Myron G. Best. Igneous and metamorphic 2003 Blackwell Science
Подождите немного. Документ загружается.

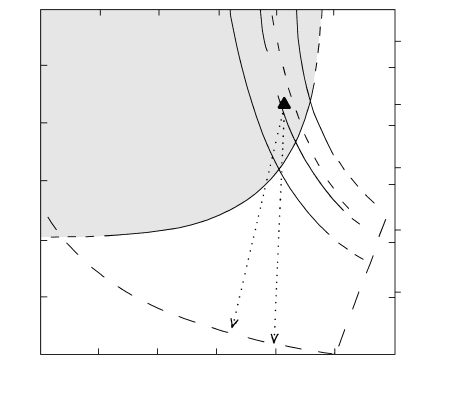
not necessarily, loss of its dissolved water. Though
many explosive pyroclastic eruptions followed the cat-
astrophic May 18, 1980, event, they were of declining
intensity and volume. Meanwhile, a viscous lava dome
slowly extruded from the vent; the magma feeding this
dome is believed to have followed a path indicated by
one of the dotted lines in Figure 5.33.
Experimental studies of the rate of amphibole
breakdown and rim production indicate ascent rates of
15–50 m/h for post–May 18 magma extrusions. These
relatively slow rates would allow some conductive cool-
ing to the wall rocks as well as cooling by the loss of wa-
ter from the magma. In contrast, ascent rates 66 m/h
must have occurred for the explosively erupted May 18
magma in which no reaction rims developed on the am-
phibole phenocrysts.
5.8 GEOTHERMOMETERS AND
GEOBAROMETERS
Determination of intensive parameters in long dead
magma systems using equilibrium assemblages of coex-
isting minerals is a major endeavor of modern petrol-
ogy. Many quantitative mineral geothermometers and
geobarometers have been calibrated by experiment
and thermodynamic models, but a detailed presenta-
tion is beyond the scope of this textbook; only a few
have been briefly introduced. Oxygen fugacity and T
can be determined from coexisting equilibrium mag-
netite and ilmenite solid solutions (Section 3.5.5). T
can be determined from coexisting alkali feldspar and
plagioclase and from clino- and orthopyroxene solid
solutions (Sections 5.5.4 and 5.6). A geobarometer for
determination of P during crystallization of some felsic
magma systems relies on the equilibrium concentration
of Al in hornblende; calibration is sensitive to T, oxy-
gen fugacity, and the crystalline phases coexising with
a water-saturated melt (Anderson and Smith, 1995).
Finally, the water fugacity in a magma can be deter-
mined from an equilibrium assemblage of biotite
magnetite K-feldspar (discussed earlier) in a rock if
P, T, and oxygen fugacity can be independently deter-
mined (Bohlen et al., 1980).
5.8.1 Assessing States of Equilibrium in Rocks
Use of mineral geothermometers and geobarometers,
as well as other interpretations of rocks, depends on a
state of stable equilibrium prevailing in the magma sys-
tem when the crystals formed. Mineral compositions
must not have been modified since that equilibrium
state and must be accurately known, and all relevant
components in the system must be accounted for
(Frost and Lindsley, 1992). Stable equilibrium is gener-
ally assumed in the case of euhedral crystals, which are
bounded on all sides by rational crystal faces, sur-
rounded by glass or aphanitic crystalline material. Such
textures are only necessary, not sufficient, to establish a
state of stable equilibrium. Various tests have been de-
vised to ascertain whether the pair of minerals used in
geothermobarometry are valid representations of equi-
librium.
States of disequilibrium, or metastable phases
frozen into the rock because of sluggish kinetics and in-
complete reaction relations in dynamic magma sys-
tems, are more readily discerned and verified than
equilibrium states. Disequilibrium states are suggested
by the following:
1. Isolated anhedral (noneuhedral) grains that re-
sulted from partial resorption or dissolution into
the melt.
2. Reaction rims of one or more phases surround-
ing another anhedral crystalline phase. Phaneritic
rocks may have reaction rims of amphibole jacket-
ing pyroxene (Plate II) and biotite surrounding am-
phibole that are created by incomplete reaction re-
lations in a fractionating magma.
3. Compositionally zoned grains also originate through
incomplete reaction relations within a single solid-
solution series, such as plagioclase. Zoning in
plagioclase is obvious in thin sections in cross-
polarized light but in other solid solution crystals is
generally less obvious. In some cases, the outermost
rims of an assemblage of zoned minerals represent
equilibrium precipitates.
Crystal-Melt Equilibria in Magmatic Systems
117
700 800 900 1000
T (°C)
0
1
2
3
P (kbar)
0
2
4
6
8
10
Depth (km)
Amphibole
Fe-Ti oxides
Pl
Cpx
Opx
Water-saturated
liquidus
Dry
solidus
Adiabat
Conductive cooling
Opx + Cpx +
Pl + Fe-Ti oxides
+ melt + fluid
Water-saturated
solidus
5.33 Phase relations for the 1980 dacite of Mount Saint Helens,
Washington. Compare Figure 5.31. Boundary lines (dashed
where approximate) are for P P
fluid
P
H
2
O
and oxygen fu-
gacity near the NNO buffer. Stability field of the amphibole in
the dacite is shaded. The filled triangle represents equilibra-
tion conditions in the magma just prior to its paroxysmal erup-
tion of May 18, 1980. Dotted lines are hypothetical paths of
ascending magmas erupted subsequently in the 1980–86 erup-
tions. (Redrawn from Rutherford and Hill, 1993.)

