Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

proportion such that the bulk composition, 50 wt.%
silica, is exactly that of the initial melt. Fractional
crystallization, in contrast, has yielded an additional
crystalline phase, cristobalite. This final cristo-
balite enstatite “rock” is silica-oversaturated,
whereas the first crystals that precipitated—
forsterite—manifest an undersaturated system.
In the model Mg
2
SiO
4
-SiO
2
system, forsterite and
enstatite constitute a reaction pair that forms across
the peritectic. In real fractionating magmas containing
additional components, a reaction pair of olivine and
orthopyroxene is manifested as a reaction rim of the
latter on the former in a single grain. On a larger
scale, this reaction pair might be manifested in layers
of olivine-bearing rock overlain by orthopyroxene-
bearing rock in a layered mafic intrusion (compare
lower-left diagram in Figure 5.9). In evolving interme-
diate composition calc-alkaline magmas, a reaction re-
lation between clinopyroxene and melt produces
hornblende. Incomplete reactions of this sort create
hornblende reaction rims around anhedral, unstable
clinopyroxene in dioritic rocks (Plate II). At lower T,
hornblende may react with a more evolved melt to
yield biotite. Two or more reaction pairs might develop
in a fractionating multicomponent magma and consti-
tute a discontinuous reaction series. Many different re-
action series are possible in magmas, depending on
their bulk chemical composition and intensive vari-
ables.
Incongruent Melting. Crystallization of a melt that
contains 59.85 wt.% silica and 40.15 wt.% MgO—the
composition of enstatite—was discussed to illustrate
the principle of the reaction relation. In the reverse
sense, if crystals of pure enstatite are heated at 1 atm,
they are found to melt in an unexpected manner. In
contrast to the familiar congruent melting of ice to liq-
uid water of the same composition, incongruent melt-
ing of enstatite yields a liquid that is slightly more
silica-rich plus forsterite crystals at its melting point of
1557°C (Figure 5.8 and reaction 5.3). As more heat is
absorbed into the 59.85 SiO
2
-40.15 MgO system,
forsterite dissolves in the silicate liquid, and eventually,
at about 1600°C, melting is complete, finally yielding a
melt of the same composition as that of enstatite.
Rather than having a unique melting T point at some
particular P, as do albite, forsterite, diopside (Figure
3.8), and some other pure end-member minerals, pure
enstatite has a melting T range through which the sili-
cate liquid, which coexists with other crystals, has a dif-
ferent composition from the initial solid. As shown
later, all solid solution minerals also melt incongruently.
It may now be realized that any “rock” mixture of
diopside and anorthite crystals also melts incongru-
ently (except for the one unique eutectic proportion) in
Figure 5.4. The initial melt has a eutectic composition
regardless of the proportion of diopside and anorthite
crystals. The incongruent melting behavior of mineral
solid solutions and rocks has profound implications
not only for magma systems but for the whole Earth.
Incongruent melting has produced the global-scale
“differentiation” of the planet into a mantle and more
silica-rich, Mg-poor crust over the course of its 4.5-Gy
evolution. Small degrees of partial melting of the peri-
dotitic mantle of basically olivine and pyroxene has
generated more Si-rich, Mg-poorer partial melts form-
ing the crust.
Liquid Immiscibility. In the Mg
2
SiO
4
-SiO
2
system a
homogeneous high-T melt lying between 70 and
100 wt.% silica will split into two stable immiscible liq-
uids upon cooling below the convex upward solvus,
shown by a dashed line in Figure 5.8. For example, an
initial liquid at 2000°C that contains 80 wt.% silica
Crystal-Melt Equilibria in Magmatic Systems
97
Fo Fo + En
High
T
Low
T
Crystalline
products
61
50
SiO
2
(wt. %)
30 40 50
MgO (wt. %)
Melt composition
FRACTIONAL CRYSTALLIZATION
En + Cr
En
Fo
Decreasing T
Crystalline
products
64
61
50
SiO
2
(wt. %)
30 40 50
MgO (wt. %)
Melt composition
Liquid line
of descent
Liquid line
of descent
5.9 Crystals and liquids produced during equilibrium crystalliza-
tion (top) and fractional crystallization (bottom) of an initial
melt that contains 50 wt.% silica in the binary system
Mg
2
SiO
4
-SiO
2
at 1 atm. In the equilibrium process, forsterite
crystals appear in the initial stage of cooling and the final crys-
talline products are forsterite plus enstatite in a proportion
whose bulk composition is exactly 50 wt.% silica. During frac-
tional crystallization, the crystalline products, which are shown
here as a hypothetical gravity accumulation, are forsterite, then
enstatite, and finally enstatite plus cristobalite with decreas-
ing T. The liquid line of descent on the MgO-SiO
2
variation
diagram resulting from fractional crystallization is more ex-
tended in composition than from equilibrium crystallization.
The increase in silica and decrease in MgO of the residual melt
during either mode of crystallization displayed here are com-
mon in many natural magmas.
splits into two melts at 1900°C; one contains 72 wt.%
silica and the other 96 wt.%. The less abundant,
more silica-rich immiscible melt will form as drops in
the mass of less siliceous liquid. Because of differing
densities, the two melts could eventually segregate
into contrasting horizontal layers in the gravity field.
Subsequently, as the two immiscible melts cool below
1700°C, cristobalite precipitates from each, but in
greatly differing amounts, found from the lever rule.
It is a property of immiscible melts that the crystal-
line phases in equilibrium with each are the same,
but in different modal proportions. Liquid immisci-
bility appears to be rare in natural multicomponent
magmas.
5.4 CRYSTAL-MELT EQUILIBRIA IN
REAL BASALT MAGMAS
The two binary systems examined so far provide valu-
able insights into crystal-melt equilibria in simple,
model mafic systems. The effect of additional chemical
constituents, such as Al, Fe, and Na on the behavior of
real mafic magmas has only been hinted. Other binary
diagrams could be explored, as could three- and four-
component (ternary and quaternary) diagrams, which
are discussed at length in Morse (1980). Instead, at
this point as a “reality check” we consider crystal-melt
equilibria in two real basalt compositions. Although
these two basalts are real rocks composed of many
components, they are only two points in the wide com-
positional spectrum of all rocks (Figure 2.4). What has
been gained over the simple binary model systems by
an examination of real multicomponent systems is lim-
ited by their unique compositions.
