn
MdorMv
89
13
95
51
18
33
85
56
97
4
83
5
35
48
20
98
6
58
55
17
24
27
29
96
66
99
68
63
100
9
87
64
31
32
79
72
2
67
1
49
53
77
26
36
57
103
82
3
71
12
25
101
227
27
243
40
75
210
138
247
9
209
11
79-81
114-1 12
40
25
1
12
140
133
35
52
59
63
247
164-162-163
254
153-151
257
19
223
12 1-1 23
166- 168-1 67
1.58-160-156
69-7
1
74-72-70
197
180-178-177
4
165
1
115
127
193-191
56
84-86
139
257
7
175
24
55
256
208-206-207
227
26.98
243
121.75
39.948
74.92
210
137.34
247
9.012
208.98
10.81
79.904
112.40
40.08
25
1
12.011
140.12
132.905
35.453
51.996
58.933
63.546
247
162.50
254
167.26
151.96
257
18.998
223
157.25
69.72
72.59
196.967
178.49
164.93
114.82
126.904
192.22
55.847
83.80
138.905
257
207.2
174.97
4.003
1.008
6.940
24.305
54.938
2.56
1.25
1.41
1.74
1.21
1.98
0.89
1.52
0.88
1.14
1.41
1.74
0.77
1.6.5
2.35
0.99
1.17
1.16
1.17
1.59
1.57
1.85
0.64
1.61
1.25
1.22
1.34
1.44
1.58
0.37
1.50
1.33
1.26
1.17
1.89
1.69
1.54
1.23
1.56
1.36
1.17
10
18
24
16
38
5
21
5
23
13
26
5
21
71
19
7
7
7
19
18
29
15
20
12
13
10
13
32
19
13
16
26
9
7
33
22
18
13
18
14
7
PROPERTIES
OF
MATERIALS
OF
THE
ELEMENTS
4-5
Electro- First Electron
Work
Function Electrochemical Equiv.
negativity, Ionization
Relative Scale Potential (eV) Thermionic Photoelectric
1.1
1.5
2.05
0
2.0
2.2
0.9
1.5
1.9
2.0
2.85
1.7
1
.o
2.6
1.1
0.7
3.15
1.6
1.8
1.9
1.2
1.2
1.1
3.9
0.65
1.1
1.6
1.9
2.4
1.3
0
1.2
2.2
1.7
2.65
2.2
1.8
0
1.1
1.8
1
.o
1.2
1.2
1.5
6.9
5.98
6.05
8.64
15.76
9.81
5.21
9.32
7.29
8.3
11.81
8.99
6.11
n
1.26
5.6
3.89
12.97
6.76
7.86
7.72
5.93
6.10
5.67
17.42
6.16
5.99
7.89
9.22
7.0
24.59
6.02
13.59
5.78
10.45
9.1
7.87
13.99
5.61
7.42
5.39
6.15
7.64
7.43
2.11
4.07
2.24
4.34
2.6
1.81
4.60
4.40
4.26
4.12
4.32
3.53
5.3
4.25
3.3
3.83
4.08
4.01
5.11
2.48
3.92
4.25
4.5
4.0
2.706
4.81
2.84
1.92
4.37
4.20
4.18
4.5
4.82
6.8
4.33
4.05
2.35
3.68
3.76
Contact
3.38
4.14
1.73
3.10
4.17
2
3.33
4.46
4.38
4.21
4.46
3.80
4.5
4.46
4.57
4.40
3.94
2.49
3.63
4.14
Valence
hvolved
3
600
J/g
3
3
5
n*
5
2
2
5
3
1
0.477
2
4
3
1
1
3
2
2
3
3
3
1
3
3
4
3
4
n*
3
1
3
1
4
3
n*
3
4
1
3
2
4
0.35
2.98
1.1
0.67
1.79
0.39
5.94
0.64
7.43
0.335
1.337
8.93
0.574
0.2
0.756
1.546
0.91
0.84
0.495
0.48
0.53
1.41
0.5 13
1.15
1.48
0.41
0.600
6.698
0.488
0.700
0.21
1
0.555
1.440
0.32
0.579
26.59
0.517
3.862
0.46
2.204
1.952
Continued
on
next
page
4-6
REFERENCE
DATA
FOR ENGINEERS
TABLE
1
(CONT).