Other indicators of disequilibrium in rocks in-
clude experimentally known unstable or unlikely (in
an evolutionary sense) mineral assemblages: for ex-
ample, a volcanic rock that contains magnesian olivine
and quartz, or magnesian olivine and/or pyroxene
with sanidine, or grains of two distinct compositions
in a single solid-solution series having no miscibility
gap or solvus. Such disequilibrium assemblages com-
monly originate through mixing of two dissimilar
magmas.
5.9 A BRIEF COMMENT REGARDING
SUBSOLIDUS REACTIONS IN
MAGMATIC ROCKS
All magmatic rocks solidified from magma at high T
must have cooled to near-atmospheric T before a
petrologist studies them. Plutonic rocks must also have
decompressed. At decreasing T, reaction rates are slug-
gish, causing metastable, magmatic phases to persist
well outside their field of stability (Section 3.6.2). Sub-
solidus mineral reactions and recrystallization are com-
monly incomplete, leading to states of disequilibrium
that can sometimes be detected by the phase rule. Sec-
ondary, subsolidus mineral grains are typically very
small and not readily identified even with a micro-
scope.
Cooling volcanic rocks emplaced on the surface of
the Earth are also subjected to a significant increase in
oxygen fugacity relative to pristine deep-seated mag-
mas, which are equilibrated generally within a few log
units of the QFM buffer (Figure 3.14). Consequently,
the predominantly ferrous Fe in primary magmatic
minerals and glass is at least partially oxidized to the
ferric state, stabilizing hematite (or at least excess mag-
netite). The tiny grains of secondary hematite redden
Fe-bearing phases, as explained for biotites. Amphi-
boles and biotites also suffer dehydration reactions be-
cause of the decrease in water fugacity that affects
erupted magmas. These subsolidus decomposition ef-
fects may be difficult to distinguish from reaction rela-
tions involving melt above the solidus. However, grain
118 Igneous and Metamorphic Petrology
Advanced Topic Box 5.4 The MELTS com-
puter software model
MELTS is a thermodynamically-based model by
Ghiorso and coworkers (e.g., Ghiorso, 1997) of
crystal-melt equilibria calibrated from thousands of
experiments. The model is menu-driven and inter-
active and computes phase compositions and pro-
portions by minimization of the chemical potential
of magma systems. MELTS models real magmas
crystallizing in either the fractional or equilibrium
way under specified conditions: initial conditions
and constraints under which the system evolves. For
example, specification of P, oxygen fugacity, and
system composition allows one to track as a func-
tion of T the progress of crystallization in a closed
system under equilibrium conditions; evolving solid
and liquid compositions, viscosities, and densities
are output data.
0 0.03mm 0 0.04mm
(a) (b)
5.34 Subsolidus decomposed hornblende phenocrysts. (a) Partially decomposed hornblende with rim of fine-grained Fe-Ti oxides, pyroxenes,
and feldspars. (b) Completely decomposed (pseudomorphed) amphibole in diorite porphyry. Fine-grained breakdown assemblage simi-
lar to that in (a) mimics shape of original mineral.

sizes tend to be larger in reaction rims, whereas sub-
solidus breakdown products are generally very fine-
grained anhydrous and oxidized secondary minerals
jacketing or completely replacing both amphiboles and
biotites (Figure 5.34).
In the plutonic environment, on the other hand,
magmatic rocks generally cool to some extent below
the solidus T before much decompression occurs, per-
haps millions of years later by tectonic uplift and ero-
sional denudation. Therefore, the P-T path followed is
much different, and so is the nature of secondary min-
eralogical changes. As the crystalline plutonic assem-
blage cools, it passes into the stability fields of lower T,
but elevated P, hydrous phases such as epidote miner-
als and other hydrated Ca-Al silicates, chlorites, and, at
lowest temperatures, clay minerals (Plate IV).
SUMMARY
Crystal-melt equilibria in simple model systems indi-
cate what is possible and impossible in chemically more
complex, multicomponent natural magma systems.
The phase rule can be used to assess states of equilib-
rium and the degrees of freedom in magmatic systems.
For example, both intensive variables may be freely
varied without changing the state of equilibrium in di-
variant stability fields in P-T or T-X space. Drawing
isopleths, isotherms, and tie lines and applying the
lever rule permit compositions and proportions of co-
existing melt and crystals to be determined in equilib-
rium systems.
Essentially isothermal decompression of initially sub-
solidus water-free rocks can lead to melting, whereas
decompression of water-saturated magmas can cause
crystallization.
Solid solutions, some pure minerals such as ensta-
tite, and polymineralic rocks melt incongruently to
a liquid and crystals whose compositions differ from
that of the starting material. Incongruent melting ac-
counts for much of the diversity of igneous rocks and
the global differentiation of the Earth into crust and
mantle.
Denser crystalline phases are stabilized in magmas
at high P. Under upper mantle conditions, a basalt
magma can contain Mg-rich garnet and a complex Na-
Al-Ca clinopyroxene instead of plagioclase, Ca-Mg-Fe
pyroxene, and olivine.
In crystallizing multicomponent magmas and in
melting source rocks, reaction relations at the ionic
level between liquid and solid solutions continuously
modify their compositions as intensive variables change.
Compounded with kinetic factors in dynamic magma
systems, reaction relations in crystallizing magmas al-
low for a wide range of possible liquid lines of descent
taken by residual melts and reaction series taken by
crystalline products. A significant kinetic factor is the
degree to which a magma fractionates during crystal-
lization because reaction relations between melt and
crystals are precluded. Fractionation can occur by
moving the crystal away from the parcel of melt where
it precipitated, by creating a reaction rim about the
metastable crystal, or by changing intensive parameters
in the magma faster than the rate at which reaction
occurs.