5.4.1 Makaopuhi Basalt
Nature provided an especially instructive experiment
for petrologists in the 1965 eruption of Kilauea, a vol-
cano on the island of Hawaii, when lava partly filled
the small preexisting Makaopuhi crater to form a lava
lake. Soon after a hard crust developed on the lake,
U.S. Geological Survey petrologists drilled into its still
molten interior. Samples of magma were collected at
various depths in the lake and temperatures were mea-
sured with a thermocouple. Photomicrographs of thin
sections made of the quenched samples (Wright and
Okamura, 1977) clearly show the sequence of precipi-
tation of crystalline phases in this basaltic magma
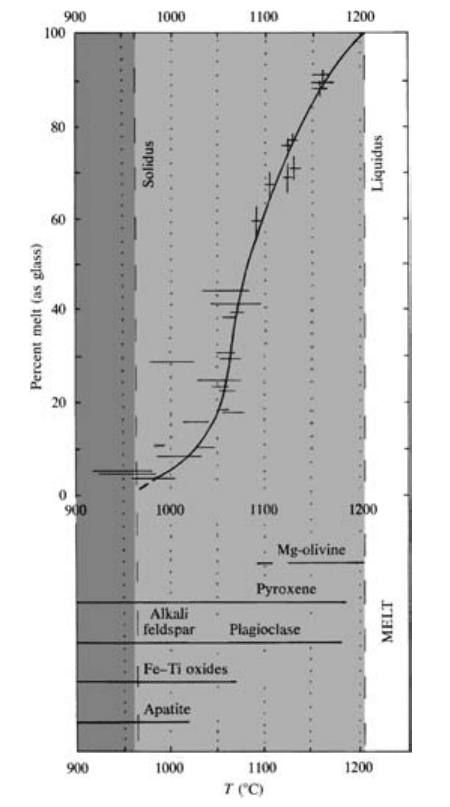
(Plate III, Figure 5.10, and Table 5.1). Each sample is
presumed to represent an equilibrium assemblage (ex-
cept as noted later) of crystals and melt at the indi-
cated T near atmospheric pressure in a closed magma
system.
The liquidus of the Makaopuhi basalt lies just above
1200°C and the solidus is at about 965°C. Throughout
most of this 235°C range of crystallization between the
98 Igneous and Metamorphic Petrology
5.10 Crystal-melt equilibria of the Makaopuhi, Hawaii, basalt at
about 1 atm. See also color photomicrographs, Plate III. Tem-
peratures, measured in drill hole, and amounts of melt, deter-
mined in thin sections as glass, are indicated by horizontal line
segments and crosses; length of line segments indicates uncer-
tainty in measured values. Heavy curve is best-fit line to data
showing how the amount of melt varies with T. Range of T
over which each mineral precipitated from the melt is shown
at the bottom. Approximate range of solid solution composi-
tions from high to low T is Fo 82 to 76 in olivine, Mg/Fe 1.2
to 0.36 in clinopyroxene, and An 71 to 30 in plagioclase. (Re-
drawn from Wright and Okamura, 1977.)
liquidus and solidus, pyroxene and plagioclase solid so-
lutions coprecipitate. In simple binary systems only one
pure crystalline phase precipitates from a melt until it
reaches a eutectic or peritectic, but with more compo-
nents in a natural magma, two or more crystalline solid
solutions can coprecipitate. The crystalline silicate
phase at the liquidus (the liquidus phase) is a Mg-rich
olivine that occurs as relatively large euhedral crystals
(Plate IIIa). At about 1180°C, much smaller, somewhat

darker clinopyroxene and colorless plagioclase crystals
begin to coprecipitate with olivine. With decreasing T,
increasing amounts of these three crystalline phases co-
precipitate. Although not visible in the photomicro-
graphs, plagioclases become more sodic and pyroxenes
and olivines become more enriched in Fe relative to
Mg: That is, the Fe/(Fe Mg) ratio increases, as the T
of crystallization decreases. Olivines below 1100°C are
corroded and embayed into anhedral shapes and there-
fore were unstable in the melt. Had there been more
time during cooling of that level of the lava lake, the re-
sorption of olivine would have been complete. Resorp-
tion of olivine into the melt with falling T is like that
seen for some compositions in the simple binary system
Mg
2
SiO
4
-SiO
2
. This confirms that the reaction relation
5.3 persists in natural mafic magmas, but only in those
of appropriate silica-saturated composition, that is,
tholeiitic basalts.
It is obvious from the changes in the color of the
glass in Plate III that the melt also changed composi-
tion as the T changed. From 1170°C to 1075°C the
glass becomes darker brown as a result of enrichment
of Fe and Ti in the residual melt. This enrichment was
created because the concentrations of these elements
are less in the crystallizing phases than in the bulk
magma. An accompanying enrichment of incompatible
Na, K, and P in the melt also occurred because the par-
tition coefficient of these elements in the coprecipitat-
ing minerals is 1 (Section 2.5.1). Ultimately, at about
1070°C, the activity of ilmenite in the melt reached 1;
that is, the melt became saturated with respect to il-
menite and it precipitated, together with magnetite.
Consequently, Fe and Ti were removed from the melt,
thus causing the color of the glass to change from red-
brown to pale gray. At about 1030°C, the melt became
saturated with respect to the phosphate mineral, ap-
atite, which then precipitated. As the residual melt be-
came sufficiently enriched in Na and K near the solidus
temperature, alkali feldspar precipitated as thin rims
on earlier-formed calcic plagioclases.
5.4.2 Basalt Magmas at High Pressures and High
Water Concentrations
Crystal-melt equilibria of naturally occurring rocks can
be determined in the laboratory by using equipment
like that used for determination of simple phase dia-
grams. However, instead of mixtures of pure reagent
compounds, finely pulverized rock is the starting mate-
rial.
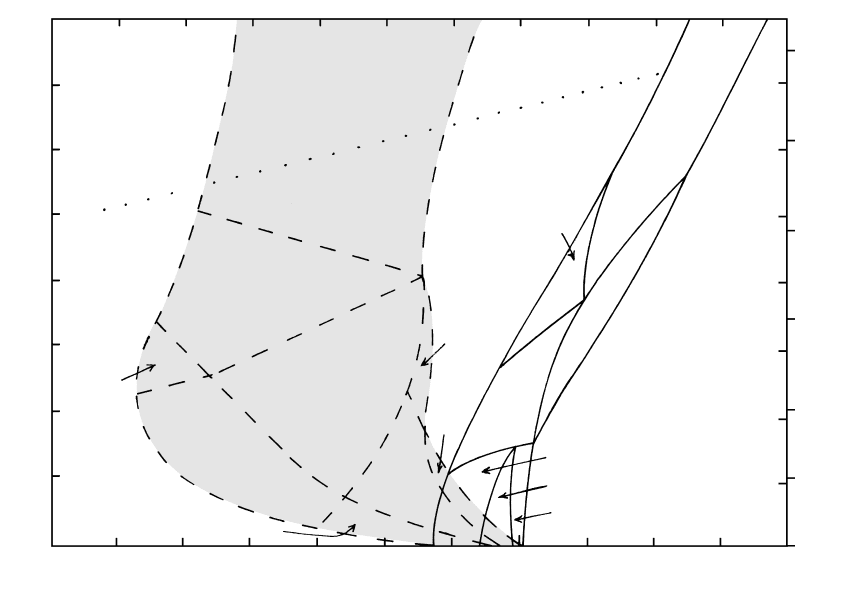
Figure 5.11 presents phase relations as a function
of P and T for a tholeiitic basalt, such as the one
listed in Table 5.1. Obviously, crystal-melt equilibria
are strikingly different for dry (water-free) and water-
saturated conditions. Relative to dry conditions, the
water-saturated liquidus and solidus are depressed
almost 600°C at 10 kbar (near the base of the aver-
age continental crust). Melting and crystallization oc-
cur over a much broader temperature range, about
400°C, in the water-saturated system, where amphi-
bole is stable over a wide range of P and T. The sta-
bility of this hydrous, aluminous phase suppresses
the stability of plagioclase, which is only stable near
the solidus at relatively low pressures in the water-
saturated system.
