Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

Increasing P drives the reaction toward the left, the
state of lesser volume. Therefore, greater concentra-
tions of volatiles can be forced into a melt at greater P.
On the other hand, increased T favors an expanded
state of greater volume; hence, the equilibrium shifts to
the right and the concentration of dissolved volatiles in
the melt is less at greater T. Thus, changes in P and T
have opposite effects on volatile solubility, but the ef-
fect of P dominates.
Water. Experimental data in Figure 4.7 show the sub-
stantial increase in water solubility with respect to in-
crease in P. On a weight percentage basis, the solubility
of water in silicic melts is slightly greater than in mafic
melts. The solubility curves in Figure 4.7 indicate the
maximal amount of water that can be contained within
the indicated melt at a particular P and T. The curves
can also be considered as saturation curves in that a
melt containing a lesser concentration of dissolved wa-
ter than the solubility value at a particular P is water-un-
dersaturated. On the other hand, a melt containing an
excess concentration is water-saturated and the magma
system consists of this saturated melt plus a separate
phase of fluid water. The analogy with the concept of
silica saturation (Section 2.4.4) should be apparent.
Many naturally occurring melts, particularly at their
site of generation from solid source rock, are probably
undersaturated in water.
Early investigations indicated that the solubility of
water in a melt is approximately proportional to
(P
H
2
O
)
0.5
, suggesting that H
2
O is dissolved in a melt ac-
cording to the reaction
4.3 H
2
O O
2
2(OH)
in melt in melt
Silicate Melts and Volatile Fluids in Magma Systems
77
Special Interest Box 4.1 Experimental determi-
nation of water solubility in a melt as a func-
tion of P and T
Rock powder or other chemical constituents of the
desired composition together with water in known
weight proportions are placed in an inert metal (Pt,
Pd, or Au) foil capsule, which is welded shut and
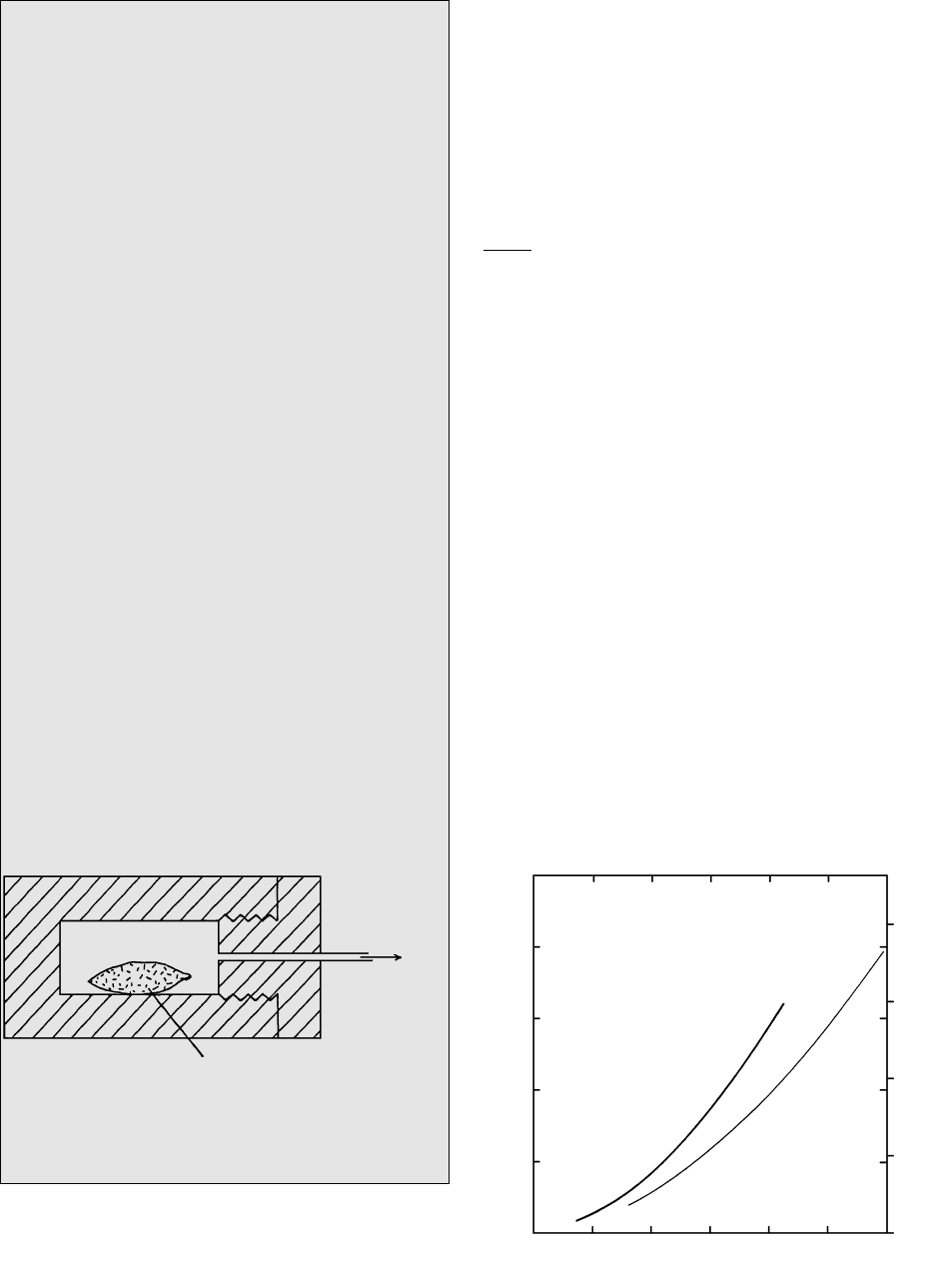
placed in a hydrothermal pressure vessel (Figure 4.6).
This vessel or “bomb” consists of a hollow cylinder of
high-strength alloy steel into which an end plug is
screwed. The plug is fitted with steel tubing to carry a
fluid (usually inert gas such as Ar) under pressure
from a pump. The bomb is placed in a furnace, at the
desired T, then the fluid pressure is raised to the de-
sired value. Inside the bomb, the fluid bears against
the flexible walls of the impervious foil capsule to
yield the desired confining pressure, P, on the mate-
rial inside. After sufficient time has passed for a state
of equilibrium to be attained within the capsule, it is
very rapidly cooled, or quenched, to room T in less
than a minute or so by dropping the bomb into a
bucket of water or by directing a blast of cold com-
pressed air at it. Any melt present in the capsule is
quenched to glass. If the concentration of water in the
capsule system exceeds the solubility in the melt at the
particular P and T of the experiment, so that the sys-
tem is oversaturated, a separate water phase exists in
the melt. Upon rapid quenching, bubbles of fluid
water are frozen into a glass as vesicles. If the con-
centration is less than the solubility, so that the sys-
tem is water-undersaturated, no vesicles are present.
By making a number of experimental runs at differ-
ent P, T, and X
water
, the solubility can be mapped
out as a function of P and T to yield a diagram like
Figure 4.7.