Fractionation extends the range of T over which
crystallization takes place, extends the range of residual
melt compositions, and creates more diverse crystalline
products, or reaction series. Liquid lines of descent
produced as solid solutions fractionate are curved on a
variation diagram. More evolved residual melts are
commonly enriched in Si and have greater (Na K)/
Ca and Fe/(Fe Mg) ratios.
Volatiles, especially water because of its high solu-
bility in melts, have a profound influence on crystal-
melt equilibria. By depolymerization of the silicate
melt, liquidus and solidus temperatures are depressed
by as much as several hundreds of degrees. Volatile-
bearing minerals, especially major rock-forming micas
and amphiboles, are stabilized in volatile-rich magmas
but decompose into volatile-free minerals at high T.
Amphibole and biotite stabilization requires 3–5 wt.%
dissolved water in andesite-dacite melts. Stability of Fe-
bearing hydrous minerals such as biotite and amphi-
bole depends on fugacities of H
2
O, O
2
, Cl, and F in
addition to P and T. Nonvolatile mineral compositions
can also be influenced by volatiles; thus, more calcic
plagioclase is stabilized in water-rich magmas. Sub-
solidus decomposition (alteration) of primary high-T
magmatic precipitates yields fine-grained aggregates of
ferric oxides and anhydrous minerals at low water and
high oxygen fugacity in extruded magma and fine-
grained, more hydrous phases in confined plutonic sys-
tems to the extent that water is available.
Under equilibrium conditions in magma and meta-
morphic systems the partitioning of chemical elements
among crystallizing solid solutions depends on the pre-
vailing P and T; hence, compositions of coexisting min-
erals serve as thermobarometers.
CRITICAL THINKING QUESTIONS
5.1 Describe how the phase rule is derived, what it
means, and how it is used in reading phase dia-
grams and interpreting magmatic mineral assem-
blages.
5.2 Contrast the effects of decompressing a mag-
matic system from a P just above the solidus un-
der water-free and water-saturated conditions.
Crystal-Melt Equilibria in Magmatic Systems
119

granitic rocks? (Hint: Write a balanced miner-
alogical reaction involving these three minerals.)
5.17 Is it true that minerals having higher melting
temperatures crystallize from magmas at rela-
tively higher temperatures? Critically evaluate
this by examining the relative melting tempera-
tures of quartz (-form in Figure 5.1), forsterite
olivine, diopside pyroxene, and albite feldspar
(Figure 3.8). With what mineral(s) does quartz
commonly coprecipitate (Figure 2.2)? What re-
ally controls crystallization temperatures of min-
erals from melts?
5.18 On the basis of the concepts of crystal-melt
equilibria set out in this chapter, how should
Figure 3.12 be modified?
PROBLEMS
5.1 Describe the crystallization and melting of a
bulk composition An
20
from above the liquidus
and from below the solidus, respectively, in the
CaMgSi
2
O
6
-CaAl
2
Si
2
O
8
system at 1 atm. What
are the proportions of phases at 1300°C?
5.2 Thousands of basaltic deposits worldwide con-
tain fragments of phaneritic rock composed of
about 65% magnesian olivine, 25% enstatite,
8% diopside, and 2% Mg-Al-Cr spinel. Name
this rock from Chapter 2, recalculate its mode
so that olivine plus enstatite equals 100%, and
draw an appropriate isopleth on Figure 5.8 for
their recalculated proportions. (Because the
densities of the two minerals are nearly the
same, no correction is needed to convert modal
volume into weight percentage.) Because these
fragments are pieces of the upper mantle from
which basalt magmas are derived by partial
melting, describe the melting relations of this re-
calculated model mantle rock. How much melt
is produced at the initial melting T? What is the
composition of the first partial melt? How does
it compare in composition with an average
basalt (Table 2.2)?
5.3 Using MELTS (Advanced Topic Box 5.4) on the
Makaopuhi basalt magma, (a) confirm that
residual melts become more enriched in Fe and
Ti until Fe-Ti oxides precipitate; (b) determine
the chemical composition of the residual melt
just above the solidus; (c) track the changing
chemical compositions of major solid solutions
during crystallization. Discuss your results.
5.4 Make photocopies of Figure 5.11. Color the sta-
bility field of amphibole. Is amphibole a stable
phase in extruded basalt magmas? Discuss. In
the dry system, contrast (by colors) the stability
fields with plagioclase and with garnet. What
5.3 Liquidus temperatures in simple binary systems
(e.g., Figures 5.4 and 5.8) used to model multi-
component magma systems are hundreds of de-
grees higher than the temperatures at which liq-
uidus phases precipitate in real magmas (e.g.,
Figures 5.10 and 5.11). Why?
5.4 A liquidus line in a binary phase diagram has
four meanings. Describe them.
5.5 Contrast the state of a water-free and a water-
saturated tholeiite system at 1100°C and 10 kbar
(Figure 5.11).
5.6 Cite two scenarios that would allow a thermally
insulated, water-rich intrusive magma to crystal-
lize rapidly—developing a fine-grained tex-
ture—as if it were extruded onto the surface of
the Earth into the cool atmosphere.
5.7 In a magmatic rock, what compositional as-
pect—modal or mineral—defines the intensive
parameters prevailing at the time the magma
crystallized? Explain.