Figure 5.11 also reveals that mineral stability in
basaltic systems is strongly dependent on P. At P
20 kbar, corresponding to depths greater than about
70 km in the upper mantle, any basalt system—wet or
dry—is dominated by clinopyroxene and garnet solid
solutions. The garnet contains a substantial proportion
of the pyrope end member (Mg
3
Al
2
Si
3
O
12
) but also Fe
and Ca. Elements normally sequestered in plagioclase
at low P, such as Ca, Na, K, and Al, occur in the high-
P clinopyroxene solid solution known as omphacite,
which contains a substantial amount of the jadeite end
member (NaAlSi
2
O
6
). Increasing P increases the activ-
ity of sixfold coordinated Al
VI
at the expense of four-
fold Al
IV
in aluminosilicate melts because the sixfold
coordination is a more compact, or smaller-volume, en-
tity. This stabilizes Al
VI
-coordinated crystalline phases
such as garnets and aluminous jadeitic pyroxenes in
Crystal-Melt Equilibria in Magmatic Systems
99
Table 5.1 Bulk Chemical Compositions (wt.%)
and Modal Composition (vol.%) of Basalts Whose
Melting Relations Are Shown in Figures 5.10 and
5.11 and Plate III (Makaopuhi). Data from Wright
and Okamura (1977) and Green (1982).
H
IGH
-A
L
O
LIVINE
M
AKAOPUHI
T
HOLEIITE
SiO
2
50.24 49.93
TiO
2
2.65 1.34
Al
2
O
3
13.32 16.75
Fe
2
O
3
1.41
FeO 9.85 11.40t
MnO 0.17 0.18
MgO 8.39 7.59
CaO 10.84 9.33
Na
2
O 2.32 2.92
K
2
O 0.54 0.37
P
2
O
5
0.27 0.19
Total 100.00 100.00
MODE
Olivine 5
Pyroxene 51
Plagioclase 30
Fe-Ti oxides 9
Glass 5
Alkali feldspar trace
lieu of Al
IV
-coordinated feldspars. With increasing P,
calcic, more aluminous plagioclases destabilize before
sodic, less aluminous plagioclases. These phase rela-
tions demonstrate why feldspars are crustal phases and
are not normally stable in the sub-continental mantle.
The dense (3.3–3.4 g/cm
3
) high-P rock made essen-
tially of red pyropic garnet and green omphacite
clinopyroxene (Appendix A) that is of basaltic bulk
chemical composition is called eclogite.
5.5 FELDSPAR-MELT EQUILIBRIA
Because nearly all magmatic rocks contain feldspar, it is
imperative that their fundamental phase relations be
understood. We begin with a discussion of the three
binary systems (Kf-An, Ab-An, Kf-Ab) and then as-
semble these into the feldspar ternary of the three-
component Ab-An-Kf.
In the two model binary systems already considered,
crystalline solids have fixed compositions with no solid
solution between end-member components. On the
other hand, the binary plagioclase system (Ab-An) ex-
hibits complete solid solution between end-member
components NaAlSi
3
O
8
(Ab) and CaAl
2
Si
2
O
8
(An)
whereas the other two binary systems exhibit only par-
tial mutual solubility. Feldspars serve as models for
other major rock-forming minerals that are also solid
solutions.
5.5.1 KAlSi
3
O
8
(Kf )-CaAl
2
Si
2
O
8
(An) Binary System:
Limited Solid Solution
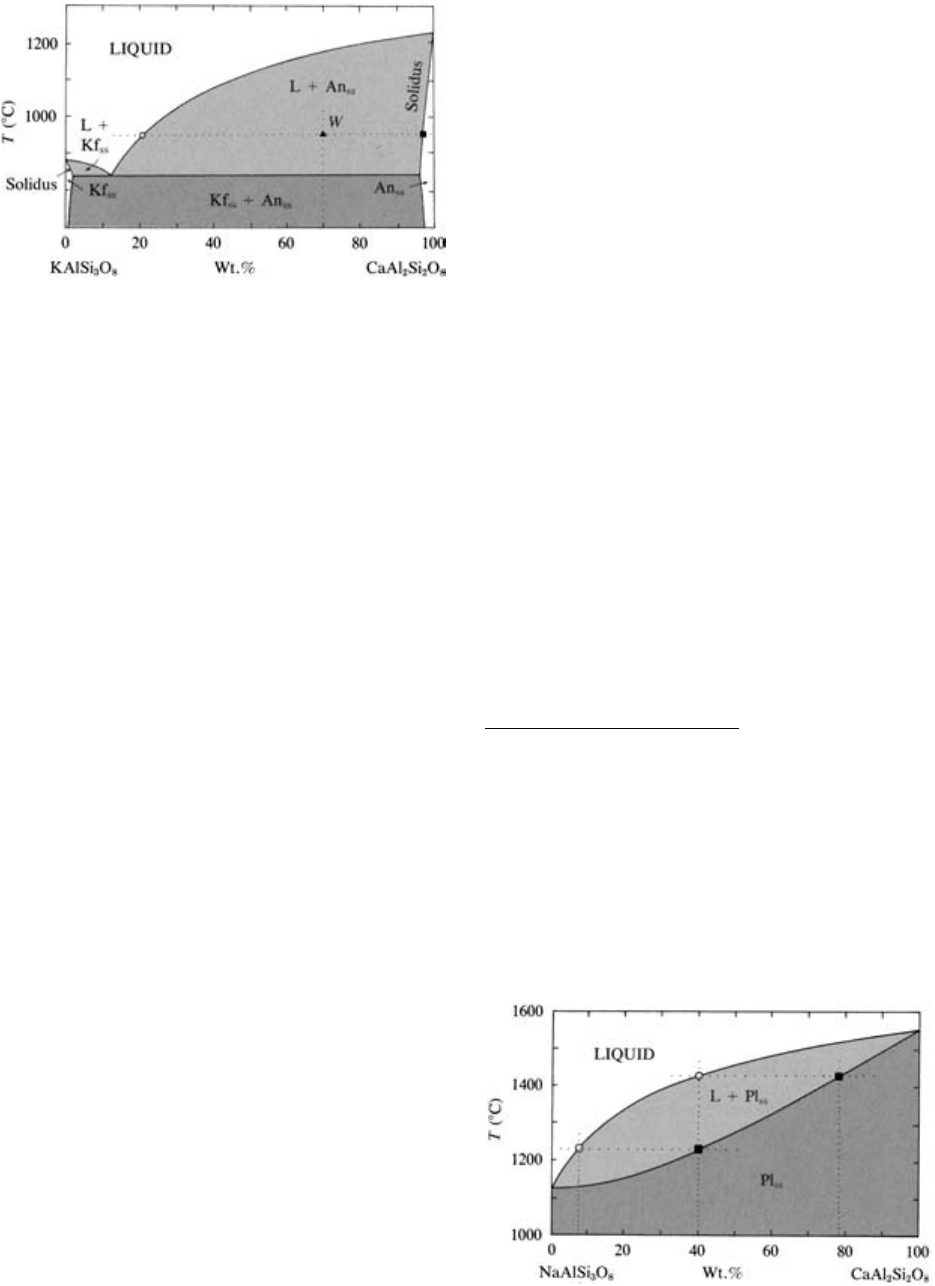
This system (Figure 5.12) resembles the CaMgSi
2
O
6
-
CaAl
2
Si
2
O
8
system except there is a limited mutual
solubility between the K- and Ca-feldspar compo-
nents of just a small weight percentage. A slender,
wedge-shaped one-phase stability field labeled Kf
ss
(K-
feldspar solid solutions) lies along the left-hand T axis
and a similar stability field labeled An
ss
(anorthite solid
solutions) lies along the right-hand T axis. Above an
isotherm through the eutectic, the boundary line be-
tween these wholly crystalline fields and the two-phase
fields of liquid Kf
ss
and liquid An
ss
is the solidus
line. The solidus line converges with the T-axes at the
melting points of pure anorthite and K-feldspar.