To
pump
Capsule containing
rock material plus water
Steel bomb
Pressure fluid
4.6 Schematic section through a hydrothermal pressure vessel,
or steel “bomb,” used to synthesize minerals and deter-
mine volatile solubilities in melts under elevated P and T.
the volume of the single-phase homogeneous melt con-
taining dissolved fluid is less than that of a two-phase
system of melt plus fluid, or
4.2 V
volatile-rich melt
(V
volatile-poor melt
V
volatile fluid
)
0246
Concentration of dissolved water (wt. %)
2.5
2.0
1.5
1.0
0.5
0
P (kbar)
0
2
4
6
8
Depth (km)
Water-undersaturated melt
Mid-ocean ridge basalt melt 1200°C
Rhyolite 850°C
Water-saturated
melt + water
(water oversaturated
magma system)
4.7 Solubility of water in silicate melts. (Redrawn from Moore et
al., 1998.)
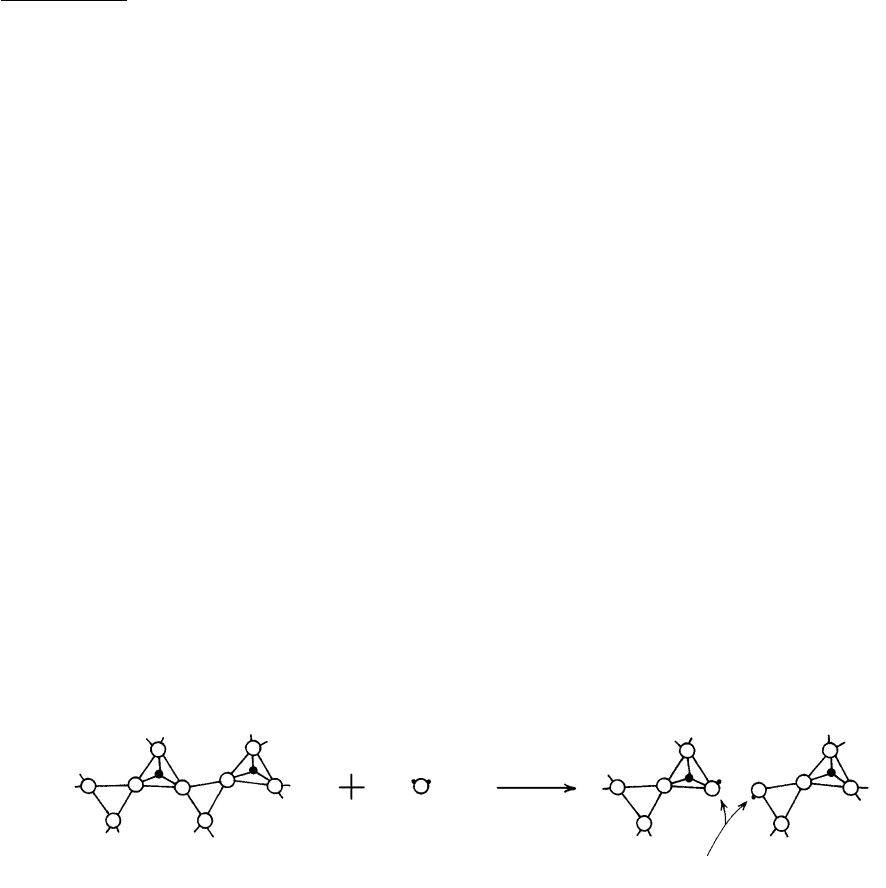
This solution process breaks silicate polymers by sub-
stituting the two hydroxyl ions for one bridging oxygen
in the melt (Figure 4.8). Even small amounts of water
by weight drastically depolymerize a silicate melt. This
effect follows from the large difference between the
weight per mole of water and the weight of condensed
constituents (Si, Ca, Fe, etc.) of silicate melts.
Continuing experimental investigations confirm that
water solubility is more complicated than suggested by
equation 4.3 (McMillan, 1994). Thus, infrared spectro-
scopic analyses indicate that an increasing proportion of
water at increasing P is dissolved in rhyolitic melts as
molecular H
2
O rather than (OH)
(Figure 4.9). The
maximal concentration of dissolved (OH)
is about
2 wt.%.
Other Volatiles. Other volatile species are generally
less abundant in magma systems than water but can,
nonetheless, be significant (Carroll and Webster, 1994).
Carbon dioxide is generally less concentrated than
water in melts, except in kimberlite, carbonatite melts,
and possibly mid–ocean ridge basalt. Like that of
water, carbon dioxide solubility in silicate melts in-
creases with increasing P and decreasing T but is about
an order of magnitude less than that of water at com-
parable P (Figure 4.10). Solubility also generally in-
creases with decreasing silica concentration and, in
some melts at high P, with increasing water content.
Infrared spectroscopy suggests that in silica-poor melts,
carbon dioxide is dissolved as (CO
3
)
2
ions, whereas
in silicic melts it is dissolved as CO
2
molecules. Appar-
ently, the dissolution mechanism depends upon the de-
gree of polymerization of the melt (Holloway and
Blank, 1994). Carbon dioxide cannot combine with
bridging oxygens in a polymerized silicic melt as water
does. In CO
2
-saturated water-bearing melts, the sepa-
rate fluid is a mixture of CO
2
and H
2
O, even though
the melt may not be water-saturated.
Precipitates of native sulfur and noxious sulfurous
fumes, such as “rotten-egg” gas, around volcanic vents
testify to ubiquitous sulfur in magmas. In most cases,
its concentration, generally less than a few thousand
parts per million, is exceeded by that of water and only
barely by that of carbon dioxide in some magmas.
Nonetheless, some calc-alkaline magmas contain signif-
icant concentrations. Sulfur solubility depends strongly
on the composition and oxygen fugacity of the magma,
in addition to P and T. Sulfur dissolves as the reduced
sulfide ion, S
2
, in generally water-poor ultramafic and
mafic magmas but as the oxidized sulfate ion, (SO
4
)
2
,
in generally more water-rich intermediate to silicic
magmas. Fluid species in reduced and oxidized systems
are H
2
S and SO
2
, respectively. In the reaction
4.4 S
2
2O
2
(SO
4
)
2
the activities of sulfide and sulfate species dissolved in
a melt are related to the square of the oxygen fugacity.
If this fugacity is below (less than) the quartz-fayalite-
magnetite (QFM) buffer (Figure 3.14), the equilibrium
shifts to the left, reducing the sulfur. Above the buffer,
it shifts to the right, oxidizing it. Another complication
is that in reduced mafic magmas an immiscible sulfide-
iron-copper melt, which depresses the concentration of
S in the melt, can form. In some silicic magmas that are
more oxidized, sulfate ions can combine with Ca, if its
activity is sufficient, to form stable anhydrite (CaSO
4
),
which also depresses the sulfur content of the coexist-
ing melt. Removal of the immiscible sulfide melt or the
anhydrite crystals from the magma will diminish the re-
maining amount of sulfur in it.
Although not generally abundant, concentrations as
much as several weight percentages of fluorine have
been found in some silicic glasses. Experimentally mea-
sured solubilities in melts can be as much as 10 wt.%;
the higher solubilities occur in hydrous melts. The
exact way in which fluorine is dissolved in silicate
melts is uncertain and probably complex, but, by what-
ever means, F causes substantial depolymerization. Al-
though the effect of P on F solubility is poorly known,
reduced P does not reduce the F solubility to the ex-
tent it does that of water (Carroll and Webster, 1994,
p. 262). Whereas at 1 atm granitic melts contain only
about 0.1 wt.% water, some simple model Na-Ca-Al-
Si-O melts contain as much as 10 wt.% F.