PROPERTIES
Mass Relative Atomic
Gram
Atomic
Atomic Number Atomic Radius Volume
Symbol Number
Z
Z+N
Mass
(
1
O-'O
m) (cm3)
80 202-200-199 200.59 1.44 14
Mercury
Molybdenum
Neodymium
Neon
Neptunium
Nickel
Niobium
Nitrogen
Nobelium
Osmium
Oxygen
Palladium
Phosphorus
Platinum
Plutonium
Polonium
Potassium
Praseodymium
Promethium
Protactinium
Radium
Radon
Rhenium
Rhodium
Rubidium
Ruthenium
Samarium
scandium
Selenium
Silicon
Silver
Sodium
Strontium
Sulfur
Tantalum
Technetium
Tellurium
Terbium
Thallium
Thorium
Thulium
Tin
Titanium
Tungsten
Uranium
Vanadium
Xenon
Ytterbium
Yttrium
zinc
Zirconium
Hg
Mo
Nd
Ne
Ni
Nb
N
No
os
0
Pd
P
Pt
Pn
Po
K
PI
Pm
Pa
Ra
Rn
Re
Rh
Rb
Ru
Sm
sc
Se
Si
Ag
Na
Sr
S
Ta
Tc
Te
Tb
T1
Th
Tm
Sn
Ti
w
U
V
Xe
Yb
Y
Zn
Zr
NP
42
60
10
93
28
41
7
102
76
8
46
15
78
94
84
19
59
61
91
88
86
75
45
37
44
62
21
34
14
47
11
38
16
73
43
52
65
81
90
69
50
22
74
92
23
54
70
39
30
40
98-96-92-95
142-144-146
20
237
58
93
14
254
192-190-189
16
31
195-1 94- 196
242
209
39
141
145
23 1
226
222
187-185
108-106- 105
103
85-87
102-104-101
152-154-147
45
80-78
28
107-109
23
88
32
181
99
130-128-126
159
205-203
232
169
120-118
48
184-186-182
238
51
132-129-13
1
174-172-173
89
64-66-68
90-94-92
95.94
144.24
20.179
237.048
58.71
92.906
14.007
254
190.2
106.4
15.999
30.974
195.09
242
210
39.098
140.907
145
23 1.036
226.025
222
186.2
102.905
85.468
101.07
150.35
44.956
78.96
28.086
107.868
22.99
87.62
32.064
180.948
98.906
127.60
15 8.925
204.37
232.038
168.934
118.69
47.90
183.85
238.029
50.94
131.30
173.04
88.906
65.38
91.22
1.29
1.64
1.31
1.15
1.34
0.70
1.26
0.66
1.28
1.10
1.29
1.53
2.03
1.65
2.14
1.28
1.25
2.16
1.24
1.66
1.44
1.17
1.17
1.34
1.57
1.92
1.04
1.34
1.37
1.59
1.55
1.65
1.56
1.40
1.32
1.30
1.42
1.22
2.09
1.70
1.62
1.25
1.45
9
21
17
6
11
14
9
11
9
17
9
46
21
45
50
9
8
56
8
20
15
16
12
IO
24
34
16
11
21
19
17
20
18
16
11
10
13
8
43
25
21
9
14
*n
=
nonvalent
PROPERTIES
OF
MATERIALS
4-7
OF
THE
ELEMENTS
Electron Work Function Electrochemical Equiv.
Electro- First
negativity, Ionization Valence
Relative Scale Potential (eV) Thermionic Photoelectric Contact Involved
3 600
J/g
1.9
1.8
1.1
0
1.3
1.8
1.6
3.05
2.2
3.5
2.2
2.15
2.2
2.0
0.8
1.1
1.1
1.5
0.9
0
1.9
2.2
0.8
2.2
1.1
1.3
2.45
1.9
1.9
0.9
1
.o
2.6
1.3
1.9
2.3
1.2
1.8
1.3
1.2
1.8
1.5
1.7
1.7
1.6
0
1.2
1.3
1.6
1.6
10.43
7.10
5.49
21.56
5.8
7.63
6.88
14.53
8.7
13.62
8.33
10.48
9.0
5.8
8.43
4.34
5.42
5.55
5.28
10.75
7.87
7.46
4.18
7.37
5.63
6.54
9.75
8.15
7.57
5.14
5.69
10.36
7.88
7.28
9.01
5.98
6.11
6.95
6.18
7.34
6.82
7.98
6.08
6.74
12.13
6.25
6.38
9.39
6.84
4.20
3.3
5.03
4.01
4.99
5.32
2.7
5.1
4.80
3.2
3.59
3.56
4.19
3.35
3.95
4.52
3.27
4.12
4.21
4.53
4.25
5.01
4.5
4.97
5.22
2.24
5.0
4.57
2.09
4.8
4.52
4.73
2.28
2.74
4.14
4.76
3.68
3.47
4.38
4.06
4.49
3.63
3.77
3.73
3.82
4.50
4.28
4.96
4.55
4.49
5.36
1.60
4.52
4.52
4.42
4.2
4.44
1.9
4.1
4.70
3.84
3.46
4.09
4.14
4.38
4.32
4.44
3.78
3.60
2 0.267
6 1.67
3 0.557
n*
1.33
2 0.913
5
9.57
4 0.56
2 3.35
4 1.005
5
4.33
4 0.549
6 0.766
1
0.685
3 0.571
5
0.580
2 0.237
n*
0.121
7 1.007
4 1.042
1
0.314
4 1.054
3 0.535
3 1.783
6 2.037
4 3.821
1 0.248
1
1.166
2 0.612
6 5.01
5
0.741
6 1.260
3 0.505
3 0.393
4 0.462
3 0.475
4 0.903
4 2.238
6 0.874
6 0.676
5
2.63
n*
0.204
3 0.465
3 0.904
2 0.820
4 1.175