5.8 Contrast crystallization in a magma system dom-
inated by a eutectic versus a system in which
continuous reaction relations take place over a
range of P-T conditions. Which has the poten-
tial to develop the most diverse crystalline prod-
ucts? Which do you think prevails in real mag-
mas? Discuss.
5.9 Describe all of the ways in which a crystallizing
magma can fractionate crystals from melt.
5.10 Contrast equilibrium versus fractional crystal-
lization with respect to operative range of T,
range of residual melt compositions, and nature
of crystalline products. Cite examples.
5.11 Describe incongruent melting and its significance.
5.12 Account for the contrasting mineralogical com-
positions of basalt and eclogite.
5.13 Why are liquid lines of descent on variation dia-
grams curved for fractionating systems involving
solid solutions?
5.14 Partially resorbed anhedral phenocrysts in vol-
canic rocks are not uncommon. Describe possi-
ble origins in both open and closed magma sys-
tems.
5.15 Contrast the changes in the magmatic system
that created the breakdown of amphibole in
Figures 5.32 and 5.34.
5.16 How common are water-saturated granitic mag-
mas? Most of the experimentally determined
phase relations for granite systems are for water-
saturated conditions. Does this necessarily mean
real magmas are water-saturated? What textures
can be found in water-saturated magmas? What
is the implication of the widespread assemblage
of biotite alkali feldspar magnetite in
120 Igneous and Metamorphic Petrology
Crystal-Melt Equilibria in Magmatic Systems
121
controls these contrasting stabilities? What is
the maximum depth to which olivine is stable in
water-free or water-saturated tholeiitic basalt?
How do you reconcile this limited stability of
olivine in basalt relative to what it is in upper
mantle peridotite (Figure 1.3)?
5.5 Prove that the mass balance equation 5.4 is valid
by multiplying the weight percentages of liquid
and crystalline phases by the weight fractions of
the NaAlSi
3
O
8
and CaAl
2
Si
2
O
8
components on
each side of the equation.
5.6 On a photocopy of Figure 5.27a, draw the path
of a fractionating melt whose initial composition
is 5 wt.% SiO
2
and 25 wt.% KAlSi
3
O
8
. Con-
struct lines of liquid descent for Na
2
O and K
2
O
versus SiO
2
for this fractionating melt. Compare
your diagram with Figure 5.27b.
5.7 Sketch the binary KAlSi
2
O
6
-SiO
2
phase diagram
at 1 atm on the basis of the following informa-
tion: Pure K-feldspar melts incongruently at
1150°C to equal amounts of liquid and leucite
crystals; melting is complete at about 1560°C.
Pure leucite (KAlSi
2
O
6
) melts at 1685°C and
pure silica at 1713°C. A binary eutectic lies at
about 990°C and 54 wt.% silica where a silica
polymorph, K-feldspar, and melt are in equilib-
rium. Describe equilibrium and fractional crys-
tallization of a system that contains 30 wt.%
silica.
5.8 Sketch the phase diagram for the binary system
Mg
2
SiO
4
(forsterite)-Fe
2
SiO
4
(fayalite) at 1 atm
keeping in mind that solid solution is complete,
as it is in the plagioclase system. The melting T
of pure fayalite is 1205°C and of pure forsterite
(Figure 3.8) about 1890°C. Describe the liquid
and crystalline products of perfect fractional
crystallization of a selected melt composition,
including a statement about the Fe/(Fe Mg)
ratio.
5.9 On a P-T diagram, sketch approximate relative
positions and slopes of liquidus and solidus
curves for a water-saturated granitic magma.
Superpose on this diagram the relative approxi-
mate positions of the breakdown curves for
muscovite, biotite, and hornblende, using the
following observations for guidelines: Pheno-
crysts of muscovite almost never occur in rhyo-
lite, but fine-grained muscovite does form as a
subsolidus alteration product. Primary mus-
covite occurs only in some peraluminous gran-
ites. Biotite and hornblende are common in
granite and as phenocrysts in rhyolite and
dacite. Amphibole is abundant in higher-T
diorites.
5.10 Using Advanced Topic Box 5.2 and Figure 5.7
as guides, draw a sequence of G-X diagrams at
different temperatures for the NaAlSi
3
O
8
-
CaAl
2
Si
2
O
8
system.

F
UNDAMENTAL
Q
UESTIONS
C
ONSIDERED IN
T
HIS
C
HAPTER
1. How do atomic-scale, time-dependent kinetic
phenomena govern changing states of melts? How
do melts flow as viscous liquids?
2. What is viscosity, what factors control the viscosity
of melts, and how does viscosity impact the
dynamic behavior of melts?
3. What is diffusion, what factors control rates of
atomic and thermal diffusion, and how do these
rates impact crystallization and vesiculation of
melts?
4. How do crystals nucleate, grow, and modify their
shape and size?
5. How do exsolved volatile bubbles nucleate, grow,
and fragment melts?
INTRODUCTION
In dynamic magmatic systems, changing intensive pa-
rameters and energy conditions cause the state of the
system to change, but only insofar as kinetic factors al-
low. Thus, the rate at which the T of a magma decreases
is commonly faster than the rate at which kinetically
controlled crystallization can progress, creating com-
positionally zoned crystals and reaction rims in the
fractionating magma. Sluggish kinetic rates prevent ho-
mogenization of the zoned crystal and equilibration via
reaction relations with the enclosing melt, even though
temperatures may be relatively high. Instantaneous
change from an initial metastable state to a new state of
more stable, perfect equilibrium almost never takes
place in magmatic systems because of slow kinetics.