78 Igneous and Metamorphic Petrology
Si−O polymer in
anhydrous melt
Water
molecule
Broken Si−O polymer
in hydrous melt
(OH)
−
4.8 Some dissolved water in silicate melts forms hydroxyl ions, (OH)
, which break O-Si-O polymers, reducing the degree of polymeri-
zation.

The maximal solubility of Cl in silicate melts ap-
pears to be less than about 2 wt.%. Cl probably bonds
with network-modifying cations, especially Na, K, and
Fe. If the magma is water-saturated so that a separate
aqueous fluid coexists with the melt, Cl partitions
strongly into the aqueous fluid phase, in which the
presence of Cl
ions enhance metal solubilities.
Still other volatiles have solubilities in melts gener-
ally less than hundreds of parts per million and are not
considered further here, except to note that dissolved
phosphorus and boron appear to enhance water solu-
bility.
4.2.3 Exsolution of Volatiles from a Melt
As magmas leave their upper mantle or lower crustal
source, rise to shallower depths and cool, their melts
can become saturated. Once saturated, the excess dis-
solved volatiles are released from the melt and separate
into a distinct coexisting fluid phase in the process
called exsolution or boiling.
Although the exsolution of volatile fluids from melts
is a complex phenomenon, two ideal end-member
processes that encompass the spectrum of real phe-
nomena can be recognized. Water is used as the sole
volatile to illustrate these two processes, as follows:
1. An ascending, decompressing, initially volatile un-
dersaturated melt can become saturated as P
decreases, exsolving fluid, generally in shallow
crustal, volcanic environments. For example, in
Figure 4.11, decompression of an initially water-
undersaturated rhyolite melt at about 850°C that
contains 4 wt.% dissolved water becomes water
saturated at 1 kbar at a depth of about 4 km.
2. A stagnant, isobaric (P constant) magma that is
initially volatile, undersaturated but losing heat to
the surroundings, and cooling can become water-
saturated by crystallization of minerals such as
olivine, pyroxene, feldspar, and quartz. As these an-
hydrous minerals crystallize, the water concentra-
tion in the residual melt increases, in some cases
sufficiently to lead to saturation, overriding the ef-
fect of increasing solubility of water in the cooling
melt. This phenomenon has been called retrograde,
resurgent, or second boiling, because it occurs by
decreasing T, the reverse of the familiar cause of
boiling water. Because all magmas eventually cool,
and most consequently crystallize, more or less iso-
baric exsolution induced by crystallization can po-
tentially affect all magma systems if volatile concen-
trations and other factors are appropriate.
Exsolution of volatiles from melts is an exothermic
process that causes them to cool in the same way that
evaporation of vapor from a body of liquid water
causes cooling. In addition to this cooling by exsolu-
tion, ascending and decompressing magmas also cool
as a result of adiabatic expansion of the melt and fluid
phases. The total refrigeration of a magma system
might be as much as hundreds of degrees in some sys-
tems. An example is afforded by kimberlite magmas
which are unusually rich in CO
2
and H
2
O that exsolve
relatively deep in the crust. Field relations indicate that
pipelike bodies of kimberlite breccia were apparently
emplaced in the shallow crust at no more than 200°C
Silicate Melts and Volatile Fluids in Magma Systems
79
0
0
2
4
6
8
24 6810
(OH)
−
and molecular H
2
O (wt. %)
Total concentration of dissolved water (wt. %)
molecular H
2
O
(OH)
−
1000°C
(OH)
−
600°C
4.9 Dissolved water in rhyolitic melts exists in ionic, (OH)
, and
molecular, H
2
O form. The concentration of (OH)
is depen-
dent upon T, but molecular H
2
O concentration is independent
of T. For example, in a melt at 1000°C that contains 4 wt.% to-
tal water, about half is dissolved as (OH)
ions and half as
molecular H
2
O. For a 1000°C melt containing 2 wt.% total
water, about 1.5 wt.% is dissolved as (OH)
and 0.5 wt.% as
molecular H
2
O. (From Silver et al., 1990.)
5
4
3
2
1
0
0
0.2 0.4 0.6
P (kbar)
4
0
8
12
16
Depth (km)
Total concentration of dissolved CO
2
(wt. %)
Undersaturated
melt
Rhyolite
Tholeiitic basalt
Basanite
CO
2
saturated
melt + CO
2
(CO
2
oversaturated
magma system)
4.10 Solubility of carbon dioxide in some silicate melts. Note that
more CO
2
can dissolve in less polymerized mafic and espe-
cially silica-undersaturated melts. (From Holloway and Blank,
1994.)
or so because of minimal thermal effects on wall rock
and the absence of thermal conversion of rare inclu-
sions of coal into coke.
After solidification, only a part of the volatiles ini-
tially dissolved in the melt is preserved in the mag-
matic body as Cl and F in accessory apatite and S in
accessory sulfides, together with H
2
O in micas, amphi-
boles, and apatite. Volatiles can be preserved in glass
formed by quenching of melt.
Interactions of Volatile Species: Composition of Ex-
solved Fluids. In most magmas, several volatile species
are dissolved in the melt. These species do not neces-
sarily exsolve individually in pure form in some sort of
sequence depending on their contrasting concentra-
tions and solubilities. Rather, interactions between
species are widespread and, because of substantial mu-
tual solubilities, exsolved fluids are generally mixtures
of two or more species.
Because of its order-of-magnitude lower solubility,
CO
2
tends to exsolve from melts at greater depths
and at lesser degrees of crystallization than does wa-
ter. Even though a melt is undersaturated in water,
small concentrations are dissolved in any exsolved
CO
2
. These mixed CO
2
-H
2
O volatile bubbles can rise
through the body of magma and collect at the top of
the chamber. Or the mixed fluid may escape entirely
from the magma chamber. In either case, the water
content of the initial magma is reduced.
Significant concentrations of sulfur are partitioned
into an aqueous fluid exsolved from silicic melts (Kep-
pler, 1999). S partitioning is governed mostly by oxy-
gen fugacity, much less by P and T. Under reducing
conditions where H
2
S is stable, an order of magnitude
more sulfur is sequestered in the fluid compared to the
fluid in equilibrium with oxidized magmas in which
SO
2
is stable. During the June 1991 explosive activity
of Mount Pinatubo in the Philippines, the 17 10
9
kg
(17 megatons) of SO
2
released into the atmosphere was
one to two orders of magnitude more than the total
amount of S that could have been degassed out of the
erupted volume of magma. The outgassed amount of
S is based on analyses of the concentration of S (60–
90 ppm) in melt inclusions entrapped in growing
phenocrysts in the preeruption magma that was subse-
quently blown out of the volcano. Calculations (Prob-
lem 4.5) show that the 17 10
9
kg could have reason-
ably been derived by scavenging S from throughout a
40–90 km
3
volume of magma beneath the volcano (in-
dicated by seismic data), rather than just the 5–10 km
3
erupted. If the calculations are creditable, it is also nec-
essary to assume that long-term migration of aqueous
fluid bubbles containing the scavenged S had occurred
and this fluid had accumulated in the upper part of the
magma chamber, which furnished most of the erupted
material.