For a magma system An
70
Kf
30
at 950°C, an isother-
mal tie line shows that crystals of An
96
Kf
4
are in equi-
librium with liquid An
21
Kf
79
. The lever rule indicates
that 64 wt.% of this system is crystals, 36 wt.% liquid.
With increasing T, the solubility of the KAlSi
3
O
8
com-
ponent in anorthite crystals decreases, from about 4
wt.% at 850°C to about 2 wt.% at 1100°C.
100 Igneous and Metamorphic Petrology
500 700 900 1100 1300 1500
0
20
40
60
80
100
120
Pl + Px + Ol + L
Pl + Ol + L
Pl + L
LIQUID
Dry liquidus
Cpx +
Grt +
L
solidus
Cpx + Grt +
Pl + L
Dry
Cpx +
Pl + L
Cpx + L
Ol + L
Ol +
Cpx +
L
Ol + Cpx + Pl + L
10
20
30
40
0
Amp +
Grt +
Cpx +
Amp +
Cpx + Pl
+ L
Amp + Cpx + L
Amp + Grt + Cpx
+ L
CRYSTALS
Cpx + Grt + L
Coesite
Quartz
Water-saturated solidus
Water-saturated liquidus
T (°C)
Depth (km)
P (kbar)
Pl + L
Cpx
+ L
5.11 Generalized crystal-melt equilibria in tholeiitic basalt. This is actually two diagrams, which have been superposed to emphasize the strik-
ing influence of water on liquidus and solidus temperatures and on crystalline phase assemblages. Equilibria in the water-free “dry” sys-
tem are shown by solid lines. Equilibria in the water-saturated system are indicated by dashed lines and shaded area. Quartz-coesite poly-
morphic transition is shown by dotted line. (Redrawn from Green, 1982.)
5.5.2 NaAlSi
3
O
8
(Ab)-CaAl
2
Si
2
O
8
(An) Binary
Plagioclase System: Complete Solid Solution
At 1 atm, the plagioclase phase diagram for mixtures
of the NaAlSi
3
O
8
(Ab) and CaAl
2
Si
2
O
8
(An) end-
member components (Figure 5.13) consists of a convex
upward liquidus and convex downward solidus be-
tween the melting points of pure anorthite and pure al-
bite. Within the univariant two-phase region, L Pl
ss
(liquid plagioclase solid solutions), specification of a
T uniquely fixes the equilibrium compositions of both
liquid and crystals, given by the ends of an isothermal
tie line where it intersects the liquidus and solidus
loops, respectively. Alternatively, specification of the
composition of one phase fixes the composition of the
other, as well as T. The modal proportion of crystals to
liquid at any particular T depends on the bulk compo-
sition of the univariant system and can be determined
from the lever rule.
At any T within the univariant two-phase region,
L Pl
ss
, plagioclase solid solutions are always more
calcic (anorthitic) than is the coexisting liquid, which is
more sodic (albitic). For example, the first crystals pre-
cipitating from a liquid An
40
have a composition of
An
78
. The progress of crystallization can be followed
by drawing a series of isothermal tie lines at decreas-
ing T. As T decreases, more plagioclase precipitates,
and it, as well as the liquid, become more albitic. The
liquid line of descent in the plagioclase system yields
more Na-Si-rich residual melts.
Any plagioclase of intermediate composition be-
tween pure end member Ab and pure An melts incon-
gruently with increasing T, yielding a liquid more al-
bitic and crystals more anorthitic than the original. For
example, initial melting of crystals An
40
yields liquid
whose composition is An
8
. As T increases, the melting
progresses, creating a larger proportion of liquid to
crystals, both of which become more enriched in
CaAl
2
Si
2
O
8
, as may be seen by drawing a series of
isothermal tie lines at successively higher temperatures.
In a sense, the process involves an infinite number of
incongruent melting steps. At 1425°C the last crystal to
be consumed, An
78
, is in equilibrium with a melt of the
original bulk composition, An
40
.
It may seem paradoxical that in a closed system
progressive crystallization makes both crystals and
liquid more albitic and both more anorthitic for pro-
gressive melting. This happens because the modal
proportions of solid and liquid solutions change sym-
pathetically and continuously with the concomitant
continuous changes in their compositions as T
changes. This phenomenon can be shown in the fol-
lowing hypothetical reaction, which is an example of a
mass-balance equation, for a system whose bulk com-
position is An
40
:
5.4 72.9 wt.% liquid An
29.2
27.1 wt.% crystals An
70.3
at 1387°C
80.6 wt.% liquid An
32.3
19.4 wt.% crystals An
73.6
at 1400°C
Continuous Reaction Relations. In order for continu-
ous sympathetic changes in compositions and in ratios
of coexisting solid and liquid solutions to occur, con-
tinuous reaction relations must take place between
them as intensive variables (T in this case) continuously
change. Continuous reaction relations in which reac-
tants and products coexist over a range of T are to be
contrasted with the discontinuous (peritectic) reaction
relation at a unique, single T in the system Mg
2
SiO
4
-
SiO
2
. To maintain a constant state of equilibrium be-
Crystal-Melt Equilibria in Magmatic Systems
101
5.12 The water-saturated system KAlSi
3
O
8
(Kf)-CaAl
2
Si
2
O
8
(An) at
5 kbar. The presence of a separate water-rich phase makes this
system ternary, but for our purposes the H
2
O component can
be ignored. At 1 atm this binary system is complicated by a
large stability field of leucite (Problem 5.7), which is elimi-
nated at high-P and water-saturated conditions as shown here.