In this chapter, we focus on kinetic factors that
operate on the atomic level in controlling two basic dy-
namic aspects of melts—solidification and vesiculation-
fragmentation. Whether the same granitic melt solidi-
fies to a massive metastable glass; to an aphanitic or to
a phaneritic aggregate of quartz, feldspar, and biotite
crystals; to highly vesicular pumice; or to fine ash par-
ticles depends on the interplay between rates of chang-
ing environmental conditions, such as T, and kinetic
(time-dependent) process rates. The nature of the ki-
netic history, or kinetic path, taken by the changing
melt during solidification or vesiculation is recorded in
the fabric of the resulting rock, discussed in the fol-
lowing chapter.
Section 3.6 introduced kinetic phenomena. A com-
prehensive treatment is Lasaga (1998).
The first three sections of this chapter deal with
three so-called transport phenomena (Figure 6.1):
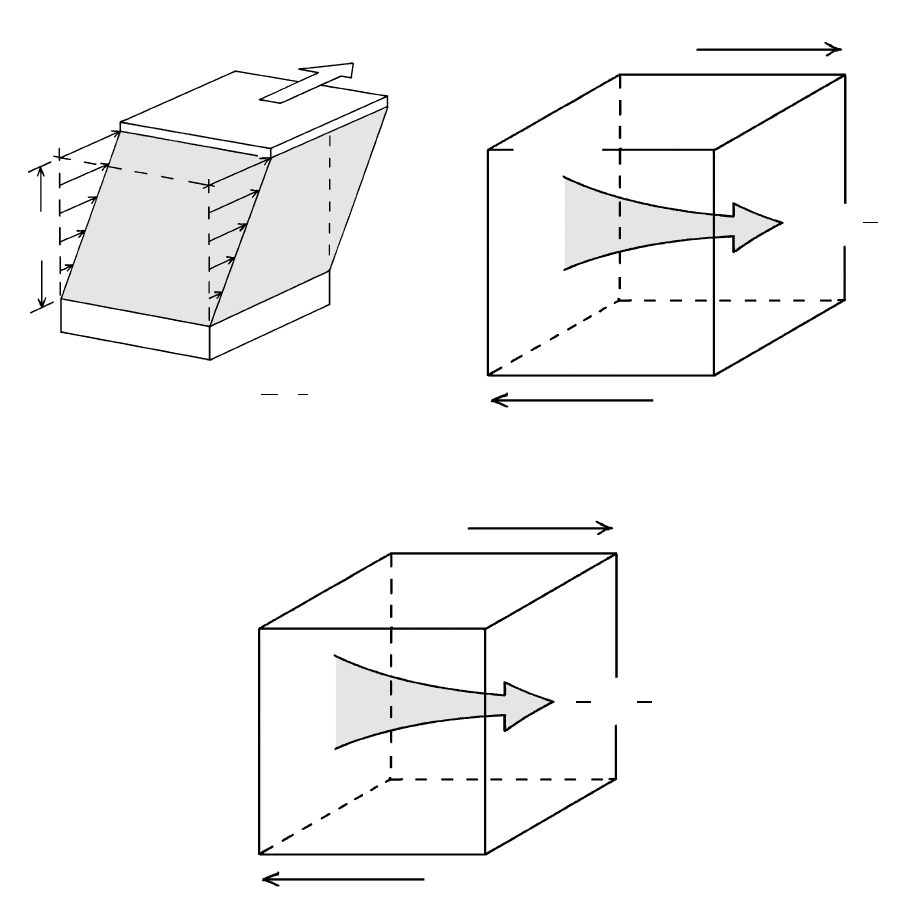
1. Transport of viscous material, sometimes referred
to as a transfer of momentum (mass velocity)
2. Transfer of atoms, usually referred to as chemical
diffusion
3. Transfer of heat by conduction (Section 1.1.3)
Each of these three transport processes has a driving
force and a proportionality constant that is an intrinsic
property of the material. Each depends in some way on
the movement of atoms.
6.1 VISCOSITY OF MELTS
On an atomic scale in melts, viscosity is a measure of
the mobility of atoms, how readily atomic bonds can
be stretched, broken, and reformed with neighboring
6
CHAPTER
Chemical
Dynamics of Melts
and Crystals
Chemical Dynamics of Melts and Crystals
123
atoms. Solidification of melts and exsolution of volatile
components from them, which are the two principal
themes of this chapter, involve the movement of atoms,
or the lack thereof. The ease with which atoms can
move about is expressed on a larger macroscopic scale
in a body of material, such as a lava flow, in its resis-
Stationary
plate
dy
dv
Viscous flow
(movement of material)
τ
τ
dv
dy
1
η
=
(a)
6.1 Three transport phenomena. All involve a gradient in some quantity that provides a driving force for transport and all follow similar laws.
(a) Viscous flow in a cube of material (dashed lines) is driven by a force applied tangentially to its upper surface area. This applied shear
stress ( force/area stress), , produces a change in the shape of the body so that the cube becomes a parallelepiped. The change in shape,
called strain, , becomes more extreme with time, that is, the applied shear stress creates a certain strain rate, d/dt, whose magnitude
depends on the viscosity of the particular material, . The strain rate is equivalent to a velocity gradient, dv/dy, which is shown by arrows
of increasing length over the distance dy. (b) Chemical diffusion is driven by a change in concentration, dc, of some particular atoms be-
tween a region of higher concentration (left face of the cubical volume of material) and lower concentration (right face); these two faces
are separated by a distance, dx. The concentration gradient, dc/dx, produces a flow of atoms, J, down the gradient. (c) Thermal diffusion,
or heat conduction, is driven by a gradient in temperature over a distance, dT/dx, and produces a flow of heat over time, dq/dt. The pro-
portionality constants for these three types of transport are intrinsic properties of the material. For viscous flow the constant is the coef-
ficient of viscosity, or viscosity, ; for chemical diffusion it is the diffusion coefficient, or diffusivity, D; and for heat conduction it is the
thermal conductivity, k.