4.3 CONSEQUENCES OF FLUID
EXSOLUTION FROM MELTS
Exsolution of volatiles in magma systems plays a sur-
prisingly varied role in geologic phenomena (Figure
4.12). Some of these occur in the shallow crustal vol-
canic environment, where the magma system can vent
to the surface and interact with atmospheric gases.
Other phenomena occur in confined plutonic magma
systems in the deeper crust, where interactions with
meteoric groundwaters are possible.
4.3.1 Explosive Volcanism
Exsolving and expanding volatiles provide the driving
force for explosive volcanic eruptions.
Hypothetical Model. This fundamental concept can be
illustrated by a hypothetical magma system that is rising
adiabatically through the crust (Figure 4.11; see also
Problem 4.6). The model system, which contains
4 wt.% dissolved water in the melt as the sole volatile,
is assumed to be closed, whereas few real systems are
truly closed. Equilibrium is assumed to prevail between
all phases, whereas in real systems, especially highly vis-
cous silicic magmas, sluggish kinetic properties retard
attainment of equilibrium (discussed later). As the
magma decompresses during ascent, the initially water-
undersaturated melt becomes water-saturated and be-
gins to exsolve water at P P
water
1 kbar at a depth
80 Igneous and Metamorphic Petrology
0
0
2
46
8
0
2
4
6
8
1
2
P (kbar)
Concentration of water (wt. %)
Depth (km)
P = P
W
Water undersaturated melt
Melt
System
Magma
(Water oversaturated
magma system)
Water
saturated
melt + water
Solubility curve
4.11 Evolution of a hypothetical closed magma system during de-
compression from an initially water-undersaturated state. The
initial magma is a crystal- and bubble-free melt at 1.6 kbar,
corresponding to a depth of 6 km, and contains 4 wt.% dis-
solved water and no other volatiles.
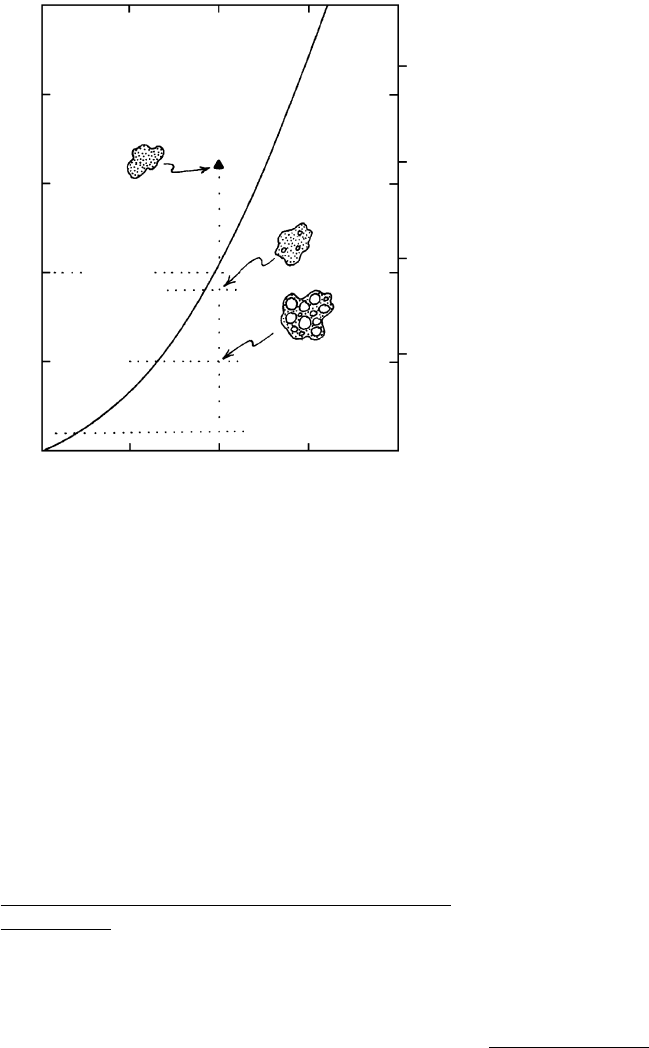
near 4 km. During continued ascent to lower P, the melt
and the magma system follow increasingly divergent
paths in the diagram. The closed water-oversaturated
magma system (magma plus separate water fluid phase)
tracks straight down the diagram along the constant
4 wt.% water line. The water-saturated melt, on the
other hand, moves down the solubility (saturation)
curve. The concentration of water that can be held in
solution in the melt decreases at lower pressures, so wa-
ter exsolves, forming bubbles of compressed steam. At
about 0.9 kbar, the melt contains about 3.6 wt.% water;
the remaining 0.4 wt.% in the closed magma system has
exsolved into the separate fluid phase. As P continues
to decrease, more water exsolves and the mass of water
in the separate fluid phase increases relative to that of
dissolved water in the melt. At 0.5 kbar, about 2.6 wt.%
water is still dissolved in the melt and 1.4 wt.% resides
in many, larger bubbles. At 0.1 kbar, 1 wt.% water is
in the melt and the remainder is in bubbles.
Not only does the mass of exsolved water increase
during adiabatic decompression in this model closed
magma system, but each mass unit also experiences a
tremendous increase in volume. Two factors are re-
sponsible for this volumetric expansion:
1. The volume of the exsolved water is much greater
than the partial molar volume of the same mass of
dissolved water in the melt: 99.3 versus 22.3 cm
3
/
mole for water in a NaAlSi
3
O
8
-H
2
O system at
950°C and 1 kbar.
2. The exsolved water expands at lower pressure ac-
cording to the perfect gas law, PV nRT. In our
model, the mass of the exsolved water has in-
Silicate Melts and Volatile Fluids in Magma Systems
81
reservoir
Gas contribution
to atmosphere
and hydrosphere
Influence on
global climate
Fumarole
Hydrothermal
alteration and
ore deposits
Magmatic
water
Breccia
Geothermal
Hot
spring
Fumarole
column
eruption
Explosive
4.12 Schematic geologic cross section through an active magmatic system showing phenomena associated with the exsolution of volatiles from
magma. An explosive eruption column of gas and pyroclastic material rises above the vent of a volcano built on an irregular erosion sur-
face (dotted line) carved into older rock. Fumaroles vent gases on the flanks of the volcano. Volcanic gases contribute to the atmosphere
and hydrosphere of the Earth and influence its global climate. Roof rocks overlying the multiple intrusive bodies have been brecciated.
Large cells of advecting water (long lines with arrow barbs) move through fractures and other open channels in the wall rocks around
the cooling intrusions, which provide heat to drive the advection. Meteoric groundwater is heated near the intrusions, expands, and rises
as cooler water moves inward to take its place, is heated, and so on. Juvenile, or magmatically derived, water is expelled from the cool-
ing magma bodies (heavy arrows). Heated meteoric and/or juvenile water constitutes a resource potentially capable of providing geo-
thermal power. Also, because the heated water carries dissolved Si, Ca, Fe, Cu, Au, Pb, and other material, it is a hydrothermal solution
that can precipitate potentially valuable ore minerals as well as quartz and other uneconomic minerals in veins and alters the rocks
through which it percolates (double-line arrows).