The symbol Or is commonly used to denote the KAlSi
3
O
8
component, but our use of Kf is a reminder that different
atomic structural forms of potassium feldspar exist; orthoclase
is only one of these. The subscript ss on the crystalline phases
An and Kf denotes solid solutions. (Redrawn from Yoder et
al., 1957.)
5.13 The binary system NaAlSi
3
O
8
(Ab)-CaAl
2
Si
2
O
8
(An) at 1 atm.
(Redrawn from Bowen, 1928.)

tween crystals and melt in the NaAlSi
3
O
8
-CaAl
2
Si
2
O
8
system there is an exchange reaction between coupled
ions
5.5 Na
Si
4
Ca
2
Al
3
Exchange must occur by migration (diffusion) of these
ions across the interface between the melt and already
formed crystals as intensive variables change. Obvi-
ously, the larger the crystals to be modified in compo-
sition; or the more viscous the melt, which makes ions
less mobile; or the faster the change in intensive vari-
ables; or combinations of these conditions, the less
chance there is for equilibrium to be maintained in the
system.
Therefore, we would expect that perfect, reversible
equilibrium crystallization in the plagioclase system
would only occur under exceptional circumstances. In-
deed, this expectation is borne out by the rocks them-
selves; perfectly homogeneous plagioclases of uniform
composition throughout are rare in magmatic rocks.
Instead, compositionally inhomogeneous, or zoned,
plagioclases are far more common and result from
incomplete reaction relations during fractional crys-
tallization as intensive variables change. Before com-
plete reaction with the melt can occur by diffusional
processes additional crystalline material of different
composition precipitates. Then, before the melt can re-
act with that newly accreted crystalline material, chang-
ing conditions inhibit further reaction. As the process
continues, the melt never has a chance to equilibrate
fully with the whole crystal, which becomes zoned as
a result. Bowen (1928) referred to such zoned solid
solution crystals as well as accumulated crystals in a
plutonic mass as a continuous reaction series (Figure
5.14).
During perfect fractional crystallization of a liquid,
such as An
40
(Figure 5.14), each liquid fraction, iso-
lated from all previously precipitated crystals, is effec-
tively a new system with no knowledge of its prior his-
tory. Because of the lack of reaction, Na
Si
4
ions
are conserved and Ca
2
Al
3
ions depleted in a rela-
tive sense in the liquid. Carried to completion, perfect
fractional crystallization theoretically creates a residual
melt that is ultimately NaAlSi
3
O
8
, at which composi-
tion it precipitates pure albite (An
0
). Hence, the
crystalline products in this hypothetical example of a
continuous reaction series range from An
78
to An
0
.
Fractionating, evolving liquids and related crystals
progress toward more sodic and silicic and less calcic
compositions. It is worth emphasizing once again here
that fractional crystallization significantly extends the
range of T over which crystals precipitate, as well as
extending the range of compositions of liquid and
solid solutions, relative to equilibrium crystallization
(Figure 5.15).
Continuous reaction relations occurred in the
Makaopuhi magma system as the melt reacted only
partially with previously precipitated olivine, pyroxene,
and plagioclase solid solutions. Though inconspicuous
to the naked eye in Plate III, continuous changes in the
chemical composition of these solid solutions are re-
vealed by microprobe analyses of the minerals in the
quenched lava lake samples. The two mafic silicates be-
come more Fe-rich at the expense of Mg and plagio-
clases become more NaSi-rich with decreasing T.
Influence of Other Components on the Plagioclase
System. Increasing P increases liquidus and solidus
temperatures in the plagioclase system by only several
degrees Celsius per kilobar. In contrast, addition of
other chemical components, especially water, to the
system depresses the liquidus and solidus by hundreds
of degrees (Figure 5.16). Addition of CaMgSi
2
O
6
(Di)
depresses anorthitic compositions but not albitic com-
positions, so that small changes in T yield large changes
in the equilibrium compositions of the coexisting melt
and crystals. Dissolved water not only depresses the
liquidus and solidus but in multicomponent real mag-
mas stabilizes more calcic plagioclase. For example, in
subduction zone basalt magmas that are typically more
water rich, crystallizing plagioclase is more anorthitic,
to An
90–95
, than is plagioclase in relatively dry mid-
ocean ridge basalt magma ( Johnson et al., 1994).