(b)
dx
Lower
concentration
concentration
dc
dx
J = − D
Higher
dc
Chemical diffusion
(movement of atoms)
Cooler
dq
dt
=
dΤ
dx
−
k
Hotter
dT
dx
Thermal diffusion
(conductive movement of
thermal energy)
(c)
tance to flow or deformation. On both scales, viscosity
is a manifestation of mobility. Less mobility corre-
sponds to greater viscosity. “Fluidity” and viscosity are
inversely related (opposites).
Unquestionably, viscosity is the most important
property of melts that controls the dynamic behavior of
magmas. Segregation of partial melts in upper mantle
and lower crustal sources, magma ascent to shallower
depths, intrusion, extrusion as lavas or as explosive
fragments, and crystallization all depend on the viscos-
ity of the melt.
Viscosity is formally defined in Figure 6.1a. A force
exerted parallel to the surface area of a viscous body
produces a shear stress, (stress force/area), that de-
forms the body by viscous flow in a time-dependent
way. This flow deformation can be measured by a
velocity gradient, dv/dy, or alternatively by a rate of
change in shape, or strain rate, d/dt. The proportion-
ality constant relating the shear stress (the “driving
force”) and the resultant flow is the coefficient of New-
tonian viscosity,
6.1
d
d
v
y
d
d
t
For brevity, the coefficient of viscosity can simply be
called the viscosity.
A convenient way to measure viscosity is by deter-
mining how fast a sphere of contrasting density falls or
rises through the material (see Stokes law in Section
8.3.3).
The unit of viscosity is the Pa s (Pascal second) or
the poise (10 poise Pa s). Typical viscosities (in Pa s)
include air 10
5
; water 10
3
; honey 10
1
; cool asphalt
(tar) 10
8
. Silicate glasses have viscosities 10
12
. In
terms of its atomic structure, glass is a liquid, but in
terms of its behavior (atomic mobility and capacity to
flow), it is a solid. One way to distinguish between a
solid and a liquid is by the time scale for flow. Liquids,
such as water and honey, can flow appreciably in min-
utes because of low viscosity, whereas solids “flow”
negligibly, or not at all, over periods measured in years.
For example, the solid mantle of the Earth, whose vis-
cosity is on the order of 10
20
Pa s, convectively flows at
a rate of a few centimeters or less per year, the rate of
plate motion.
The two principal factors governing viscosity of
melts are their composition—as it controls the de-
gree of polymerization—and their T. More polymer-
ized, more silica-rich melts, which have smaller ratios
of nonbridging oxygens to network-forming cations
(Section 4.1.1), are more viscous. Ions cannot move
about readily, especially those that make up polymers,
because the strong Si O and Al O bonds must be
stretched or broken. Less polymerized silica-poor
melts and silica-rich melts that contain dissolved
water and/or fluorine have lower viscosities. For any
melt composition, higher T reduces the viscosity by
“loosening” the melt structure through the increased
kinetic energy of the atoms; ionic mobility is en-
hanced.
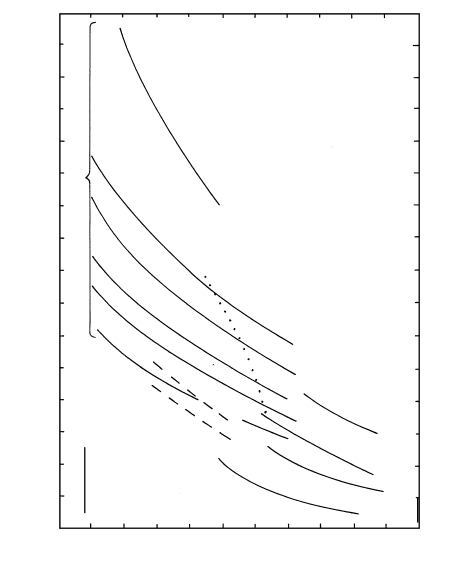
Figure 6.2 illustrates the strong dependence of melt
viscosity on T and composition. For example, the de-
pendence on T is shown by the fact that between
700°C and 1000°C the viscosity of a water-free rhyolite
melt decreases by six orders of magnitude, from about
10
15
to 10
9
Pa s. The dependence on composition is
shown by the fact that there is a difference of about 10
5
Pa s in viscosities at 1200°C between a silica-poor (46
wt.%) alkali-olivine basalt melt and a silica-rich (77
wt.%) rhyolite melt, both water-free. Besides the silica
concentration factor, dissolved water drastically lowers
the viscosity of highly polymerized, silica-rich melts be-
cause the polymers are broken by hydroxyl ions. Addi-
tion of 6 wt.% water to a rhyolite melt with 77 wt.%
124 Igneous and Metamorphic Petrology
600
−1
1
3
5
7
9
11
13
15
800 1000 1200 1400 1600
T (°C)
log
10
viscosity, η (Pa s)
Alkali
carbonatite
Rhyolite 77 wt. % SiO
2
1
2
4
10
6
0
74, 76
73
8
Andesite 61
Tholeiitic
basalt
51
Alkali basalt
46
basalt
~48
Komatiite
6.2 Newtonian viscosities of some crystal- and bubble-free melts
as a function of T at 1 atm. P dependences are negligible. Some
viscosities pertain to metastable melts at temperatures below
their liquidus where they would contain crystals under equi-
librium conditions. Concentrations of silica in weight percent-
age are indicated as well as of water in weight percentage at
low-T end of curves. (Data from Shaw, 1965; Murase and
McBirney, 1973; Webb and Dingwell, 1990; Dawson et al.,
1990; Huppert and Sparks, 1985.) Viscosities for rhyolite
melts that contain 1.5 wt.% fluorine and 6 wt.% water at
10 kbar are dashed lines (peraluminous, 74 wt.% silica; meta-
luminous, 76; peralkaline, 73). (Data from Baker and Vaillan-
court, 1995.) The dotted line indicates the viscosity of residual
melts resulting from fractional crystallization of Makaopuhi
basalt melt (Table 5.1) with 0.5 wt.% water on the QFM
buffer calculated from the MELTS program; the most evolved
melt has 73 wt.% silica and 0.3 wt.% water.