ent, nonexplosive lava flow. Vesicles in the solidified
lava, which define vesicular fabric, are remnants of the
gas bubbles. On the other hand, in a highly viscous sili-
cic magma cooling and crystallizing in a shallow crustal
chamber, and perhaps decompressing, slow release of
dissolved volatiles from the highly polymerized melt
could lead to a state of disequilibrium in which the
volatile pressure in the magma system exceeds the con-
fining pressure on it so that it is an overpressured sys-
tem. In other words, water exsolution lags behind that
dictated by decreasing P (Figure 4.11). Slow release of
volatiles is exacerbated by the fact that as water exsolves
the melt becomes more polymerized and more viscous
so that the release is further decelerated. Overpressured
systems can rupture the overlying roof rocks, as a lid on
a pressure cooker can fail. Or the system might, for an-
other reason, suddenly be unroofed, as at Mount Saint
Helens in 1981, when a moderate earthquake shook the
oversteepened volcano summit, causing it to slide off
the top of the bulging magma chamber. Whether the
overlying load of roof rock is removed or ruptures, the
magma is suddenly decompressed, as is a can of soda
pop from which the lid is removed. Overpressured bub-
bles of volatile fluid rupture their intervening melt
walls, producing fragments of melt that quench to form
vitroclasts, plus possible phenocrysts and phenocryst
fragments, or phenoclasts (Figure 4.13). All these bits
and pieces of the former coherent magma, together with
possible fragments of rock torn from the explosive con-
duit and vent, are collectively called pyroclasts; a de-
posit made of them has pyroclastic fabric.
Such overpressured volcanic systems illustrate that
the pressure of a magma system cannot be assumed
equal to the confining pressure, P, evaluated from the
geobaric gradient (Section 1.2). In this case, the con-
fining pressure due to the load of overlying rock, P, is
less than fluid pressure, P
f
, in the magma system. Burn-
ham (1985) showed that exsolution and expansion of
2 wt.% water from a crystallizing silicic magma at
depths of no more than a few kilometers have the ca-
pacity to do PV work, rupturing the roof rocks overly-
ing the magma body. In other words, P
f
P strength
of roof rocks. As rocks fracture, openings are created,
decompressing the magma system, leading to further
exsolution and, in some instances, explosive venting of
the gas-charged magma. Fracturing creates breccia—
rock fragments ranging widely in size but commonly
several centimeters in diameter—and brecciated fabric.
Void spaces between fragments serve as channels for
advective heat transfer and migration of hydrothermal
solutions and provide openings for deposition of met-
als from them (Figure 4.12).
4.3.2 Global Atmosphere and Climate
There is wide consensus among geologists that the at-
mosphere and hydrosphere of the Earth were pro-
creased during decompression; that is, the number
of moles of water, n, has increased. Because RT re-
mains essentially constant and P has decreased,
V increases accordingly, hundreds of times (Figure
4.4). It is not surprising that relatively small vol-
umes—on the order of 1–10 km
3
—of erupting hy-
drous magma can generate the gigantic cauliflower
clouds of ash-laden steam rising tens of kilometers
above a volcano that are familiar hallmarks of
countless climactic explosive eruptions, such as
those of Mount Saint Helens and Mount Pinatubo.
This compounding of factors—continued exsolu-
tion and expansion of the exsolved water—reduces the
density of the magma, promoting, at least, extrusion of
buoyant, bubble-bearing magma as a lava flow, but, in
many instances, leading to explosive eruption.
Real Magma Systems. Of course, in real, generally
open, magma systems, exsolution is a complex inter-
play of many factors, including decreasing P and T, ini-
tial volatile concentration in the melt, types of volatile
species, changing solubilities, and interactions with the
atmosphere and surrounding wall rocks. These factors
modify the details of explosive eruption, even though
the tremendous volumetric expansion of the magma
still occurs.
Most magmas cool and crystallize en route to the
surface, augmenting exsolution due to decompression.
Most magmas do not behave as perfectly closed sys-
tems. Every volcano vents gas before and after explo-
sive events. Not all of this gas is juvenile, that which re-
sides in the melt from its place of origin in the deep
crust or upper mantle. Some vented gas may be heated
meteoric groundwater derived from atmospheric pre-
cipitation, and some may be atmospheric gases. Mix-
tures of all these fluids are typical.
Ample evidence indicates that many magma bodies,
especially silicic ones, have higher concentrations of
volatiles, especially water, in their upper part than in
their lower. Hence, eruptions are initially highly explo-
sive as the uppermost, volatile-rich part of the magma
chamber is tapped, but as eruptions continue, they
tend to be less explosive as less volatile-rich magma is
erupted.
Additional, often very significant factors in volcanic
eruptions are kinetic. The most important of these ki-
netic factors are the viscosity of the melt and the rate of
ascent of the magma body. These two factors can con-
spire to cause different dynamic scenarios. On the one
hand in a slowly ascending and decompressing low-
viscosity mafic magma, exsolving volatile fluid can read-
ily segregate into bubbles that may be able to escape
from the magma into openings in the surrounding wall
rocks or escape relatively harmlessly out of a vent. Such
magma might extrude from a volcanic vent as a coher-