5.5.3 NaAlSi
3
O
8
(Ab)-KAlSi
3
O
8
(Kf )
Binary Alkali Feldspar System
Dry, or with only small concentrations of water at rela-
tively low P, this system is complicated by a large sta-
bility field of leucite (KAlSi
2
O
6
), whose composition
cannot be expressed in terms of the components
Ab and Kf. However, at between 2 and 3 kbars under
water-saturated conditions the stability field of leu-
cite disappears and the system becomes truly binary
(Figure 5.17). Solid solution is complete between the
NaAlSi
3
O
8
and KAlSi
3
O
8
components; the solidus and
liquidus form loops on each side of a minimum-melting
composition. This minimum resembles a eutectic, in
that evolved residual liquids move to it and there pre-
cipitate an alkali feldspar solid solution, about Kf
30
. At
P
H
2
O
3 kbar (water-saturated conditions at 3 kbar)
any feldspar precipitated from a melt is a homogeneous
alkali feldspar solid solution. As any feldspar cools be-
low the solidus, its isopleth eventually intersects the
convex-upward solvus, below which the single feldspar
unmixes, or exsolves, under equilibrium conditions,
into two stable alkali feldspar solid solutions. For ex-
ample, at 600°C an initially homogeneous feldspar Kf
60
exsolves into a K-rich feldspar solid solution Kf
68
and
a Na-rich feldspar solid solution Kf
21
. The lever rule in-
dicates that their proportions are about 83 wt.% and
102 Igneous and Metamorphic Petrology
Crystal-Melt Equilibria in Magmatic Systems
103
An
78
An
43
An
15
An
0
Fractional
crystallization by
gravitative settling
(b)
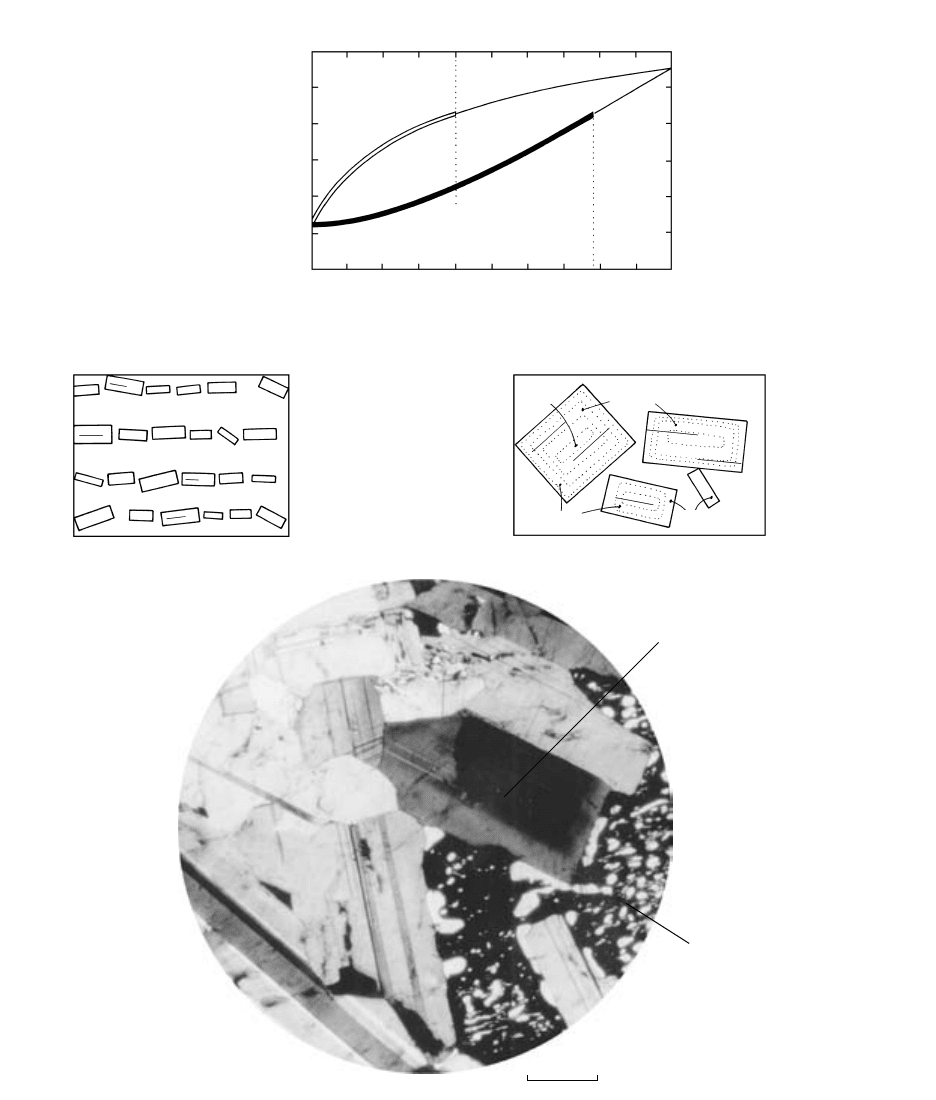
5.14 Fractional crystallization in a plagioclase model magma system whose composition is An
40
. (a) The range of transient liquid and crystal
compositions during cooling of the magma is indicated by the double and thick black lines, respectively, along the liquidus and solidus.
(b) Stacks of crystals (a continuous reaction series) that are more albitic toward the top representing schematically the product of frac-
tional crystallization by gravitative segregation of crystals in a less dense melt. The same sort of sequence, but rotated 90°, could occur
by side-wall crystallization along the steep border of an intrusion, as more albitic plagioclases would precipitate into the intrusion.
(c) Schematic product of fractional crystallization resulting from incomplete reaction relations; zoned crystals are more albitic toward their
margins. All An values are arbitrary except for initial crystals, which are An
78
. (d) Normal compositional zoning in plagioclase under cross-
polarized light in gabbro, Skaergaard intrusion, Greenland. Twinned grain in center of view has been carefully oriented to show lighter
gray interference color in slightly more albitic rim. Very slow interdiffusion of NaSi and CaAl ions in plagioclase has not taken place to
erase its zoning. Adjacent pyroxene grain (black in extinction orientation) has experienced subsolidus exsolution by means of more rapid
diffusion of Ca-rich and Ca-poor phases, forming a “blebby perthitic” intergrowth (white) within the original homogeneous crystal.
"Perthitic"
pyroxene
Zoned
plagioclase
0 0.5mm
(d)
An
17
An
2
An
38
An
78
Fractional
crystallization by
incomplete chemical
reaction forming
zoned crystals
(c)
0
1000
1200
1400
1600
20 40 60 80 100
Wt. % CaAl
2
Si
2
O
8
NaAlSi
3
O
8
T (°C)
(a)
L + Pl
ss
LIQUID
Pl
ss
17 wt.%, respectively. These two feldspars produced
by the exsolution process usually segregate within the
original crystal as thin subparallel lamellae, forming the
intergrowth known as perthite. At lower temperatures
the mutual solubility decreases, that is, the miscibility
gap widens, as the feldspar structure tightens, so that at
500°C, for example, Kf
75
and Kf
8
coexist at equilib-
rium. Perthitic intergrowths in volcanic rocks are gen-
erally not visible even with a microscope, because the
magmas cool so quickly that the diffusion-controlled
exsolution does not create visible lamellae; these ex-
ceedingly fine, cryptoperthitic intergrowths can, how-
ever, be discerned by X-ray diffraction analysis. Per-
thite is commonly visible in more slowly cooled plutonic
rocks, even with the naked eye. Sluggish rates of diffu-
sion within the crystal that preserve metastable compo-
sitions prevent the use of perthites as a geothermometer,
but they do furnish information on cooling rates.
At higher pressures, such as 5 kbar, in water-
saturated systems the solidus loop is depressed so far
that it intersects the solvus, forming an isothermal
boundary line between the two-feldspar and liquid
feldspar fields (Figure 5.17b). In this case, the mini-
mum in the liquidus is a eutectic. The phase diagram
now resembles the Kf-An diagram (Figure 5.12), ex-
cept that the extent of solid solution between the two
end members is greater in the alkali feldspars. Starting
liquids that lie between about Kf
19
and Kf
52
and crys-
tallize in the equilibrium manner, as well as liquids of
any composition undergoing extreme fractional crystal-
lization, ultimately yield two feldspars, Kf
19
and Kf
52
.
Each of these phases may subsequently experience
slight exsolution upon cooling below the solvus.
The contrasting phase relations depicted in Figure
5.17 prompted Tuttle and Bowen (1958) to classify
granites into two textural categories, hypersolvus and
subsolvus (Figure 5.18). Hypersolvus granites, and
some syenites, crystallize from relatively dry magmas
whose phase relations are governed as are those in
Figure 5.17a where the chief or sole feldspar is
perthite; accompanying mafic minerals are commonly
anhydrous. Subsolvus granites and other felsic rocks
have two distinct feldspars that crystallize directly
from the melt (Figure 5.17b); accompanying mafic
minerals are commonly hydrous amphiboles and bi-
otite.