Chemical Dynamics of Melts and Crystals
125
silica at 800°C lowers the viscosity by seven orders of
magnitude, from about 10
12
to 10
5
Pa s. Most of the re-
duction in viscosity in hydrous silicic melts occurs in
the first 2 wt.% or so of added dissolved water because
that first 2% is mostly (OH)
(Section 4.2.2). Dis-
solved water has less effect on basalt melts because they
are less polymerized regardless of volatile content. Dis-
solved fluorine also reduces polymerization and viscos-
ity, but, unlike water, which has very limited solubility
in melts at low P, fluorine can remain in solution in the
melt and can therefore enhance the mobility of lavas
extruded onto the surface of the Earth. Rhyolite melts
having the lowest viscosity are those that contain both
dissolved water and fluorine. The lowest viscosities of
any known terrestrial melts are found in ultramafic
high-T komatiites and essentially silica-free, alkali car-
bonatites extruded at about 585°C from Oldoinyo
Lengai volcano in the East African Rift (Dawson et al.,
1990). The 15 orders of magnitude difference in vis-
cosity between carbonatite and dry rhyolite melts ex-
truded near 600°C is illustrated by the contrasting
morphological characteristics of their extruded lavas—
the former having the appearance of muddy water and
the latter forming steep bulbous domes that can be
higher than their horizontal diameter. Widespread
basaltic lavas that are no more than a couple of orders
of magnitude more viscous than cooler carbonatitie
melts form sheetlike flows, again in striking contrast
with mushroom-shaped rhyolite extrusions.
Melt viscosity is only weakly dependent on P. An
isothermal increase in P of 20 kbar reduces the viscos-
ity of mafic melts by less than 50% and silicic melts by
a slightly greater factor (Richet and Bottinga, 1995).
This negative dependence on P follows because bridg-
ing oxygens have a greater molar volume than non-
bridging, and, therefore, from Le Chatelier’s principle,
a positive increment in P increases the ratio of non-
bridging to bridging oxygens that depolymerizes the
melt, reducing the viscosity.
The viscosity of melts that carry suspended crystals
(i.e., magma) and contain bubbles of volatile fluid, as
well as those that have an intrinsic yield strength do not
follow the form of Equation 6.1 (see Section 8.2.2 for a
discussion of non-Newtonian magmas).
If a melt is deformed slowly, its distorted atomic
structure has time to adjust or relax; it behaves as a liq-
uid. However, for increasingly rapid deformation (in-
creasing strain rate) at constant T, particularly in cooler
more viscous polymerized melts, the melt crosses a
threshold between liquid and solid behavior, called the
glass transition, defined as the T at which 10
12
Pa s.
If rapid deformation takes place at viscosities above
this glass transition, the melt cannot adjust: its relax-
ation “time” is exceeded. It behaves as if it were a
solid, breaking as any brittle solid does if the applied
stress is excessive.
Advanced Topic Box 6.1 Calculation of
melt viscosity
Shaw (1972) developed a model from which the
viscosity of homogeneous melts can be calculated
from their chemical composition. This model as-
sumes the viscosity follows an Arrhenius relation
A exp (E
a
/RT) where A is a constant, E
a
is
the activation energy of viscous flow, R is the gas
constant, and T is in degrees Kelvin. Although many
melts depart to varying degrees from Arrhenian be-
havior, Shaw’s model has nonetheless proved useful.
The full details cannot be dealt with here; only a
“cookbook” presentation is given. The viscosity,
, is found from the equation
2.303 log
10
s(10
4
/T) 1.5s 6.4
For example, calculation of the viscosity of a
hydrous rhyolite (granite) melt at T 1073K
(800°C) is presented in Table 6.1, where determi-
nation of the value of s is described (minor con-
stituents, such as MnO and P
2
O
5
, are ignored).
Numbers in the Moles column are found by di-
viding the Wt.% values by the Formula wt. of
the corresponding oxide. Note that the moles of
Al
2
O
3
and Fe
2
O
3
are doubled. The mole fraction,
X, is found by dividing the Moles value by the
sum of Moles, or 1.908. In the next column, X
i
, the
mole fractions of Fe
2
O
3
, FeO, and MgO are added
together (giving 0.009), as are CaO and TiO
2
(0.004), and Na
2
O and K
2
O (0.057). Shaw’s values
of the partial molar activation energies of SiO
2
in
binary systems with categories of other oxides, s
i
o
,
are listed in the next column. These values multi-
plied by the mole fraction of SiO
2
in the particular
melt are listed in the s
i
o
X
SiO
2
column. Finally, at the
bottom of Table 6.1, the sum of the X
i
(s
i
o
X
SiO
2
)
values listed in the last column is divided by (1
X
SiO
2
) to give s, which is inserted into the viscosity
equation. The viscosity calculated from this equa-
tion is 10
5.24
poise 10
4.24
Pa s. The viscosity mea-
sured by Shaw (reference in his 1972 paper) is
10
4.63
Pa s.