82 Igneous and Metamorphic Petrology
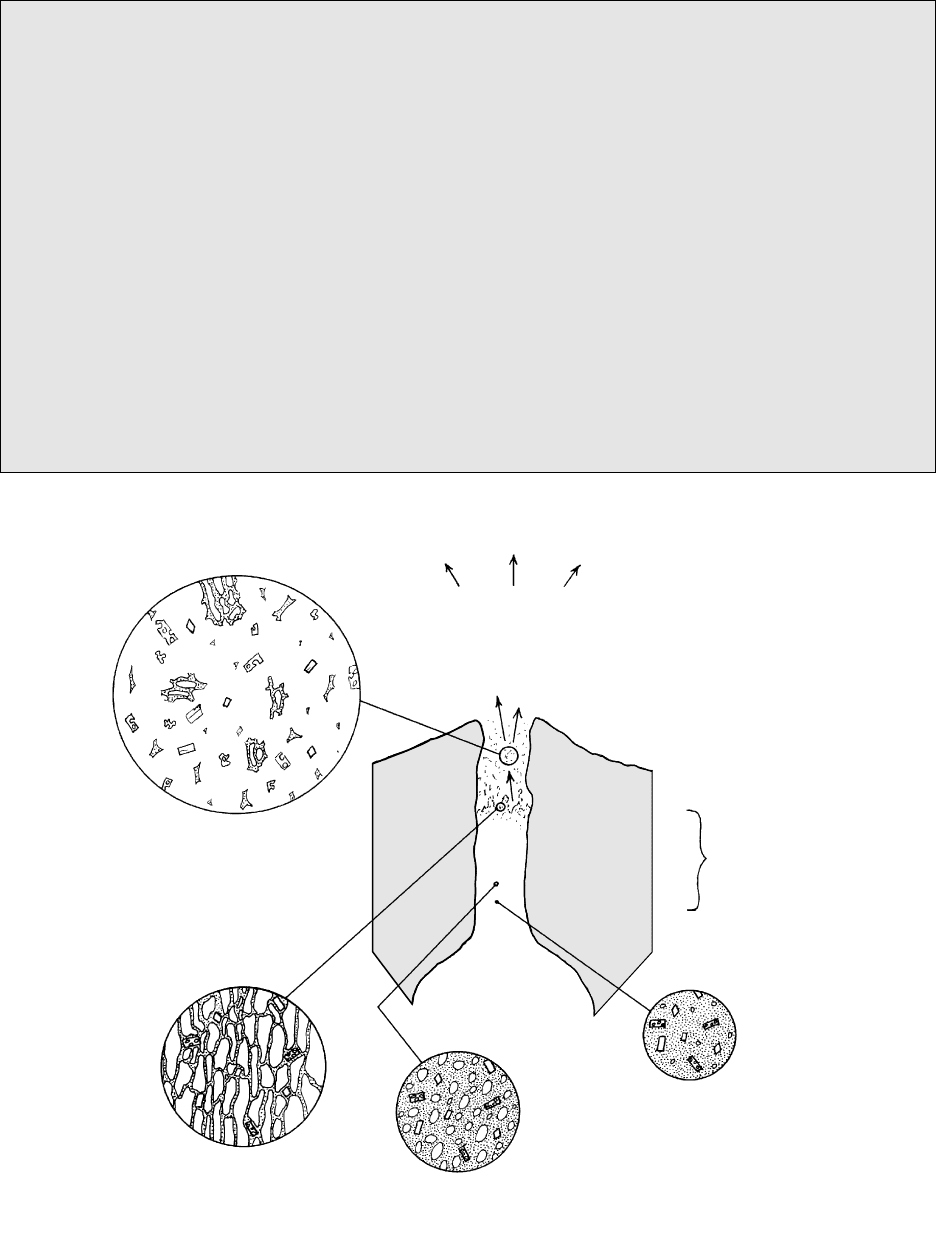
Silicate Melts and Volatile Fluids in Magma Systems
83
DISPERSED
PYROCLASTS IN A
GREATLY EXPANDED
CONTINUOUS GAS PHASE
Unstable foam
with overpressured
bubbles (+ crystals)
Larger, more abundant
bubbles (+ crystals) in
melt
Sparse, small bubbles
(+ crystals) in melt
DISPERSED
BUBBLES IN A
CONTINUOUS
MELT
BUBBLE -
FREE MELT
+ CRYSTALS
Pyroclasts consisting of
(1) euhedral phenocrysts
(2) irregular, exploded
phenoclasts
(3) exploded vitroclasts
4.13 Schematic cross section through the volcanic conduit of an exploding magma system. Circular diagrams are “snapshots” of the state of
the expanding magma as a function of depth and P in the conduit. Because of limitations of the diagram area, the hundreds-fold expan-
sion of the volatile fluid phase cannot be accurately represented.
Special Interest Box 4.2 Volcanic hazards,
monitoring, and forecasting
A very brief comment will serve to draw atten-
tion to the impact of volcanoes on human society, the
focus of a rapidly expanding discipline that is cov-
ered in numerous recent articles and books (e.g.,
Decker and Decker, 1998). Tilling (1989) estimates
that since 1000
A
.
D
. more than 300,000 people have
lost their lives directly or indirectly as a result of vol-
canic debris, lava, and pyroclastic flows and associ-
ated tsunami. For example, a small eruption at the
5321-m summit of the Nevado del Ruiz volcano in
Colombia in 1985 melted part of the icecap, creating
a disastrous muddy debris flow that killed about
23,000 people downslope. Financial burdens can be
staggering even in the absence of fatalities; the cost
to repair engines on one jumbo-jet aircraft that in-
advertently flew into an ash cloud from the 1989
eruption of Redoubt volcano, Alaska, exceeded $80
million.
Monitoring and forecasting eruptions of active
and dormant volcanoes are obviously high priorities
for both scientists and government officials. In addi-
tion to seismic and geodetic (tilt) monitoring, remote
sensing from aircraft and satellites and direct sam-
pling of emitted gas at volcanic vents, especially CO
2
and SO
2
, have proved to have significant potential
(Symonds et al., 1994). Variations in the flux of a
particular gas species during eruptive waxing and
waning appear to be related to interactions with the
surrounding hydrothermal system and to sealing,
pressure buildup, and failure of the cap rock overly-
ing a shallow magma body. Presence of vesicular glass
in the vented ash, as contrasted with rock fragments
torn from vent walls, implies tapping of a juvenile
mass of volatile-saturated melt potentially capable of
exploding.
duced over the 4.5 Gy of its evolution by degassing of
the interior through generation of volatile-bearing
magmas and varying degrees of exsolution of these
volatiles during magma ascent and cooling.
There is a correlation between some types of volcan-
ism and global climate, as first suggested by Benjamin
Franklin, the early American statesman and scientist.
After the eruption of 12.3 km
3
of basalt lava and about
0.3 km
3
of ash at Laki (Lakagígar), Iceland, beginning
June 1783 (Francis, 1993; Rampino et al., 1988),
Franklin noted a persistent “dry” fog and faint sun dur-
ing the severe 1783–84 winter in Europe and eastern
North America. In contrast to this predominantly lava
eruption, the Tambora, Indonesia, eruption in April
1815 was probably the largest ash-producing eruption
in the past 10,000 years. Its eruption column of gas and
pyroclastic material may have reached a height of 50 km
from an estimated 50 km
3
of pyroclastic material, caus-
ing darkness for as much as 2 days 600 km away. The
following year, 1816, was the notorious “year without a
summer” when mean temperatures in Europe were
about 1°C cooler and repeated summertime frosts in
New England caused devastating crop failures.
The contrasts between the Laki and Tambora erup-
tions suggest that the amount of ash inserted into the
atmosphere cannot be responsible for global climate
change. The real culprit—sulfur—was only identified
after predominantly ash eruptions at Mount Saint
Helens, Washington, in 1980 and at El Chichón, Mex-
ico, in 1982. Although of similar, small volume, about
0.35 km
3
each, the global cooling effect in the north-
ern hemisphere from Mount Saint Helens was nil, but
that of El Chichón was about 0.5°C. The erupted El
Chichón magma was unusually rich in sulfur; con-
tained as much as 2.5 wt.% total SO
2
; included phe-
nocrysts of pyrrhotite (Fe
1x
S) and anhydrite (CaSO
4
);
and injected about 10
13
g of H
2
SO
4
into the strato-
sphere, about one hundred times more than Mount
Saint Helens. The magma in the 1991 eruption of
Pinatubo in the Philippines was similarly sulfur-rich,
contained anhydrite phenocrysts, and produced about
twice as much stratospheric H
2
SO
4
as El Chichón, but
an order of magnitude less than that estimated for
Tambora and Laki.
Therefore, more important than dust-sized ash par-
ticles, which fall to the ground in a few months, are
acid aerosols. In the stratosphere, about 25 km above
the ground, SO
2
reacts with (OH)
, created by photo-
dissociation of water vapor, to form micrometer-size
droplets of sulfuric acid, H
2
SO
4
. This aerosol, which
continues to form for several years after an eruption, is
not washed out by atmospheric precipitation to form
acid rain, as is the 10–20 times greater amount of
man-made SO
2
injected into the lower atmosphere
(Symonds et al., 1994). The H
2
SO
4
aerosol absorbs
and backscatters incoming solar radiation, heating the
stratosphere but restricting normal solar heating of the
atmosphere.