5.5.4 KAlSi
3
O
8
(Kf )-NaAlSi
3
O
8
(Ab)-CaAl
2
Si
2
O
8
(An)
Ternary Feldspar System
The ternary feldspar system is of paramount impor-
tance in petrology because most rocks contain ternary
feldspar solid solutions. It also serves as an introduc-
tion to “reading” ternary phase diagrams. For realism
and for hastening of reaction rates, laboratory studies
of feldspars have involved water, making the system
quaternary. However, to keep the discussion simple,
water pressure is assumed constant and can be ignored
in an isobaric diagram.
104 Igneous and Metamorphic Petrology
0
1000
1200
1400
1600
20 40 60 80 100
Wt.%
T (°C)
LIQUID
L + Pl
ss
Pl
ss
Equilibrium crystallization
produces homogeneous
crystals An
40
CaAl
2
Si
2
O
8
NaAlSi
3
O
8
5.15 Equilibrium crystallization in a plagioclase model magma sys-
tem whose composition is An
40.
The final products of equilib-
rium crystallization are homogeneous crystals An
40.
0
600
800
1000
1200
1400
1600
20 40 60 80 100
NaAlSi
3
O
8
Wt. % CaAl
2
Si
2
O
8
T (°C)
LIQUID
1 atm
1 atm
+ Di
5 kbar
water-saturated
+ Quartz, 5 kbar
water-saturated
5.16 Comparison of liquidus and solidus temperatures in the pla-
gioclase system at 1 atm with projected liquidi and solidi
curves in systems with additional components. (Redrawn from
Johannes, 1978.) Dashed curves labeled Di are for a system at
1 atm in which diopside coprecipitates. (Redrawn from Morse,
1980.)
In ternary phase diagrams, T varies along an axis
perpendicular to an equilateral triangle on which are
represented the proportions of the three components
(review Figure 2.3b), in this case Kf, Ab, and An. Thus,
the four intensive variables T, X
Kf
, X
Ab
, and X
An
form
a triangular prism whose three side faces are the three
binary feldspar systems in Figures 5.12, 5.13, 5.17, and
5.19a. The upper bounding surface of the prismatic
volume (Figure 5.19b) is the liquidus surface, above
which, at higher temperatures, any mixture of compo-
nents is liquid. This three-dimensional liquidus surface
is analogous to the familiar topographic surface of the
Earth in that it has hills and valleys whose exact con-
figuration can be represented by isothermal contour
lines drawn on the surface. A contour line on the liq-
uidus surface is the intersection of an isothermal plane
Crystal-Melt Equilibria in Magmatic Systems
105
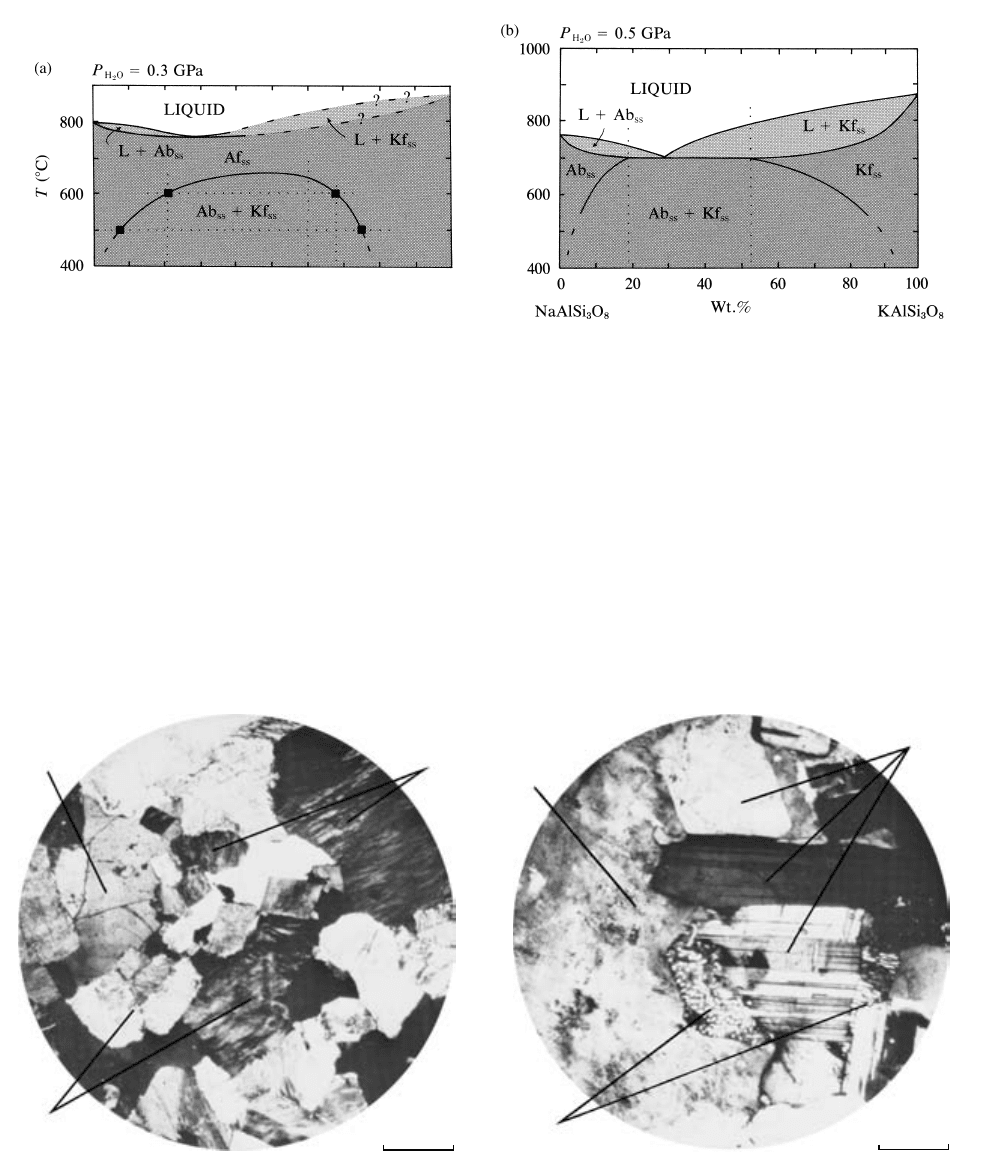
5.17 The NaAlSi
3
O
8
(Ab)-KAlSi
3
O
8
(Kf) system under water-saturated conditions at 3 and 5 kbar (0.3 and 0.5 GPa). Granite magmas crys-
tallizing under conditions like those in (a) yield hypersolvus textures, whereas in (b) they yield subsolvus textures (Figure 5.18 and Plate
IV). Dashed lines in (a) are inferred liquidus and solidus where experimental data are lacking. Subscript ss refers to solid solutions. (Re-
drawn from Yoder et al., 1957; Morse, 1970.)