Hess and Dingwell (1996) and Baker (1996) have
devised models based on experimental data that ac-
count for the non-Arrhenian T dependence of vis-
cosity for hydrous rhyolite (granite) melts.
In the Shaw, Hess-Dingwell, and Baker models
no dependence on P is included, as this is negli-
gible.
Ryan and Blevins (1987) have compiled experi-
mentally determined viscosities of a wide range of
melt compositions.
6.2 CHEMICAL DIFFUSION
Diffusion, the second transport phenomenon (Figure
6.1b), is the movement of individual atoms or molecules
through a group of atoms and driven by a concentration
gradient. The material in which atoms are moving does
not itself necessarily move. Atoms and molecules diffuse
quickly through gaseous bodies where the atoms are
widely separated and loosely associated; for example,
aromatic molecules from brewing coffee disperse many
meters in minutes into the surrounding air. Diffusion is
much slower in a liquid and depends strongly on its vis-
cosity and T; higher T expands the atomic structure and,
together with lower viscosity, allows greater freedom of
movement for diffusing atoms. Diffusion is still slower in
solids where atoms are tightly bonded and have limited
kinetic motion relative to those in liquids.
Bowen (1921) first drew attention to the significance
of diffusion in magma systems. But it was not until a
half-century later, beginning with Shaw (1974), that
other experimental petrologists began to explore this
important transport process.
Diffusion plays many roles in petrologic processes,
some of which are growth of crystals from melts and
other multicomponent solutions, exsolution of vola-
tiles from a melt, reaction relations between melt and
crystals to produce equilibrium compositions among
major as well as trace elements, mixing and contamina-
tion of magmas, and isotopic exchange between crys-
tals and fluids. Because the distance over which diffu-
sion takes place depends on time and T, measurement
of small-scale compositional inhomogeneities within
rock-forming minerals and glasses, such as by an elec-
tron microprobe, can provide important constraints on
their thermal history if the relevant diffusional proper-
ties are known. The closure temperature at which
T-dependent diffusion effectively ceases, or becomes
nil in a geologic time frame, must be taken into account
in interpreting isotopic ages, mineral geothermometers
and geobarometers, and any other aspect of a “frozen-
in” state of compositional equilibrium.
If rates of diffusion (and other kinetic processes)
were infinitely fast, thermodynamic equilibrium would
be accomplished instantaneously as changes in inten-
sive variables occurred. However, diffusion is slow in
melts and can be extremely slow in crystals, so that ves-
tiges or relics of prior states may be preserved in mag-
matic rocks. Fortunately for the petrologist, sluggish
rates of diffusion can provide insights into prior
metastable states and make possible inferences about
petrologic history.
6.2.1 Types of Diffusion
Three routes of diffusion are possible in an aggregate
of mineral grains, a rock.
1. Surface diffusion occurs essentially over a two-
dimensional surface area, but generally has a com-
ponent of atomic movement in the third dimension.
Thus, ions can move about over the free surface of
a mineral grain through a static liquid in contact
with it.
2. Grain-boundary (intergranular) diffusion occurs
along mutual boundaries between contacting min-
eral grains. Because of the hindering effects of the
adjacent grain, diffusion is slower than for surface
diffusion, though both utilize the exterior of a
grain. A very thin film of fluid along grain bound-
aries may facilitate diffusion.
3. Volume diffusion occurs within any single homoge-
neous phase, such as a crystal or a melt body. Vol-
ume diffusion through solids is generally the slow-
126 Igneous and Metamorphic Petrology
Table 6.1 Calculation of the viscosity of a hydrous rhyolite melt at 1073K as described in
Advanced Topic Box 6.1.
W
T
.% F
ORMULA
W
T
.M
OLES
XX
iS
i
°
S
i
°
X
SiO
2
Xi(
S
i
°
X
SiO
2
)
SiO
2
71.90 60.085 1.195 0.627
Al
2
O
3
12.15 101.961 0.238 0.125 0.125 6.7 4.201 0.525
Fe
2
O
3
0.57 159.692 0.007 0.004 0.009 3.4 2.132 0.019
FeO 0.52 71.846 0.007 0.004
MgO 0.04 40.311 0.001 0.001
CaO 0.27 56.079 0.005 0.003 0.004 4.5 2.82 0.011
TiO
2
0.09 79.899 0.001 0.001
Na
2
O 3.94 61.999 0.064 0.033 0.057 2.8 1.756 0.100
K
2
O 4.32 94.203 0.046 0.024
H
2
O 6.20 18.015 0.344 0.180 0.180 2.0 1.254 0.226
SUM 100.00 1.908 0.881
s [ X
i
(S
i
°
X
SiO
2
)] (1 X
SiO
2
) 0.881 0.373 2.362. 2.303 log
10
s (10
4
/T) 1.5s 6.4 2.362 (10
4
/1073) (1.5 2.362) 6.4 12.07.
10
5.24
poise 10
4.24
Pa s.
Data from Shaw (1972).