4.3.3 Fumaroles, Hydrothermal Solutions, Ore
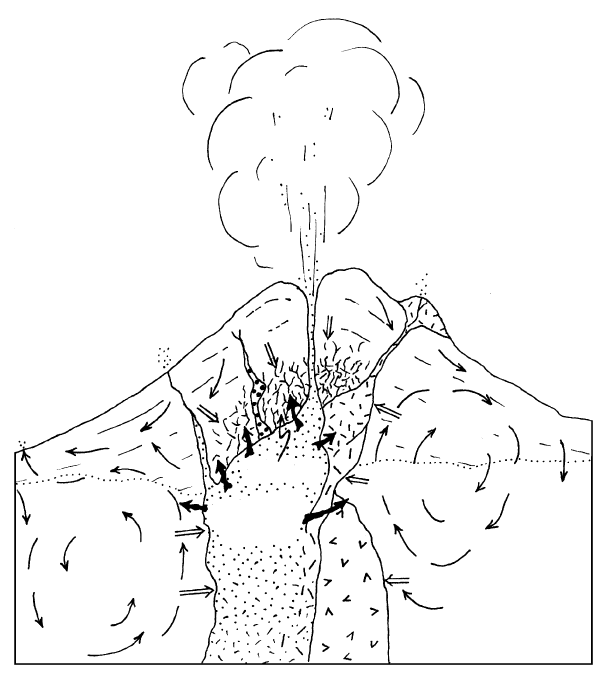
Deposits, and Geothermal Reservoirs (Figure 4.12)
Exsolved volcanic gases can vent to the atmosphere in
large volumes at a rapid rate in explosions, or much
more slowly but in at least comparable volumes over
many years from fumaroles (called solfataras if sul-
furous). Fumaroles can be located at a summit crater or
on the flank of an active volcano and in recently em-
placed extrusions of lava and pyroclastic material.
Cooling intrusive magma heats adjacent wall rock and
any meteoric groundwater included (Figure 4.12). Hot
fluids exsolved from the magma can mix with meteoric
water, both surface water and groundwater, to create
hot springs, such as the famous Yellowstone National
Park in the United States and the Wairakei area on the
North Island of New Zealand. Because of extensive
contamination with near-surface meteoric water, sea-
water, and atmospheric gases, which are highly oxidiz-
ing, the exact nature of the exsolved gas species and
their solubilities are difficult to determine. However,
thermodynamic modeling (e.g., Symonds et al., 1994)
indicates many elements are transported at 800–900°C
as chloride complexes (NaCl, KCl, FeCl
2
, ZnCl
2
,
PbCl
2
, CuCl, SbCl
3
, MnCl
2
, NiCl
2
, MoO
2
Cl
2
), and
some elements as sulfide, fluoride, and carbonate com-
plexes.
Beneath the surface of the Earth at elevated P hot
aqueous fluids called hydrothermal solutions (Barnes,
1979; Henley et al., 1984; Brimhall and Crerar, 1987)
carry many of the same elements as fumarolic gases.
These solutions are essentially brines whose total dis-
solved solids range to as much as 50 wt.%. The dis-
solved species are mostly Na, K, Ca, and Cl and lesser
amounts of Mg, Br, SO
4
, H
2
S, CO
2
, and possibly NH
3
but include concentrations as high as 1000 ppm of ele-
ments such as Au, Ag, Cu, Zn, Pb, and Mo. Fluids ex-
solved from decompressing and cooling magmas are
more or less neutral in pH, but as their T decreases, Cl-
and S-bearing species ionize and the hydrothermal so-
lutions become carriers for metals as well as developing
acid potential for wall-rock interaction. Partition of
metals such as Cu from the silicate melt into the aque-
ous fluid can be nearly complete: The fluid/melt parti-
tion coefficient is nearly infinite. Juvenile magmatic flu-
ids, as well as heated meteoric water or groundwater
and by-product fluids derived from metamorphic
reactions, are sources of hydrothermal solutions. The
oxygen isotopic composition of rocks and minerals
(Section 2.6.1) demonstrates that
18
O-enriched mete-
oric water can advect through rock openings for tens of
kilometers around intrusive magma bodies (Figure
4.14). Thermal energy released from the cooling in-
84 Igneous and Metamorphic Petrology
trusions supplies heat that powers these circulating
water systems. Hot springs (see Figure 11.17) on the
seafloor along oceanic spreading ridges are obvious ex-
amples of advective water systems driven by magmatic
heat. Because of their solvent capabilities at high T,
hydrothermal solutions advectively migrating through
large volumes of rock over tens of thousands of years
can leach significant amounts of valuable metal ions
from the wall rocks through which they percolate.
Whether derived from wall rock or partitioned into
exsolving fluids from the crystallizing melt, or both,
dissolved metals can be precipitated from hydrother-
mal solutions to form economically significant hy-
drothermal ore deposits (e.g., Guilbert and Park, 1986;
Brimhall and Crerar, 1987; Clarke, 1992). Commonly
associated with these hydrothermal deposits are tab-
ular or sheetlike veins of various minerals, such as
quartz. These owe their origin largely to the strongly
T-dependent solubilities of compounds in water. Also
associated with the deposits are surrounding, more ex-
tensive and obvious halos of hydrothermally altered
wall rock produced by thermal and chemical interac-
tion of the migrating acid fluids with their wall rock
(Rose and Burt, 1979). These altered rocks serve to
indicate the presence and character of the smaller,
more focused ore deposit. Widespread replacement of
magmatically crystallized alumino-silicate minerals—
especially feldspars—by clay minerals and other low-T
sheet silicates creates argillically altered rock. Replace-
ment of feldspars, micas, amphiboles, and pyroxenes
by epidote, actinolite, chlorite, albite, and calcite cre-
ates propylitically altered rocks.
Another economically significant resource related to
magma systems is the geothermal reservoir; this con-
sists of a large volume of underground supercritical wa-
ter, largely if not entirely of meteoric origin, lodged in
open spaces within the rock, which can be tapped and
used to drive electric power turbines. Geothermal re-
sources have been developed in regions of young—
generally 1 Ma—volcanism because associated un-
derground bodies of unerupted magma that are invari-
ably present have not cooled sufficiently for the ther-
mal energy to be dissipated.
SUMMARY
Magma is high-T mobile rock material that generally
includes crystals and always includes melt, a silicate
liquid solution that contains dissolved volatiles, chiefly
H
2
O and many other compounds of H, C, O, and S.
The kinetic properties of a magma are largely deter-
mined by the atomic structure of the melt, which re-
sembles crystalline silicates but lacks their symmetric
long-range order. Network-forming Si and Al cations
are linked to bridging oxygen anions to form polymers.
Silicic melts are polymerized to varying degrees; silicic
melts in which the ratio of nonbridging oxygens to net-
work-forming ions is small are most polymerized and
consist of three-dimensional ionic networks that pos-
sess high viscosity. In less silicic, more mafic melts,
there are more nonbridging oxygens, the degree of
polymerization is less, and melts are less viscous. Dis-
solved water and fluorine depolymerize melts, reducing
their viscosity.