Perthite
Quartz
Perthite
0
1
mm
(a)
Myrmekite
Plagioclase
0
1
mm
(b)
K-rich
feldspar
5.18 Textural types of granites formed at contrasting water pressures during crystallization. Photomicrographs of thin sections under cross-
polarized light. (a) Hypersolvus granite formed under conditions like that in Figure 5.17a where only one initially homogeneous alkali
feldspar precipitated from the melt. This single phase subsequently unmixed at temperatures below the solvus from perthite, an inter-
growth of Na-rich and K-rich alkali feldspars. Associated mafic minerals are commonly anhydrous: Fe-rich pyroxenes and olivines.
(b) Subsolvus granite formed under conditions like that in Figure 5.17b, where two discrete feldspars—a sodic plagioclase and a potas-
sic alkali feldspar—coprecipitated from the melt; each may experience slight unmixing at lower temperatures, but this may not be obvi-
ous under the microscope. Associated mafic minerals are typically hydrous: biotite and amphibole. Turbid appearance of alkali feldspar
is caused by clay alteration and possible minute fluid inclusions. Myrmekite is a vermicular intergrowth of quartz and sodic plagioclase.
106 Igneous and Metamorphic Petrology
T
O
U
E
V
S
KAlSi
3
O
8
S
M
A
T
NaAlSi
3
O
8
A
T
O
CaAl
2
Si
2
O
8
(a)
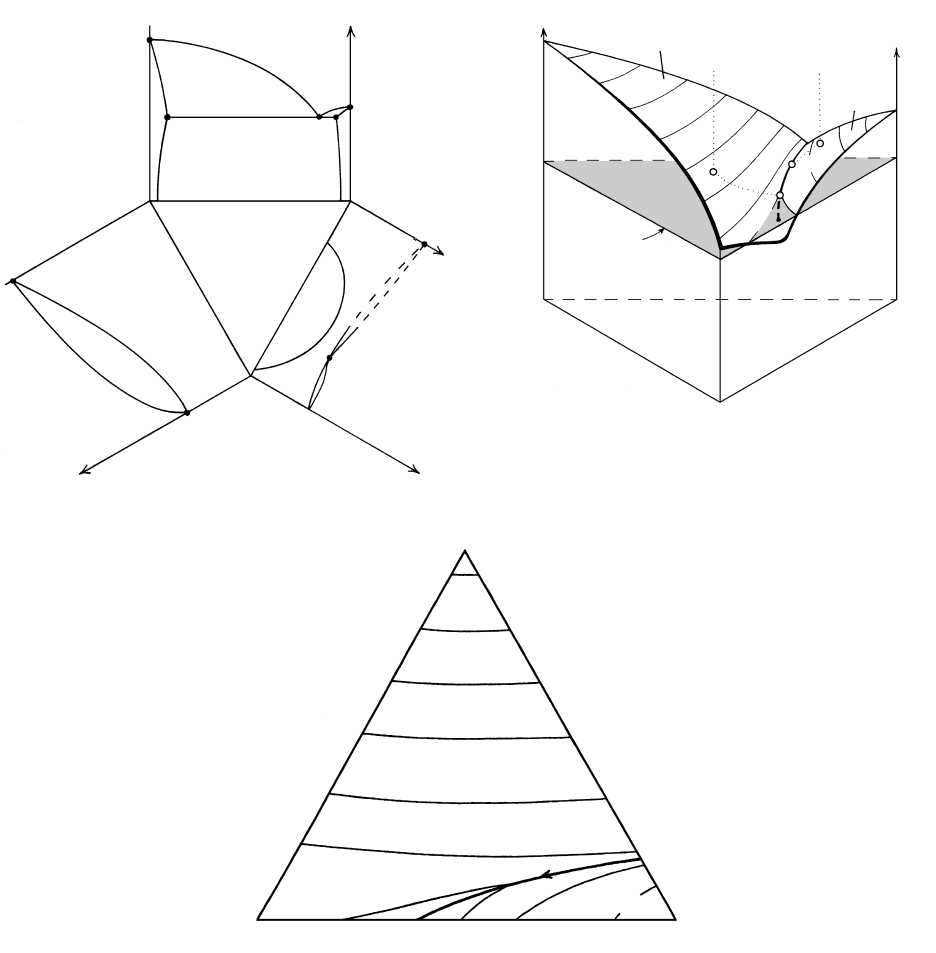
5.19 The system KAlSi
3
O
8
(Kf)-NaAlSi
3
O
8
(Ab)-CaAl
2
Si
2
O
8
(An)-H
2
O at moderately high P appropriate to shallow plutonic conditions.
(a) Three binary systems shown in previous figures are linked around a compositional equilateral triangle in which proportions of the
three components CaAl
2
Si
2
O
8
, KAlSi
3
O
8
, and NaAlSi
3
O
8
are represented. The binary Kf-An system has been slightly distorted for clar-
ity. Points A, O, S, V, and so on, refer to points in subsequent figures of this ternary system. (b) Perspective view of the three binary sys-
tems folded up to form a three-dimensional triangular prism whose axis represents T. The underlying solidus and solvus surfaces
(Figure 5.20) have been omitted for clarity. The upper surface of the prism is the liquidus surface in three-dimensional T-X space on
which curved isothermal contour lines are drawn parallel to the compositional base of the prism. One isothermal plane (shaded) paral-
lel to the base is shown cutting the liquidus surface along isothermal contour lines on each side of the two-feldspar-liquid boundary line,
EM
1
. This line lies in the thermal valley of the liquidus surface in which falling T is toward M
1
. The liquidus phases to the left of the
boundary line are plagioclase solid solutions; these coexist stably with any melt on this part of the liquidus surface. The liquidus phases
to the right of the boundary are alkali feldspar solid solutions. Melts lying on the boundary are in equilibrium with both feldspar solid
solutions. The end point of the boundary line does not lie on the NaAlSi
3
O
8
-KAlSi
3
O
8
join, but within the ternary with a small amount
of dissolved CaAl
2
Si
2
O
8
. (See Nekvasil and Lindsley, 1990; Brown, 1993, for a discussion of the complexities at the termination of the
boundary line.) (c) Projection of isothermal contour lines and boundary line EM
1
from the triangular prism in (b) onto its base.
T
O
CaAl
2
Si
2
O
8
NaAlSi
3
O
8
KAlSi
3
O
8
S
Pl
ss
+ liquid
alkali
feldspar
ss
+ liquid
Z
Z
1
Z
2
Y
2
Y
1
Isothermal
plane
M
1
E
Y
T
A
(b)
An
L + Pl
ss
Ab
M
Kf
L + Kf
ss
E
(c)