Volatiles such as H
2
O, CO
2
, and SO
2
are gases at
magmatic temperatures and near-atmospheric pres-
sures but become compressed into denser fluids more
than a kilometer below the surface of the Earth, where
there is no physical distinction between a supercritical
gas and fluid. Because dissolved volatiles have smaller
partial molar volumes in melts relative to a separate
pure phase at corresponding P and T, their solubilities
in melts increase substantially with increasing P and
less markedly at decreasing T. Concentrations of sev-
eral weight percentages of dissolved water, as both
molecular H
2
O and hydroxyl ion, (OH)
, can occur in
natural melts at high P and depolymerize them because
bridging oxygens are replaced by (OH)
. Modest
weight concentrations of water translate into larger
molar concentrations because of the contrast in the
molar weight of silicate and water. Depolymerizing
fluorine, in concentrations of several weight percent-
ages, also occurs in some melts, but, unlike water, these
concentrations can be retained in the melt even at at-
mospheric P. Carbon dioxide, sulfur, and chlorine sol-
Silicate Melts and Volatile Fluids in Magma Systems
85
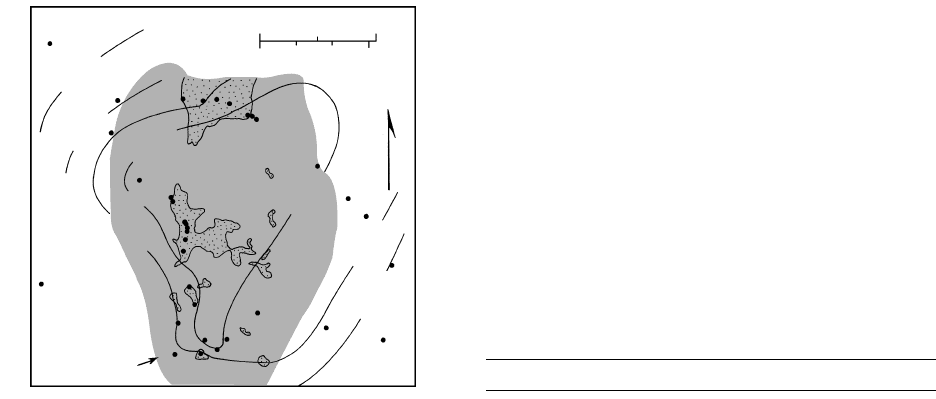
δ = 6
δ = 4
δ = 0
δ = 2
δ = 0
δ = 2
δ = 4
Sample
locality
Area of
propylitic
alteration
N
30km
0mi2
4.14 Effects of meteoric water-rock interactions around cooling
magmatic intrusions in the Bohemia mining district, Oregon.
Volcanic country rocks (unpatterned) are enriched in
16
O to-
ward dioritic intrusions (stippled) because of exchange reac-
tions between the country rocks ( 6) and heated meteoric
groundwater ( 9). Shaded area delineates propylitically
altered rocks.
18
O/
16
O (see Section 2.6.1). (Redrawn
from Taylor, 1974.)
4.9 How does volatile exsolution from magmas in-
fluence global climate?
4.10 What economic benefits accrue from volatile
fluids?
PROBLEMS
4.1 Make a schematic sketch of three different five-
phase magma systems after the style of Figure
4.1. Label the phases represented.
4.2 Verify that 5 wt.% water in a NaAlSi
3
O
8
melt is
equivalent to 15 wt.% on a molar basis. (Hint:
Convert the chemical analyses in weight per-
centage of albite [Appendix A] plus 5 wt.% wa-
ter to mole percentage by dividing each oxide
weight by its formula weight. Recalculate the to-
tal molar weight to 100.00%.)
4.3 Show from equation 4.3 why the solubility of
water, as (OH)
, should be proportional to
(P
H
2
O
)
0.5
.
4.4 In a plot of P versus concentration of dissolved
volatiles in melt draw schematic solubility
curves for CO
2
and H
2
O. If an initially volatile-
undersaturated melt decompresses at more or
less constant T, which volatile, CO
2
or H
2
O, ex-
solves first?
4.5 In the erupting 1991 Mount Pinatubo magma
assume that the partition coefficient of SO
2
is
D C
fluid
/C
melt
47, appropriate to a rela-
tively oxidized state (Keppler, 1999). From
the maximal concentration of S in the melt
(90 ppm), what was the weight percentage SO
2
in the coexisting aqueous fluid phase? If it is as-
sumed that 1 wt.% water was released from an
all-melt magma body whose volume was 90
km
3
, how many kilograms of SO
2
was released?
(Use 2.2 g/cm
3
for the melt density.) Discuss
your results.
4.6 A body of silicic melt that contains 5 wt.% wa-
ter and has a density of 2.2 g/cm
3
occupies a
volume of 1 km
3
at 1000°C in a crustal magma
chamber. What is the volume of melt plus ex-
solved water at the surface (1 atm), assuming all
5 wt.% water exsolves and T remains 1000°C?
What is the ratio of the volume of steam to melt
at the surface? What is the ratio if T 700°C
upon eruption? What is the ratio if only 4 wt.%
water has exsolved in a nonequilibrium system
at 1 atm and 700°C? Discuss your answers in
terms of the gigantic “cauliflower” clouds of ash
and steam that typically develop over exploding
volcanoes.
ubilities are about an order of magnitude less than
those of water and fluorine.
Most melts are probably initially undersaturated in
volatiles where magmas are generated in the crust or
upper mantle. However, as magmas ascend, decom-
press, cool, and crystallize, melts become volatile satu-
rated and a separate volatile fluid phase exsolves from
the melt. Ascending melts saturate in CO
2
, S, F, and Cl
at greater depth than they do in H
2
O because of their
lesser solubilty.
Exsolution of volatile fluids from magmas is associ-
ated with several significant geologic phenomena. Ex-
plosive volcanism is driven by volatile exsolution and
drastic volumetric expansion of the exsolved volatile
fluid. These processes create pyroclastic deposits and
fabrics, have contributed to the formation of the at-
mosphere and hydrosphere of the Earth throughout its
long history, and can influence global climate, chiefly
because of SO
2
ejected high into the stratosphere,
where it forms H
2
SO
4
aerosols that block solar heating
of the surface of the Earth. Volatile exsolution in shal-
low magma bodies can generate sufficient PV energy to
fracture overlying roof rocks, producing a mass of brec-
ciated rock or at least channelways for emplacement of
veins of quartz and ore minerals. Hydrothermal solu-
tions alter preexisting rocks and form ore deposits.
CRITICAL THINKING QUESTIONS
4.1 Describe the nature of magmas and the combi-
nations of solid, liquid, and gaseous phases that
are possible.
4.2 Describe the atomic structure of silicate melts
and the role played by different ions in govern-
ing the degree of polymerization.
4.3 Describe the polymerization of Mg
2
SiO
4
melt.
(You may need to consult a mineralogy text to
refresh your memory of the atomic structure of
forsterite olivine.)
4.4 Characterize volatile fluids and indicate how
they are distinguished from silicate liquids
(melts).
4.5 Describe the volatile species that are commonly
dissolved in silicate melts, their concentrations,
the factors that control their solubilities, and
their effects on the atomic structure of the melt.
4.6 Discuss how volatiles exsolve from melts and
variations in the concentrations of H
2
O, CO
2
, F,
Cl, and S in mixed volatile fluid phases exsolved
from melts.
4.7 Describe factors governing explosive volcanism.
4.8 Describe fabrics that are a direct consequence
of volatile exsolution.
86 Igneous and Metamorphic Petrology
